Functionalization of Graphene Oxide with Low Molecular Weight Poly (Lactic Acid)
Abstract
:1. Introduction
2. Experimental Methods
2.1. Materials
2.1.1. Preparation of Raw Materials
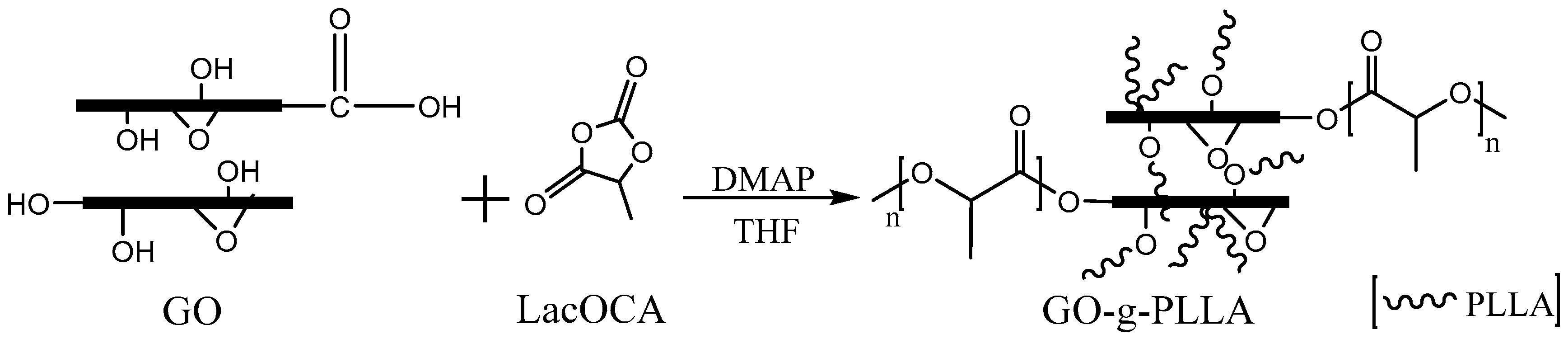
2.1.2. Preparation of GO-g-PLLA
2.1.3. Preparation of GO-g-PLLA/PLLA Composite Material
2.1.4. Instruments and Characterization
3. Results and Discussion
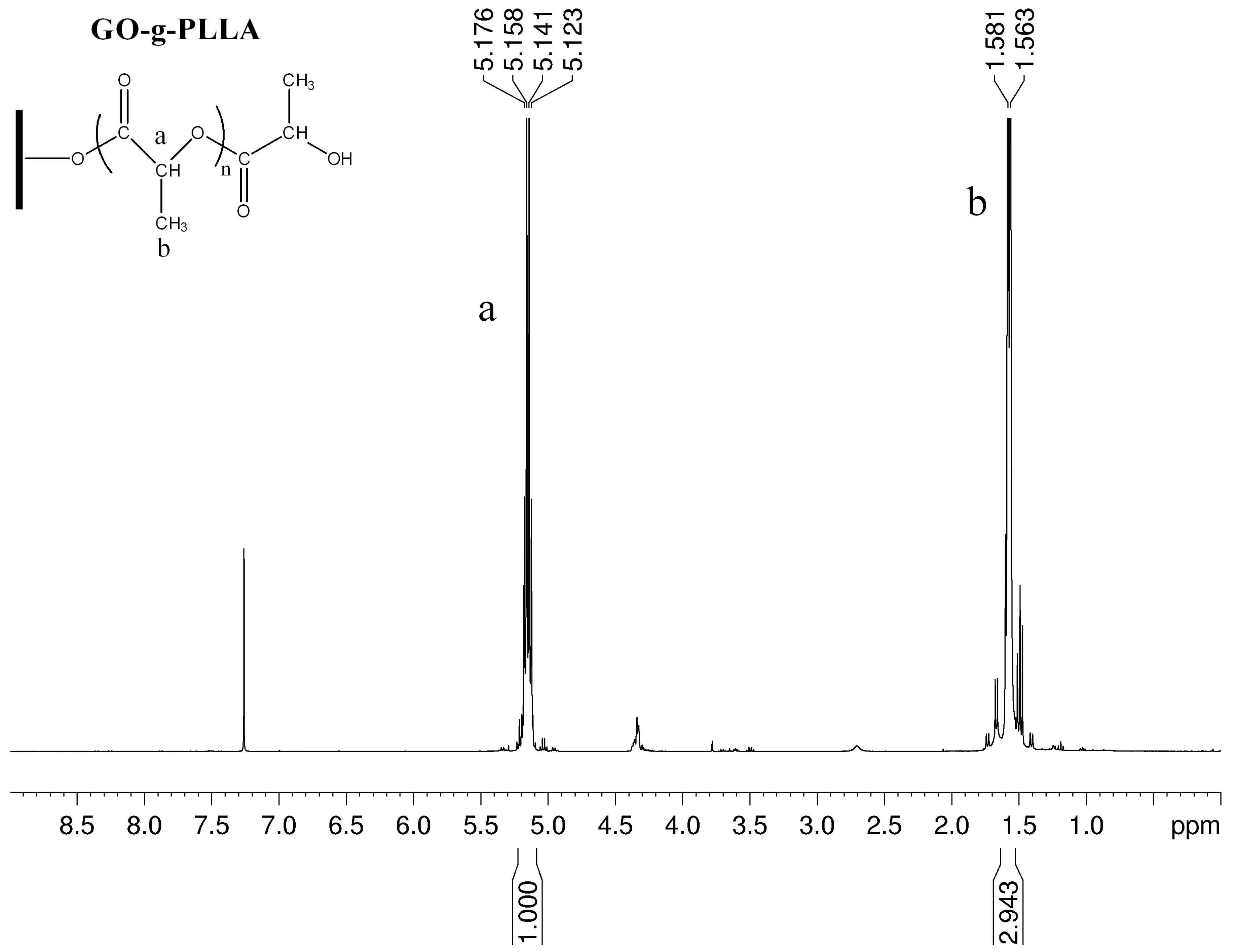
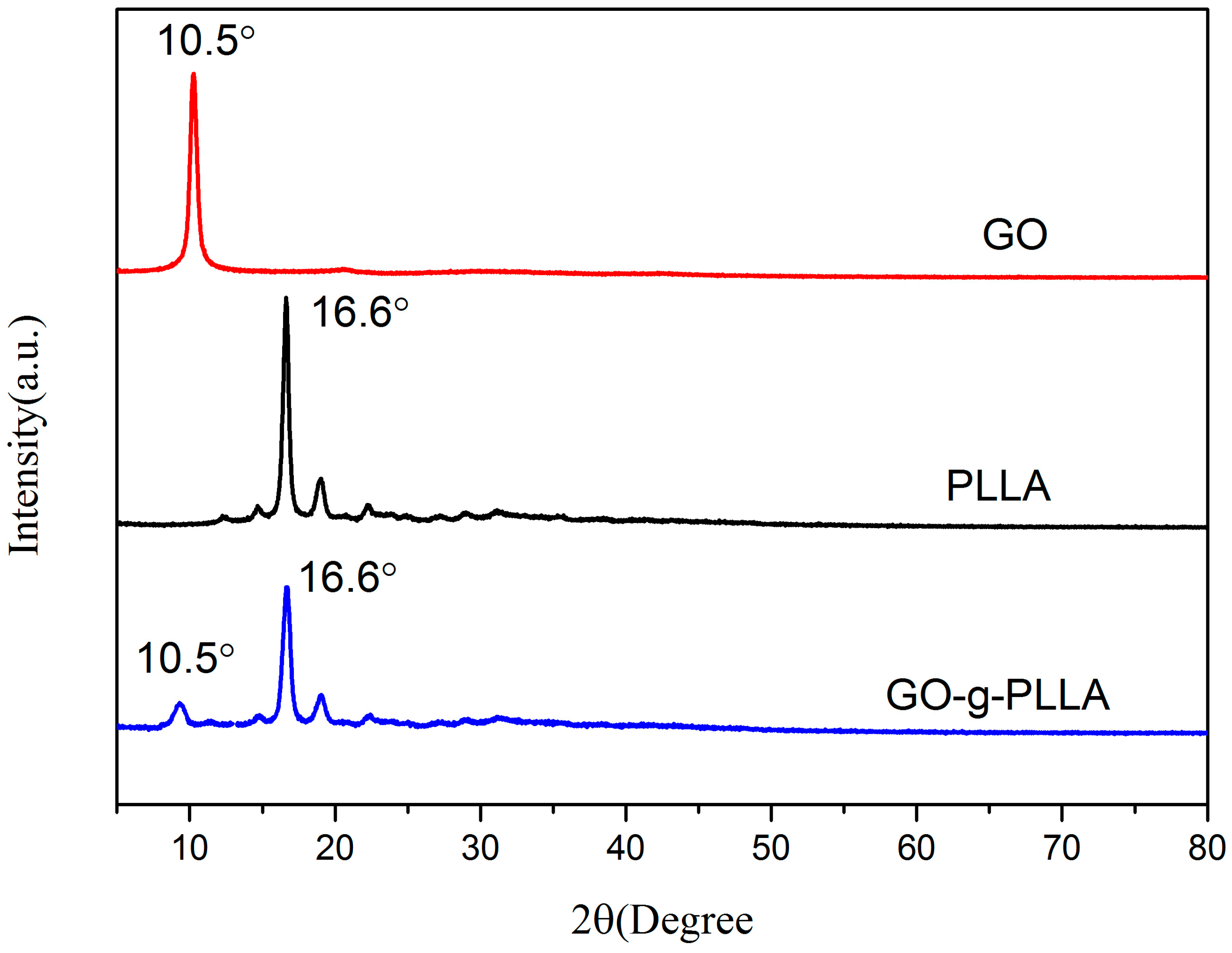
3.1. Characterization of GO-g-PLLA
3.2. Determination of Molecular Weight by Gel Permeation Chromatography (GPC)
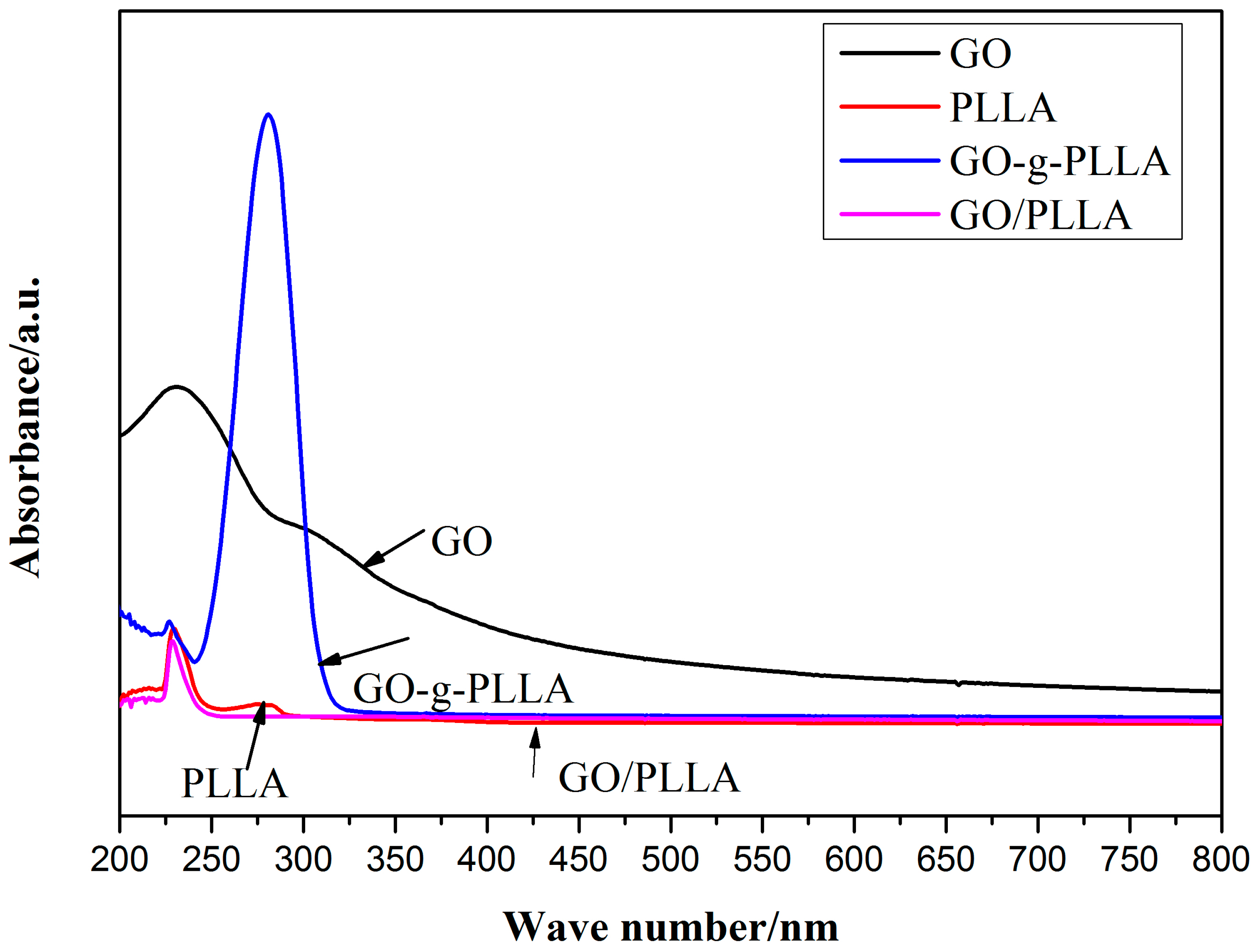
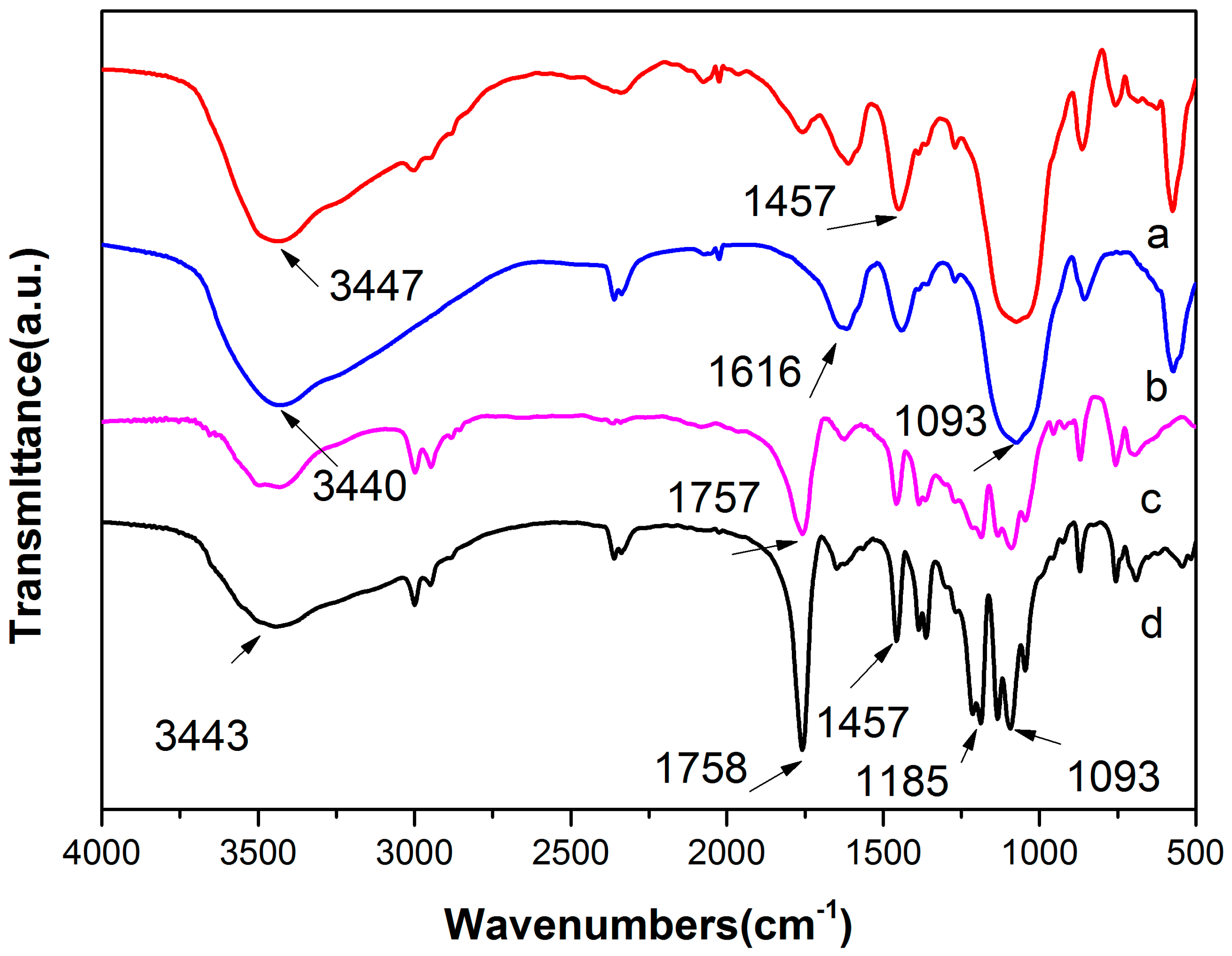
3.3. Infrared (IR) Spectroscopy
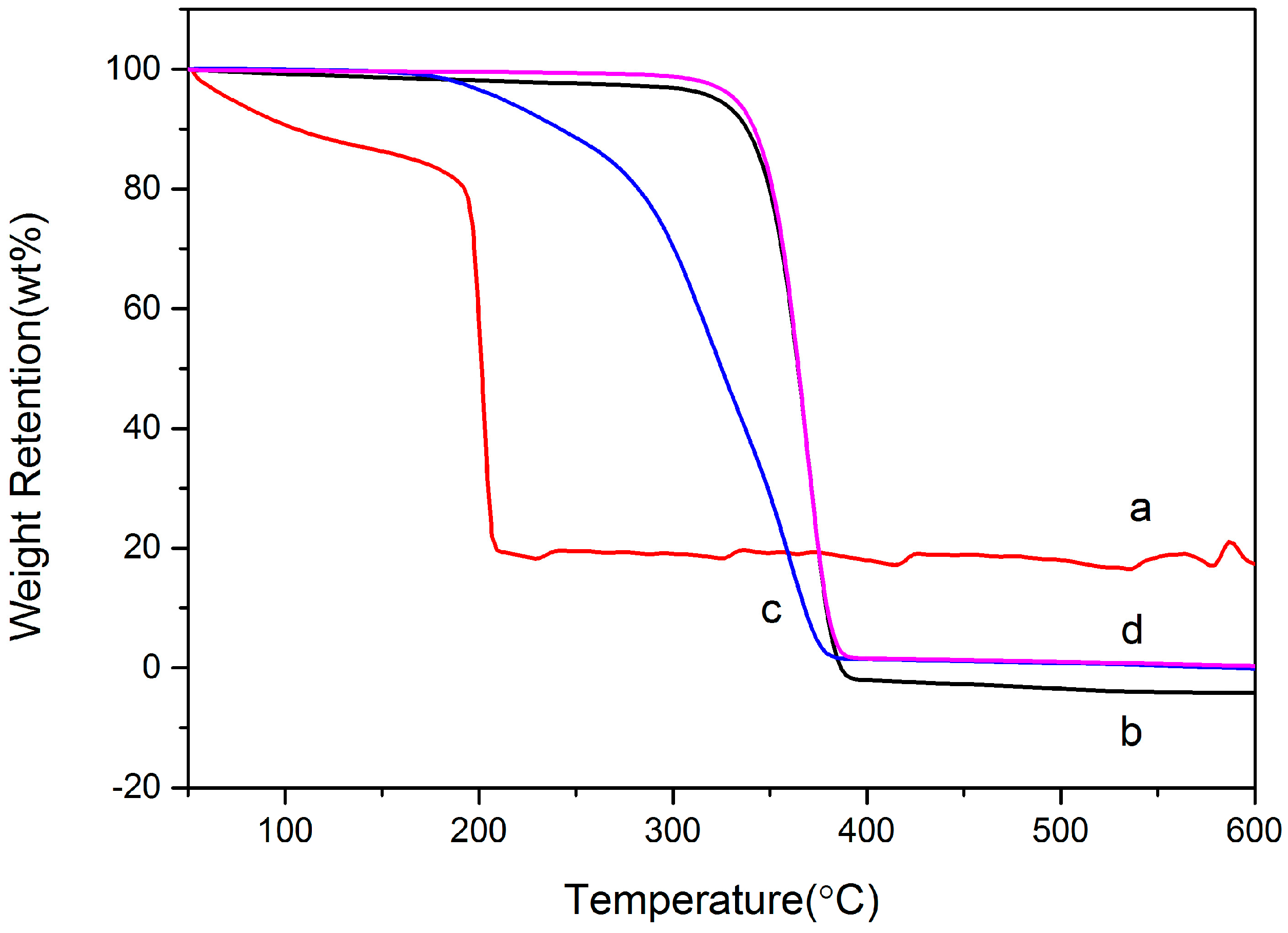
3.4. Thermogravimetric Analysis (TGA)
3.5. X-ray Photoelectron Spectroscopy (XPS)
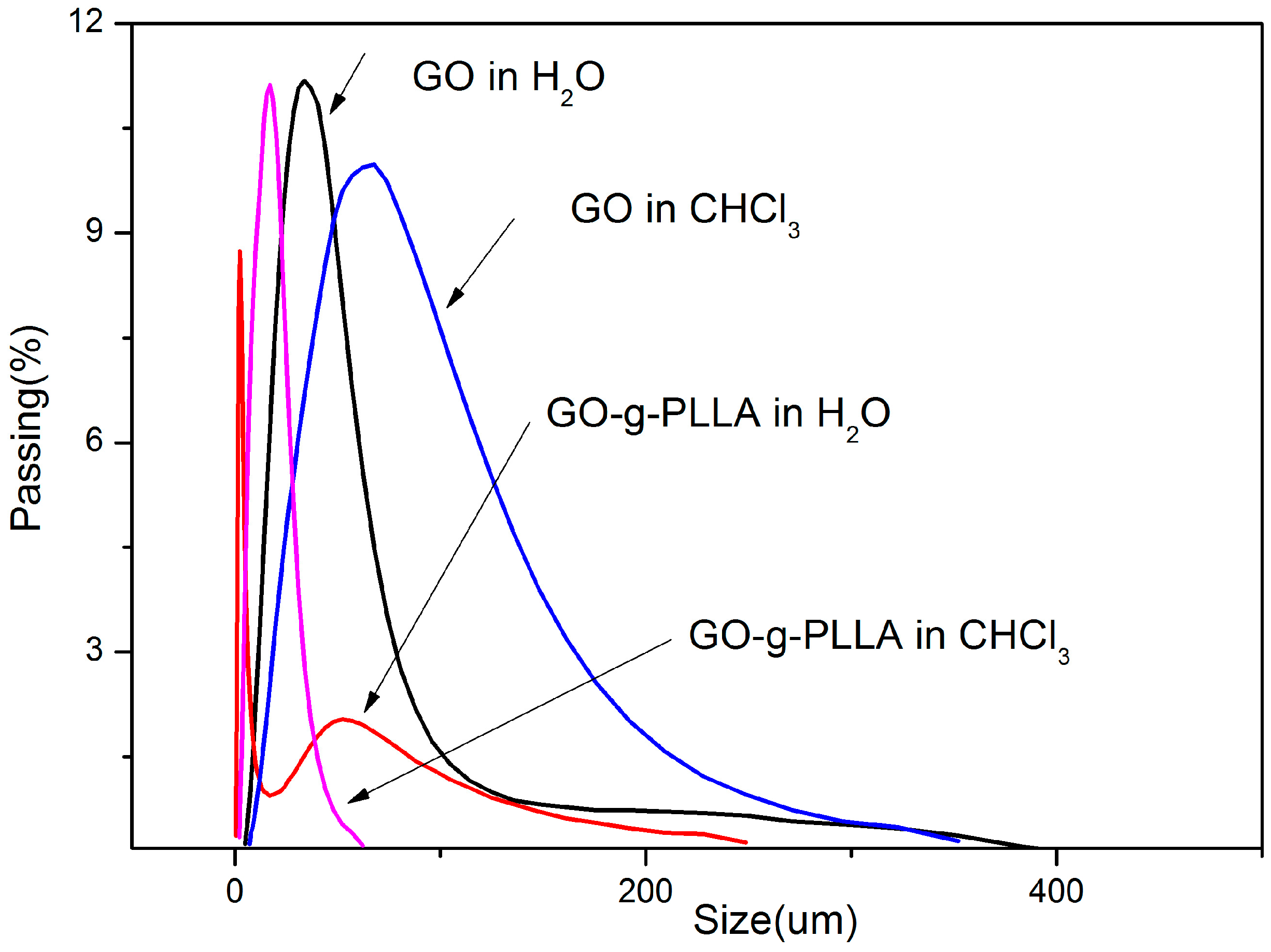
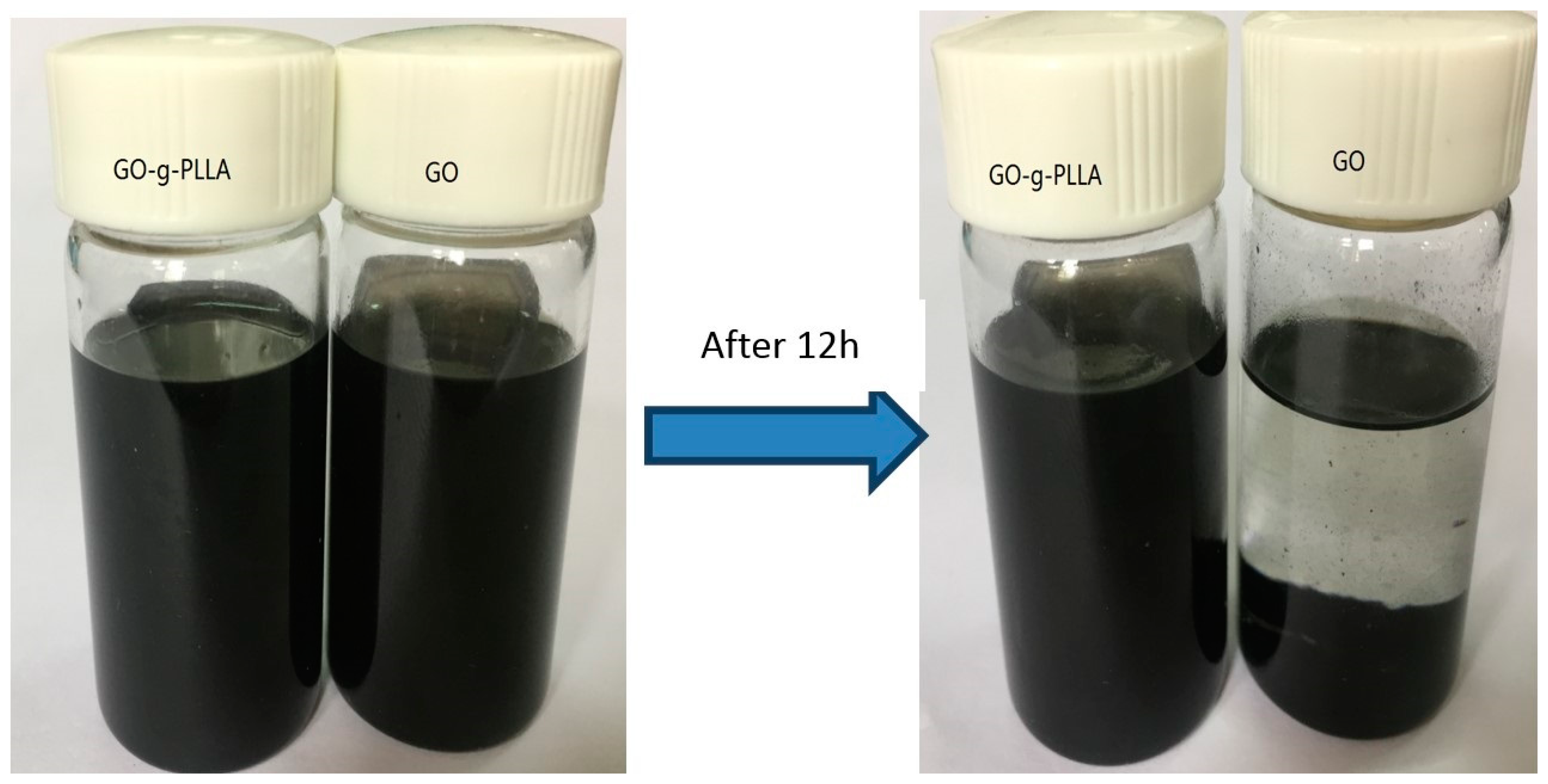
3.6. Characterization of Physical Properties
3.7. Differential Scanning Calorimetry (DSC) of PLLA and Its Composites
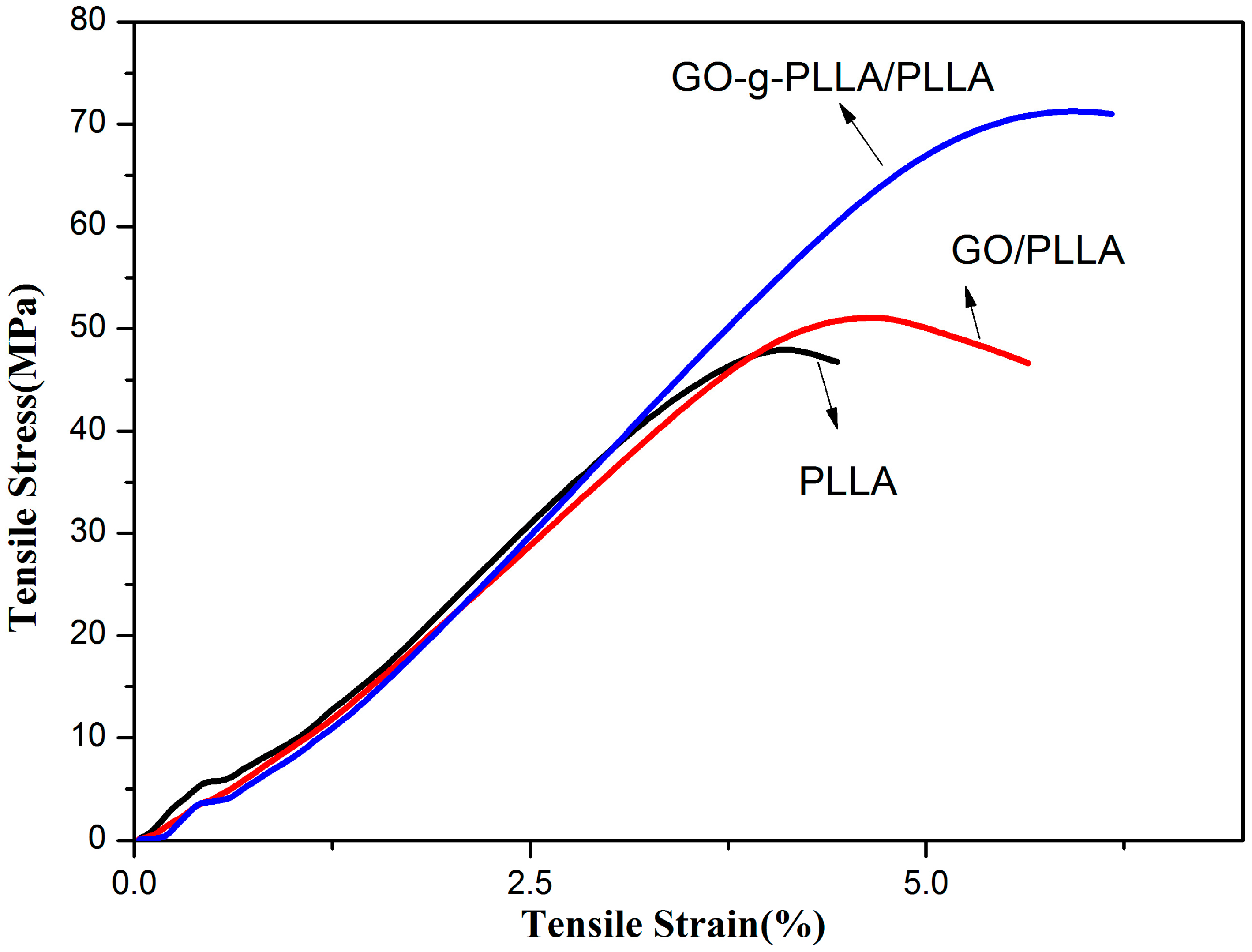
3.8. Characterization of the Mechanical Properties of PLLA and Its Composite
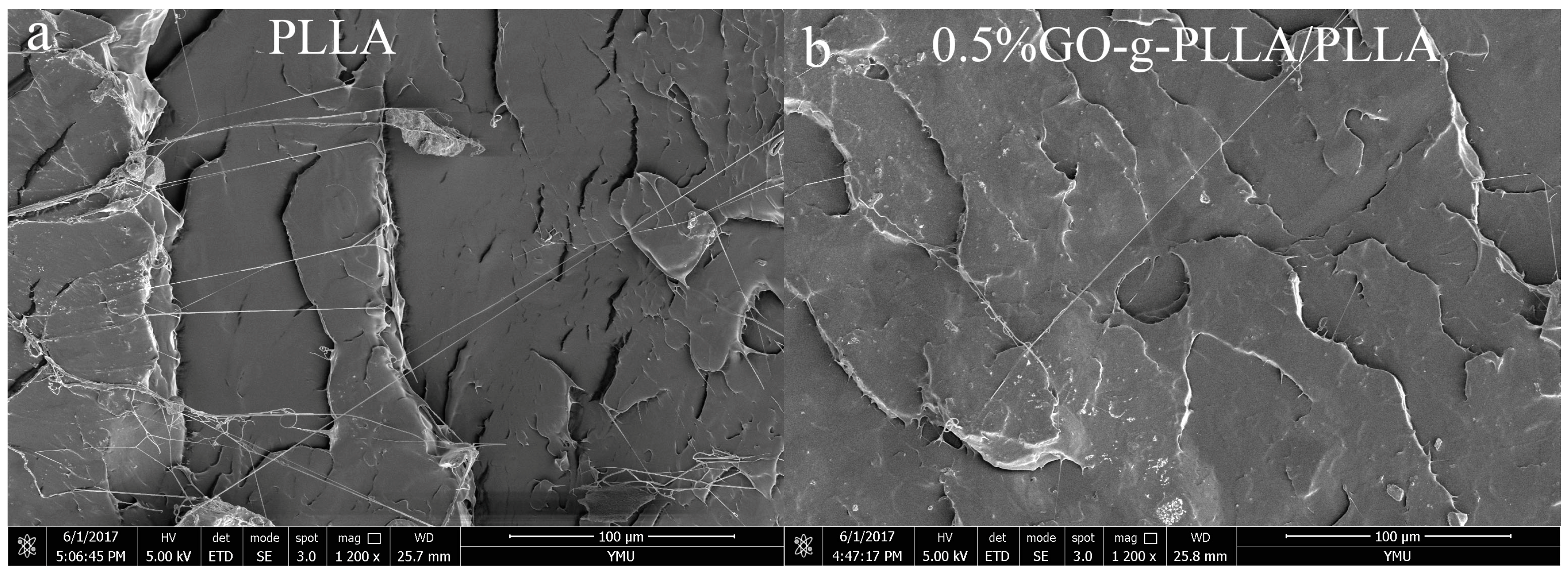
3.9. Characterization of the Morphology of PLLA and Its Composite
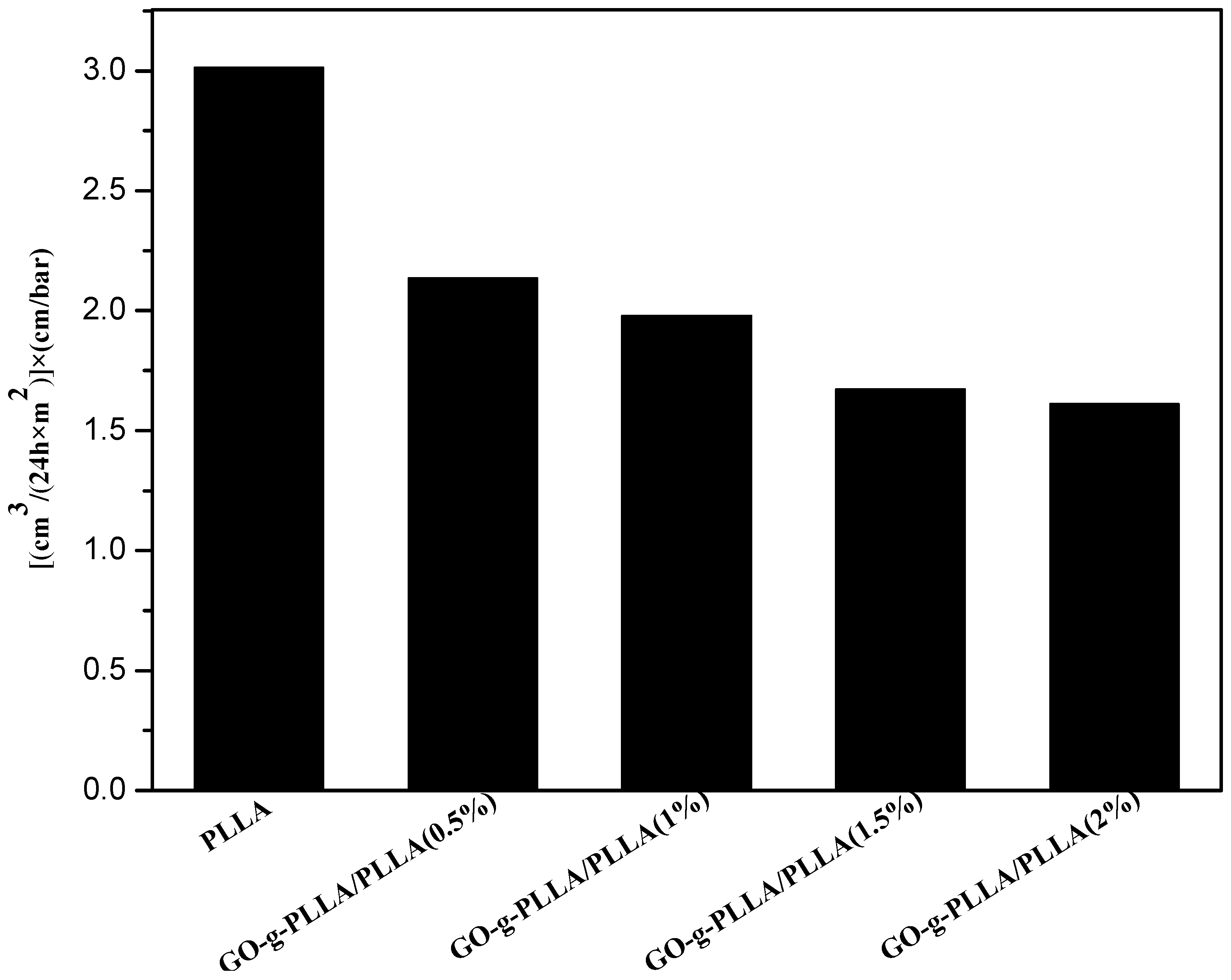
3.10. Barrier Tests
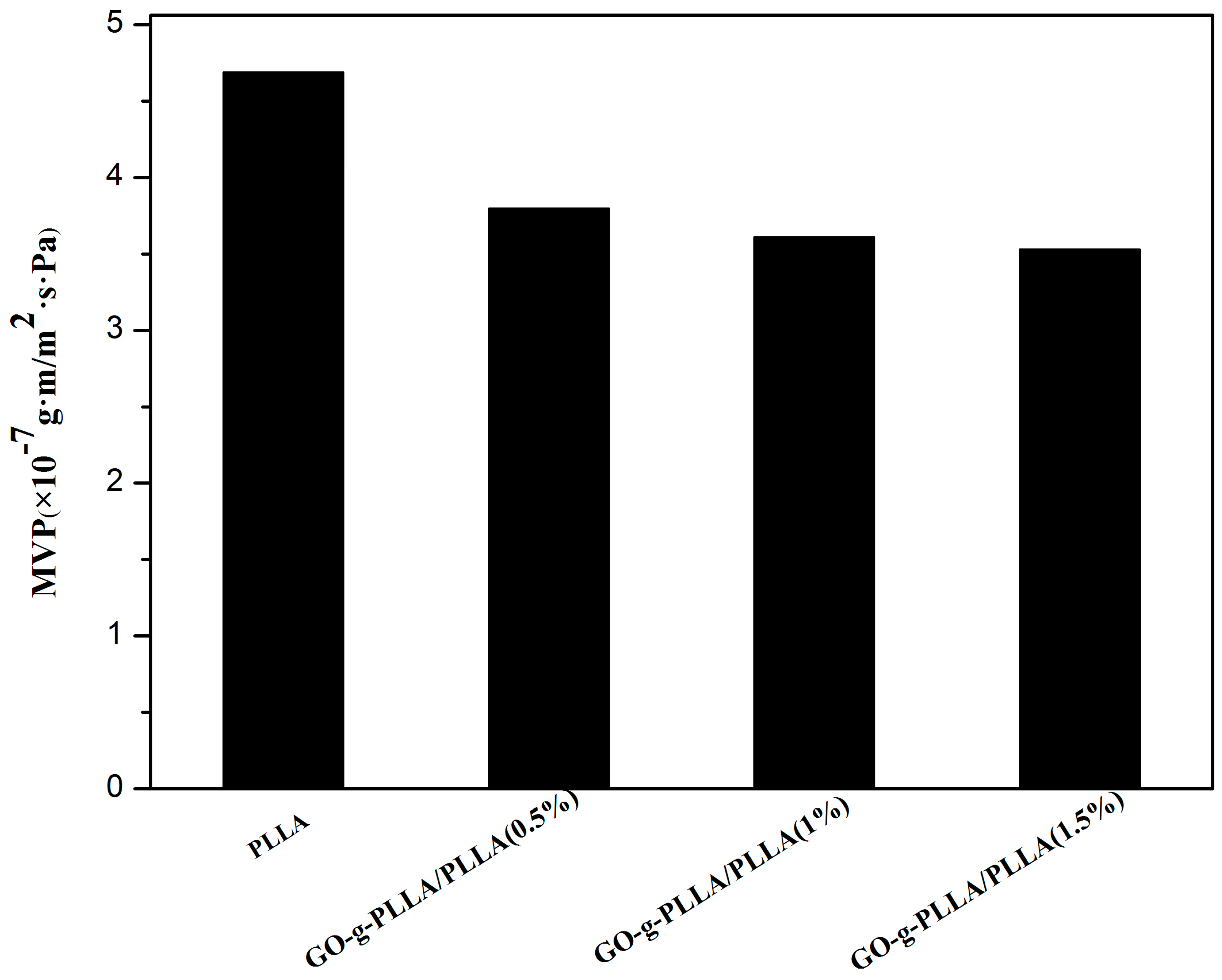
3.10.1. Water Vapor Permeability (WVP) Test
3.10.2. Oxygen Permeability (PO2) Test
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly(lactic acid) modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Anderson, K.S.; Schreck, K.M.; Hillmyer, M.A. Toughening polylactide. Polym. Rev. 2008, 48, 85–108. [Google Scholar] [CrossRef]
- Xu, J.-Z.; Chen, T.; Yang, C.-L.; Li, Z.-M.; Mao, Y.-M.; Zeng, B.-Q.; Hsiao, B.S. Isothermal crystallization of poly(l-lactide) induced by graphene nanosheets and carbon nanotubes: A comparative study. Macromolecules 2010, 43, 5000–5008. [Google Scholar] [CrossRef]
- Murariu, M.; Dechief, A.L.; Bonnaud, L.; Paint, Y.; Gallos, A.; Fontaine, G.; Bourbigot, S.; Dubois, P. The production and properties of polylactide composites filled with expanded graphite. Polym. Degrad. Stab. 2010, 95, 889–900. [Google Scholar] [CrossRef]
- Cao, Y.; Feng, J.; Wu, P. Preparation of organically dispersible graphene nanosheet powders through a lyophilization method and their poly(lactic acid) composites. Carbon 2010, 48, 3834–3839. [Google Scholar] [CrossRef]
- Wang, H.; Qiu, Z. Crystallization kinetics and morphology of biodegradable poly(l-lactic acid)/graphene oxide nanocomposites: Influences of graphene oxide loading and crystallization temperature. Thermochim. Acta 2012, 527, 40–46. [Google Scholar] [CrossRef]
- Wang, H.; Qiu, Z. Crystallization behaviors of biodegradable poly(l-lactic acid)/graphene oxide nanocomposites from the amorphous state. Thermochim. Acta 2011, 526, 229–236. [Google Scholar] [CrossRef]
- Liu, S.; Gordiichuk, P.; Wu, Z.-S.; Liu, Z.; Wei, W.; Wagner, M.; Mohamed-Noriega, N.; Wu, D.; Mai, Y.; Herrmann, A.; et al. Patterning two-dimensional free-standing surfaces with mesoporous conducting polymers. Nat. Commun. 2015, 6, 8817. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, J.; Dong, R.; Gordiichuk, P.; Zhang, T.; Zhuang, X.; Mai, Y.; Liu, F.; Herrmann, A.; Feng, X. Two-dimensional mesoscale-ordered conducting polymers. Angew. Chem. Int. Ed. 2016, 55, 12516–12521. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; He, C. Synthesis and stereocomplex crystallization of poly(lactide)-graphene oxide nanocomposites. ACS Macro Lett. 2012, 1, 709–713. [Google Scholar] [CrossRef]
- Arias, V.; Odelius, K.; Hoglund, A.; Albertsson, A.-C. Homocomposites of polylactide (PLLA) with induced interfacial stereocomplex crystallites. ACS Sustain. Chem. Eng. 2015, 3, 2220–2231. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wu, D.; Yang, X.; Xie, L.; Hakkarainen, M. Thermostable and impermeable “nano-barrier walls” constructed by poly(lactic acid) stereocomplex crystal decorated graphene oxide nanosheets. Macromolecules 2015, 48, 2127–2137. [Google Scholar] [CrossRef]
- Xu, H.; Xie, L.; Wu, D.; Hakkarainen, M. Immobilized graphene oxide nanosheets as thin but strong nanointerfaces in biocomposites. ACS Sustain. Chem. Eng. 2016, 4, 2211–2222. [Google Scholar] [CrossRef]
- Xu, H.; Feng, Z.-X.; Xie, L.; Hakkarainen, M. Graphene oxide-driven design of strong and flexible biopolymer barrier films: From smart crystallization control to affordable engineering. ACS Sustain. Chem. Eng. 2016, 4, 334–349. [Google Scholar] [CrossRef]
- Hua, L.; Kai, W.; Yang, J.; Inoue, Y. A new poly(l-lactide)-grafted graphite oxide composite: Facile synthesis, electrical properties and crystallization behaviors. Polym. Degrad. Stab. 2010, 95, 2619–2627. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Jonte, J.M. New polymer syntheses. 8. Synthesis and polymerization of l-lactic acid O-carboxyanhydride (5-methyl-dioxolan-2,4-dione). Polym. Bull. 1983, 9, 276–283. [Google Scholar]
- Bonduelle, C.; Martin-Vaca, B.; Bourissou, D. Lipase-catalyzed ring-opening polymerization of the O-carboxylic anhydride derived from lactic acid. Biomacromolecules 2009, 10, 3069–3073. [Google Scholar] [CrossRef] [PubMed]
- Thillaye du Boullay, O.; Marchal, E.; Martin-Vaca, B.; Cossio, F.P.; Bourissou, D. An activated equivalent of lactide toward organocatalytic ring-opening polymerization. J. Am. Chem. Soc. 2006, 128, 16442–16443. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, L.; Chen, H.; Li, H.; Yuan, M. Synthesis and solubility of polylactide-co-poly(amino acid) random copolymer. Polym. Bull. 2013, 70, 1739–1751. [Google Scholar] [CrossRef]
- Yuan, M.L.; Li, X.H.; Xiong, C.D.; Deng, X.M. Polymerization of lactides and lactones—5. Ring-opening polymerization of epsilon-caprolactone and dl-lactide by rare earth 2-methylphenyl samarium. Eur. Polym. J. 1999, 35, 2131–2138. [Google Scholar] [CrossRef]
- Deng, X.M.; Yuan, M.L.; Xiong, C.D.; Li, X.H. Polymerization of lactides and lactones. II. Ring-opening polymerization of epsilon-caprolactone and dl-lactide by organoacid rare earth compounds. J. Appl. Polym. Sci. 1999, 71, 1941–1948. [Google Scholar] [CrossRef]
- Yuan, M.L.; Xiong, C.D.; Li, X.H.; Deng, X.M. Polymerization of lactides and lactones. III. Ring-opening polymerization of dl-lactide by the (eta(3)-c3h5)(2)sm(mu(2)-cl)(2)(mu(3)-cl)mg(tmed)(mu(2)-cl)mg(tmed) complex. J. Appl. Polym. Sci. 1999, 73, 2857–2862. [Google Scholar] [CrossRef]
- Deng, X.M.; Yuan, M.L.; Xiong, C.D.; Li, X.H. Polymerization of lactides and lactones. IV. Ring-opening polymerization of epsilon-caprolactone by rare earth phenyl compounds. J. Appl. Polym. Sci. 1999, 73, 1401–1408. [Google Scholar] [CrossRef]
- Deng, X.M.; Yuan, M.L.; Li, X.H.; Xiong, C.D. Polymerization of lactides and lactones vii. Ring-opening polymerization of lactide by rare earth phenyl compounds. Eur. Polym. J. 2000, 36, 1151–1156. [Google Scholar] [CrossRef]
- Yuan, M.L.; Wang, Y.H.; Li, X.H.; Xiong, C.D.; Deng, X.M. Polymerization of lactides and lactones. 10. Synthesis, characterization, and application of amino-terminated poly(ethylene glycol)-co-poly(epsilon-caprolactone) block copolymer. Macromolecules 2000, 33, 1613–1617. [Google Scholar] [CrossRef]
- Fang, M.; Wang, K.; Lu, H.; Yang, Y.; Nutt, S. Covalent polymer functionalization of graphene nanosheets and mechanical properties of composites. J. Mater. Chem. 2009, 19, 7098–7105. [Google Scholar] [CrossRef]
- Wang, J.; Shi, Z.; Ge, Y.; Wang, Y.; Fan, J.; Yin, J. Functionalization of unzipped carbon nanotube via in situ polymerization for mechanical reinforcement of polymer. J. Mater. Chem. 2012, 22, 17663–17670. [Google Scholar] [CrossRef]
- Song, W.; Zheng, Z.; Tang, W.; Wang, X. A facile approach to covalently functionalized carbon nanotubes with biocompatible polymer. Polymer 2007, 48, 3658–3663. [Google Scholar] [CrossRef]
- Yoon, J.T.; Lee, S.C.; Jeong, Y.G. Effects of grafted chain length on mechanical and electrical properties of nanocomposites containing polylactide-grafted carbon nanotubes. Compos. Sci. Technol. 2010, 70, 776–782. [Google Scholar] [CrossRef]
- Goffin, A.-L.; Raquez, J.-M.; Duquesne, E.; Siqueira, G.; Habibi, Y.; Dufresne, A.; Dubois, P. From interfacial ring-opening polymerization to melt processing of cellulose nanowhisker-filled polylactide-based nanocomposites. Biomacromolecules 2011, 12, 2456–2465. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.T.; Jeong, Y.G.; Lee, S.C.; Min, B.G. Influences of poly(lactic acid)-grafted carbon nanotube on thermal, mechanical, and electrical properties of poly(lactic acid). Polym. Adv. Technol. 2009, 20, 631–638. [Google Scholar] [CrossRef]
- Amirian, M.; Chakoli, A.N.; Sui, J.H.; Cai, W. Enhanced mechanical and photoluminescence effect of poly(l-lactide) reinforced with functionalized multiwalled carbon nanotubes. Polym. Bull. 2012, 68, 1747–1763. [Google Scholar] [CrossRef]
- Olalde, B.; Aizpurua, J.M.; Garcia, A.; Bustero, I.; Obieta, I.; Jurado, M.J. Single-walled carbon nanotubes and multiwalled carbon nanotubes functionalized with poly(l-lactic acid): A comparative study. J. Phys. Chem. C 2008, 112, 10663–10667. [Google Scholar] [CrossRef]
- Chen, G.X.; Kim, H.S.; Park, B.H.; Yoon, J.S. Controlled functionalization of multiwalled carbon nanotubes with various molecular-weight poly(l-lactic acid). J. Phys. Chem. B 2005, 109, 22237–22243. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xu, Z.; Li, J.; Li, Y.; Shan, M.; Wang, C.; Wang, Z.; Guo, Q.; Liu, L.; Chen, G.; et al. A facile strategy to prepare functionalized graphene via intercalation, grafting and self-exfoliation of graphite oxide. J. Mater. Chem. 2012, 22, 13460–13463. [Google Scholar] [CrossRef]
- Yang, J.-H.; Lin, S.-H.; Lee, Y.-D. Preparation and characterization of poly(l-lactide)-graphene composites using the in situ ring-opening polymerization of PLLA with graphene as the initiator. J. Mater. Chem. 2012, 22, 10805–10815. [Google Scholar] [CrossRef]
- Li, W.; Xu, Z.; Chen, L.; Shan, M.; Tian, X.; Yang, C.; Lv, H.; Qian, X. A facile method to produce graphene oxide-g-poly(l-lactic acid) as an promising reinforcement for plla nanocomposites. Chem. Eng. J. 2014, 237, 291–299. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; He, C. Synthesis, stereocomplex crystallization, morphology and mechanical property of poly(lactide)-carbon nanotube nanocomposites. RSC Adv. 2013, 3, 2219–2226. [Google Scholar] [CrossRef]
- Shen, J.; Hu, Y.; Shi, M.; Lu, X.; Qin, C.; Li, C.; Ye, M. Fast and facile preparation of graphene oxide and reduced graphene oxide nanoPLLAtelets. Chem. Mater. 2009, 21, 3514–3520. [Google Scholar] [CrossRef]
- Cao, A.; Liu, Z.; Chu, S.; Wu, M.; Ye, Z.; Cai, Z.; Chang, Y.; Wang, S.; Gong, Q.; Liu, Y. A facile one-step method to produce graphene-cds quantum dot nanocomposites as promising optoelectronic materials. Adv. Mater. 2010, 22, 103–106. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Sun, J.; Wang, X.; Fan, X.; Zhao, Q.; Cai, L.; Song, R.; Ma, Z.; Huang, W. Unzipped multiwalled carbon nanotubes-incorporated poly(l-lactide) nanocomposites with enhanced interface and hydrolytic degradation. Mater. Chem. Phys. 2012, 134, 1059–1066. [Google Scholar] [CrossRef]
- Leszczynska, A.; Pielichowski, K. Application of thermal analysis methods for characterization of polymer/montmorillonite nanocomposites. J. Therm. Anal. Calorim. 2008, 93, 677–687. [Google Scholar] [CrossRef]
- Suk, J.W.; Piner, R.D.; An, J.; Ruoff, R.S. Mechanical properties of mono layer graphene oxide. ACS Nano 2010, 4, 6557–6564. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, T.; Abdala, A.A.; Stankovich, S.; Dikin, D.A.; Herrera-Alonso, M.; Piner, R.D.; Adamson, D.H.; Schniepp, H.C.; Chen, X.; Ruoff, R.S.; et al. Functionalized graphene sheets for polymer nanocomposites. Nat. Nanotechnol. 2008, 3, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, R.; Bhattacharya, M.; Bandyopadhyay, S.; Bhowmick, A.K. A review on the mechanical and electrical properties of graphite and modified graphite reinforced polymer composites. Prog. Polym. Sci. 2011, 36, 638–670. [Google Scholar] [CrossRef]

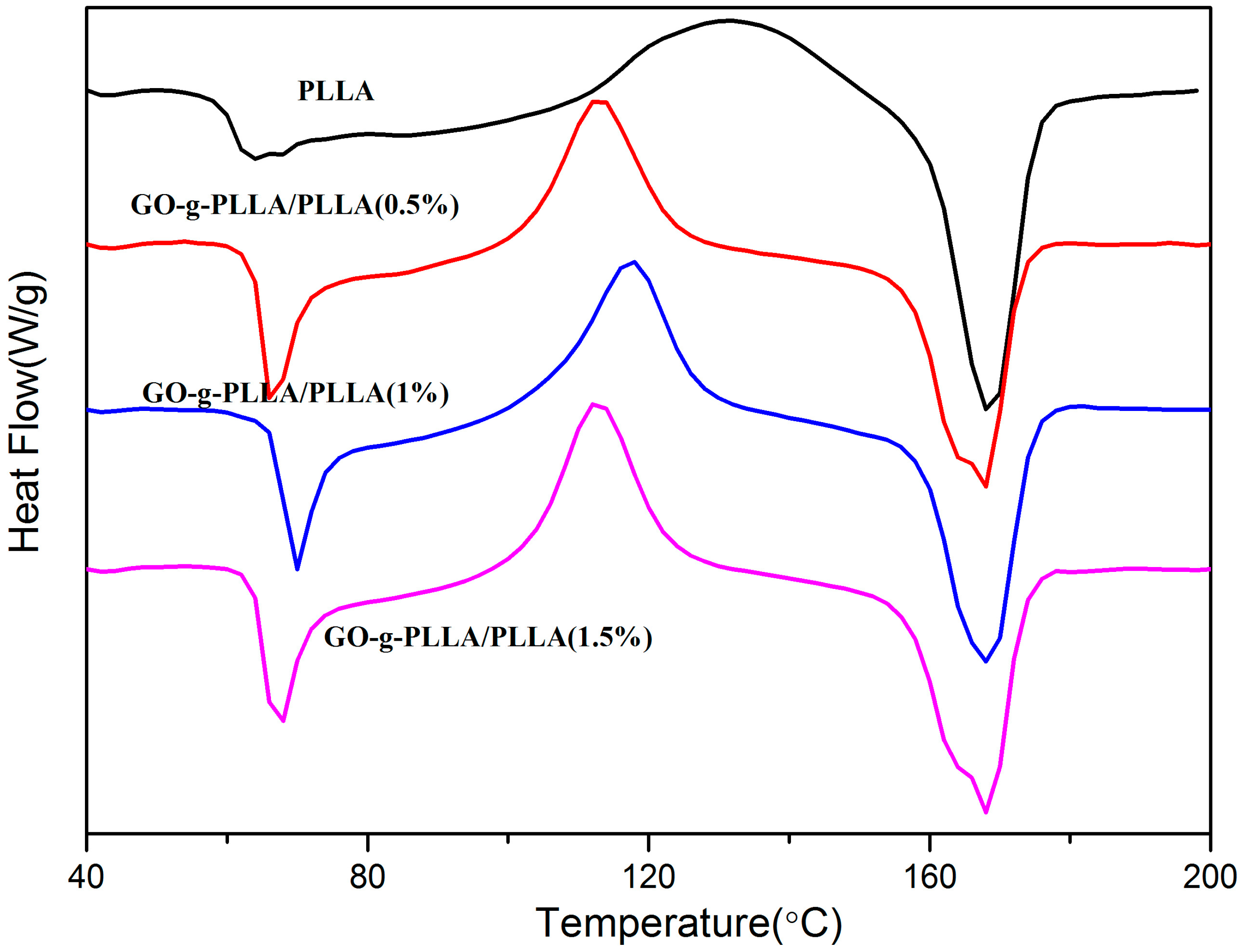
| Sample | Tg (°C) | Tm (°C) | ΔHm (J/g) | Xc (%) |
|---|---|---|---|---|
| PLLA | 63 | 167.8 | 23.84 | 25.6 |
| GO-g-PLLA/PLLA (0.5%) | 68 | 168 | 35.67 | 38.3 |
| GO-g-PLLA/PLLA (1%) | 68 | 170 | 39.51 | 42.4 |
| GO-g-PLLA/PLLA (1.5%) | 62 | 170 | 37.13 | 39.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, M.; Chen, Y.; Yuan, M.; Li, H.; Xia, X.; Xiong, C. Functionalization of Graphene Oxide with Low Molecular Weight Poly (Lactic Acid). Polymers 2018, 10, 177. https://doi.org/10.3390/polym10020177
Yuan M, Chen Y, Yuan M, Li H, Xia X, Xiong C. Functionalization of Graphene Oxide with Low Molecular Weight Poly (Lactic Acid). Polymers. 2018; 10(2):177. https://doi.org/10.3390/polym10020177
Chicago/Turabian StyleYuan, Mingwei, Yike Chen, Minglong Yuan, Hongli Li, Xiansong Xia, and Chengdong Xiong. 2018. "Functionalization of Graphene Oxide with Low Molecular Weight Poly (Lactic Acid)" Polymers 10, no. 2: 177. https://doi.org/10.3390/polym10020177





