Conjugate of PAMAM Dendrimer, Doxorubicin and Monoclonal Antibody—Trastuzumab: The New Approach of a Well-Known Strategy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
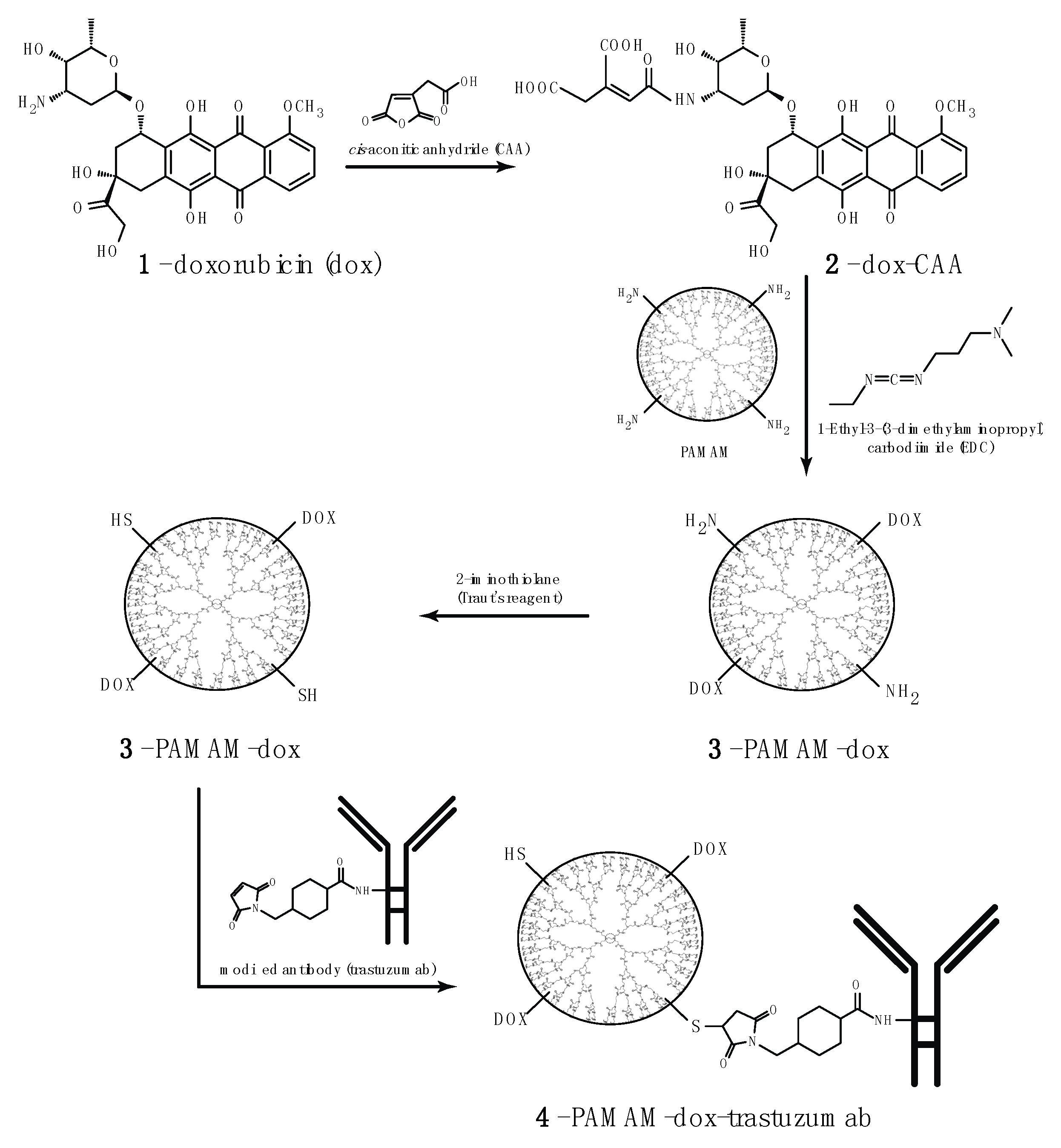
2.2. Synthesis of PAMAM Doxorubicin Conjugate
2.3. Synthesis of PAMAM–dox–trastuzumab Conjugate
2.4. Cell Culture
2.5. Determination of Cytotoxicity
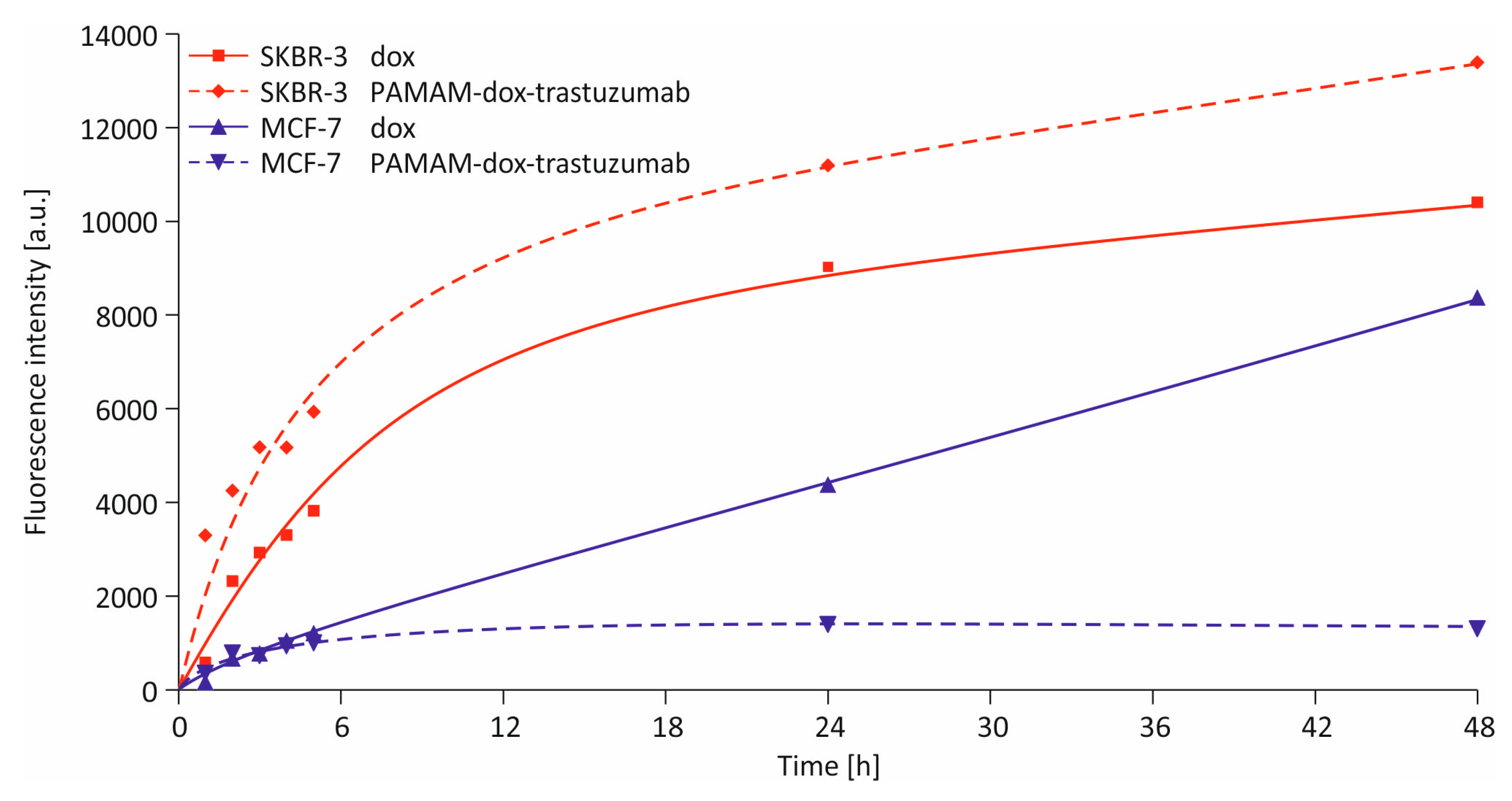
2.6. Cellular Uptake Detection
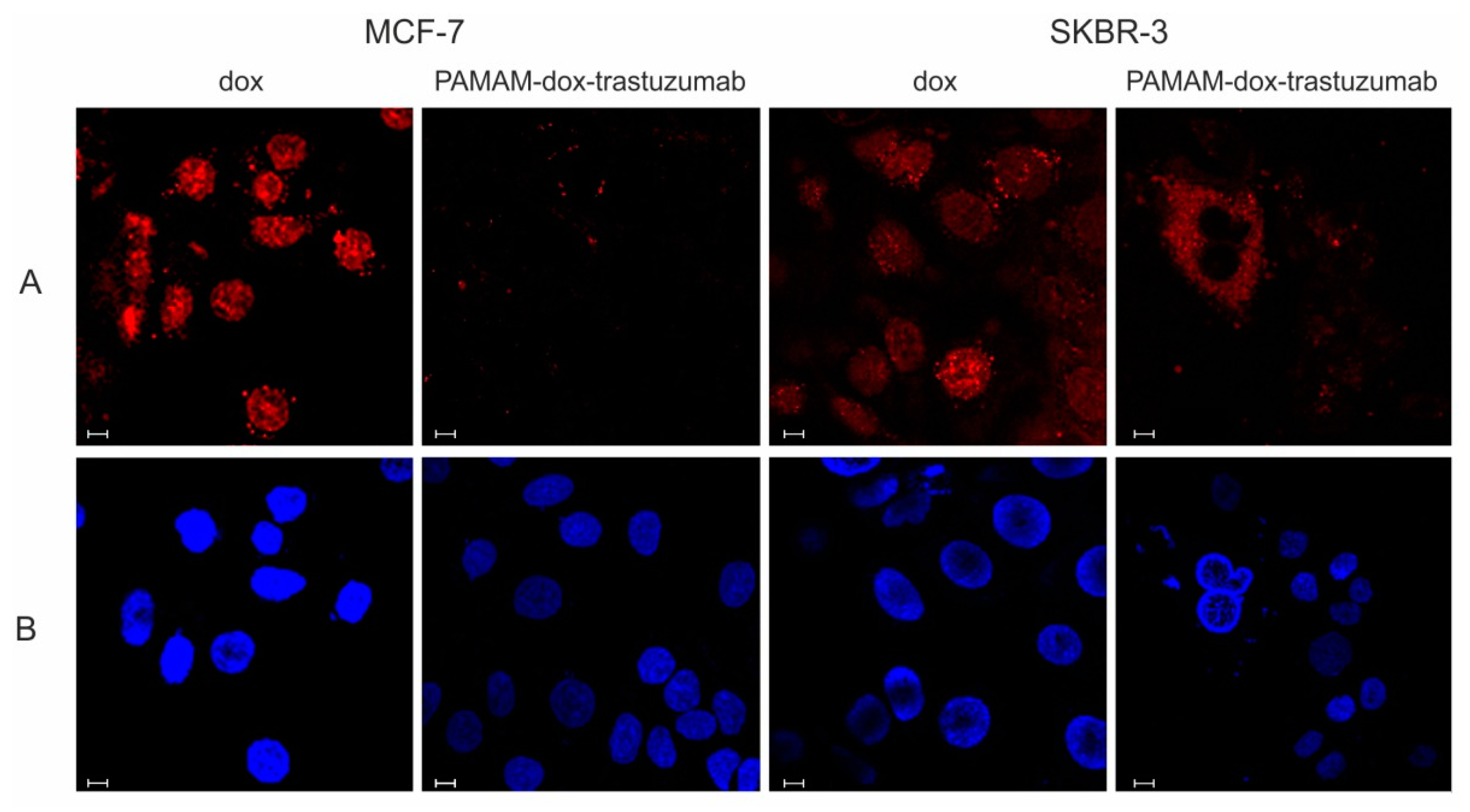
2.7. Confocal Microscopy
2.8. Statistical Analysis
3. Results and Discussion
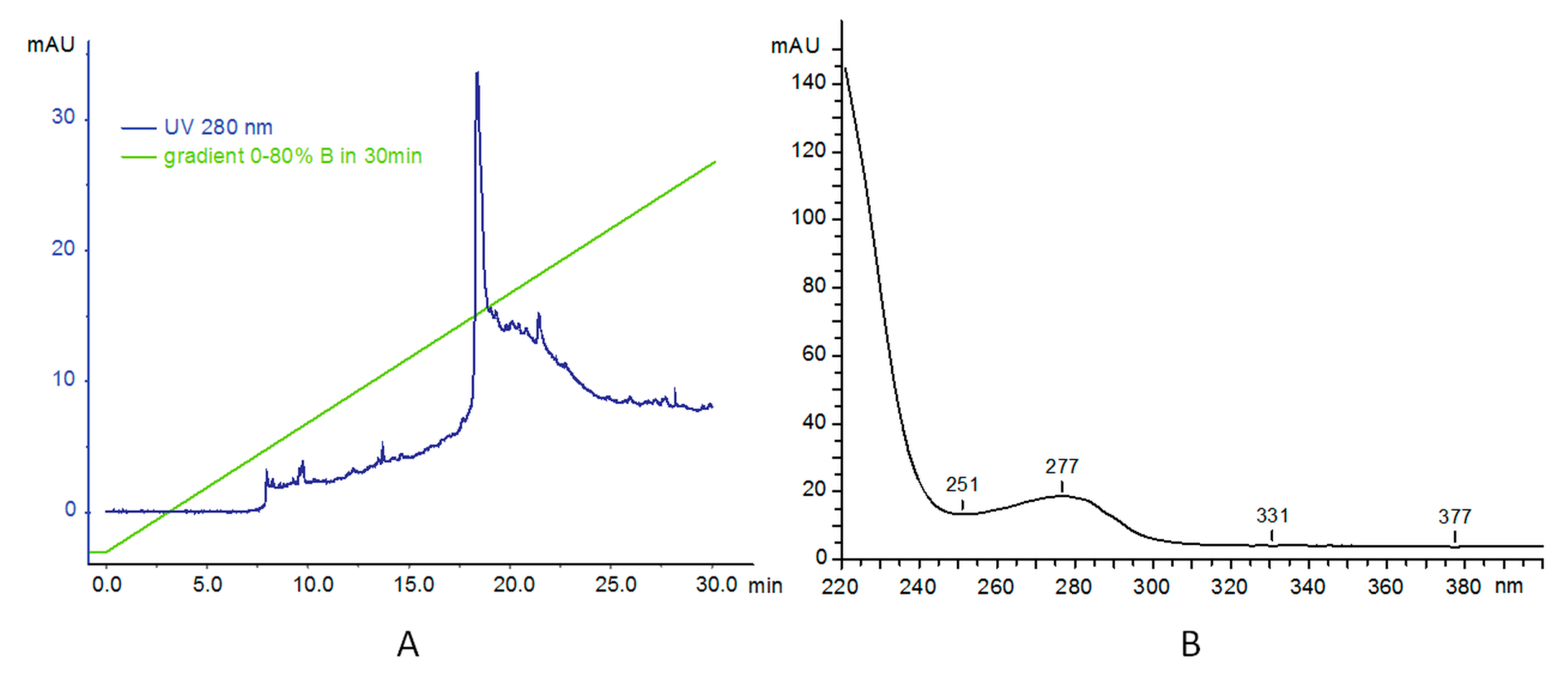
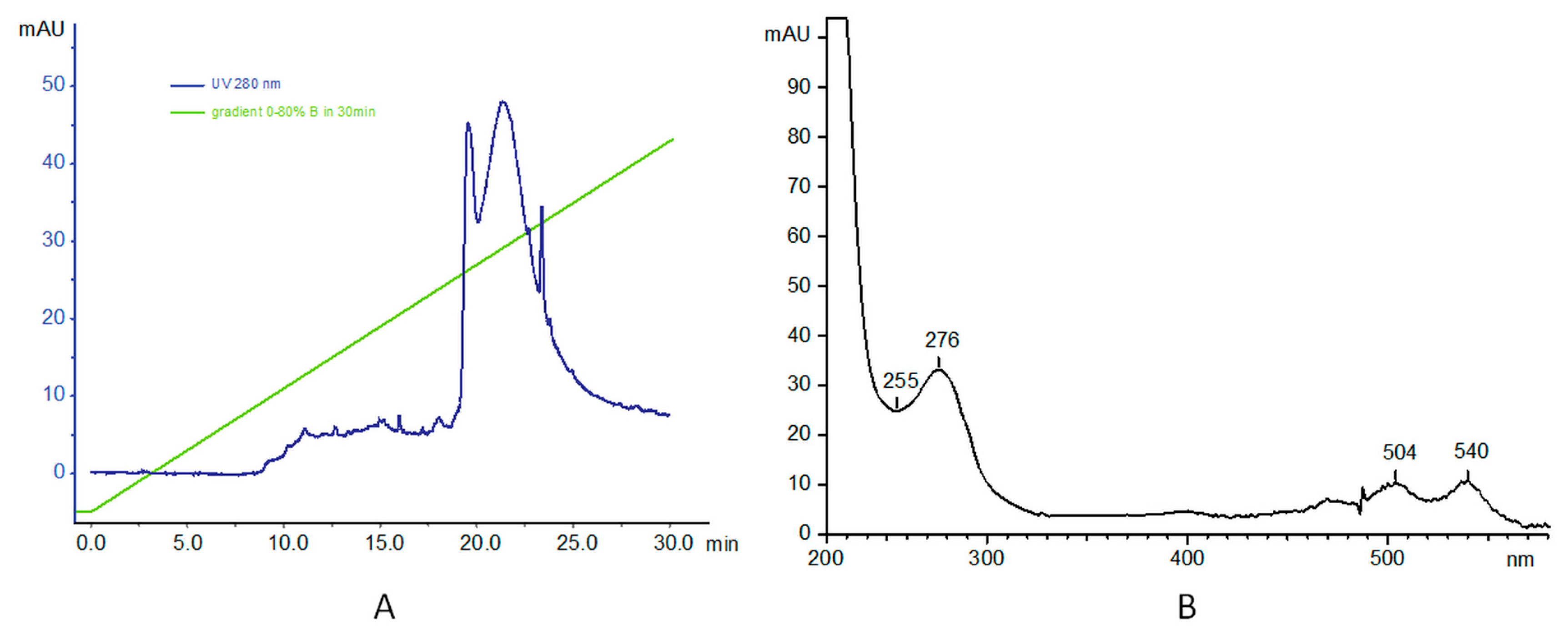
3.1. Synthesis and Characterization of PAMAM–trastuzumab and PAMAM–dox–trastuzumab Conjugates
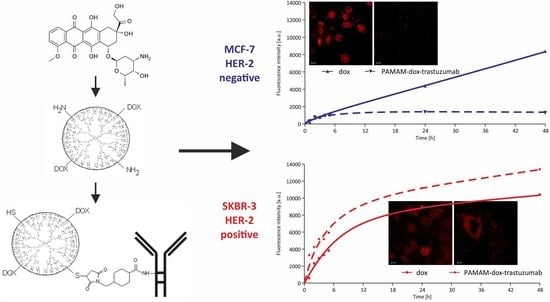
3.2. In Vitro Studies
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Donepudi, M.S.; Kondapalli, K.; Amos, S.J.; Venkanteshan, P. Breast cancer statistics and markers. J. Cancer Res. Ther. 2014, 10, 506–511. [Google Scholar] [PubMed]
- Zhang, Y.; Hong, H.; Cai, W. Tumor-targeted drug delivery with aptamers. Curr. Med. Chem. 2011, 18, 4185–4194. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.M.; Allison, J.P.; Wolchok, J.D. Monoclonal antibodies in cancer therapy. Cancer Immun. 2012, 12, 14. [Google Scholar] [PubMed]
- Fountzilas, G.; Tsavdaridis, D.; Kalogera-Fountzila, A.; Christodoulou, C.; Timotheadou, E.; Kalofonos, C.; Kosmidis, P.; Adamou, A.; Papakostas, P.; Gogas, H.; et al. Weekly paclitaxel as first-line chemotherapy and trastuzumab in patients with advanced breast cancer. A Hellenic Cooperative Oncology Group phase II study. Ann. Oncol. 2001, 12, 1545–1551. [Google Scholar] [CrossRef] [PubMed]
- Pegram, M.; Hsu, S.; Lewis, G.; Pietras, R.; Beryt, M.; Sliwkowski, M.; Coombs, D.; Baly, D.; Kabbinavar, F.; Slamon, D. Inhibitory effects of combinations of HER-2/neu antibody and chemotherapeutic agents used for treatment of human breast cancers. Oncogene 1999, 18, 2241–2251. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.M.; Senter, P.D. Arming antibodies: Prospects and challenges for immunoconjugates. Nat. Biotechnol. 2005, 23, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Wilczewska, A.Z.; Niemirowicz, K.; Markiewicz, K.H.; Car, H. Nanoparticles as drug delivery systems. Pharmacol. Rep. 2012, 64, 1020–1037. [Google Scholar] [CrossRef]
- Jackson, J.L.; Chanzy, H.D.; Booy, F.P.; Drake, B.J.; Tomalia, D.A.; Bauer, B.; Amis, E.J. Visualization of dendrimer molecules by transmission electron (TEM): Staining methods and Cryo-TEM of vitrified solutions. Macromolecules 1998, 31, 6259–6265. [Google Scholar] [CrossRef]
- Kesharwani, P.; Jain, K.; Jain, N.K. Dendrimer as nanocarrier for drug delivery. Prog. Polym. Sci. 2014, 39, 268–307. [Google Scholar] [CrossRef]
- Alper, J. Rising chemical “stars” could play many roles. Science 1991, 251, 1562–1564. [Google Scholar] [CrossRef] [PubMed]
- Boas, U.; Heegaard, P.M.H. Dendrimers in drug research. Chem. Soc. Rev. 2004, 33, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Svenson, S.; Tomalia, D.A. Dendrimers in biomedical applications—Reflections on the field. Adv. Drug Deliv. Rev. 2005, 57, 2106–2129. [Google Scholar] [CrossRef] [PubMed]
- Menjoge, A.R.; Kannan, R.M.; Tomalia, D.A. Dendrimer-based drug and imaging conjugates: Design considerations for nanomedical applications. Drug Discov. Today 2010, 15, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Barratt, G.M. Therapeutic applications of colloidal drug carriers. Pharm. Sci. Technol. Today 2000, 3, 163–171. [Google Scholar] [CrossRef]
- Byrne, J.D.; Betancourt, T.; Brannon-Peppas, L. Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv. Drug Deliv. Rev. 2008, 60, 1615–1626. [Google Scholar] [CrossRef] [PubMed]
- Talekar, M.; Kendall, J.; Denny, W.; Garg, S. Targeting of nanoparticles in cancer: Drug delivery and diagnostics. Anticancer Drugs 2011, 22, 949–962. [Google Scholar] [CrossRef] [PubMed]
- Esfand, R.; Tomalia, D.A. Poly(amidoamine) (PAMAM) dendrimers: From biomimicry to drug delivery and biomedical applications. Drug Discov. Today 2001, 6, 427–436. [Google Scholar] [CrossRef]
- Yabbarov, N.G.; Posypanova, G.A.; Vorontsov, E.A.; Popova, O.N.; Severin, E.S. Targeted delivery of doxorubicin: Drug delivery system based on PAMAM dendrimers. Biochemistry 2013, 78, 884–894. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Hong, M.; Zhang, L.; Tang, G.; Jiang, Y.; Pei, Y. PEGylated PAMAM dendrimer-doxorubicin conjugates: In vitro evaluation and in vivo tumor accumulation. Pharm. Res. 2010, 27, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Thomas, T.P.; Desai, A.M.; Kotlyar, A.; Park, S.J.; Baker, J.R. HER2 specific delivery of methotrexate by dendrimer conjugated anti-HER2 mAb. Nanotechnology 2008, 19, 295102. [Google Scholar] [CrossRef] [PubMed]
- Kulhari, H.; Pooja, D.; Shrivastava, S.; Kuncha, M.; Naidu, V.G.M.; Bansal, V.; Sistla, R.; Adams, D.J. Trastuzumab-grafted PAMAM dendrimers for the selective delivery of anticancer drugs to HER2-positive breast cancer. Sci. Rep. 2016, 6, 23179. [Google Scholar] [CrossRef] [PubMed]
- Miyano, T.; Wijagkanalan, W.; Kawakami, S.; Yamashita, F.; Hashida, M. Anionic amino acid dendrimer-trastuzumab conjugates for specific internalization in HER2-positive cancer cells. Mol. Pharm. 2010, 7, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Zhang, X.; Ni, L.; Li, J.; Zhang, F.; Wang, Z.; Lian, S.; Sun, K. Targeted delivery of polyamidoamine-paclitaxel conjugate functionalized with anti-human epidermal growth factor receptor 2 trastuzumab. Int. J. Nanomed. 2015, 10, 2173–2190. [Google Scholar] [CrossRef] [PubMed]
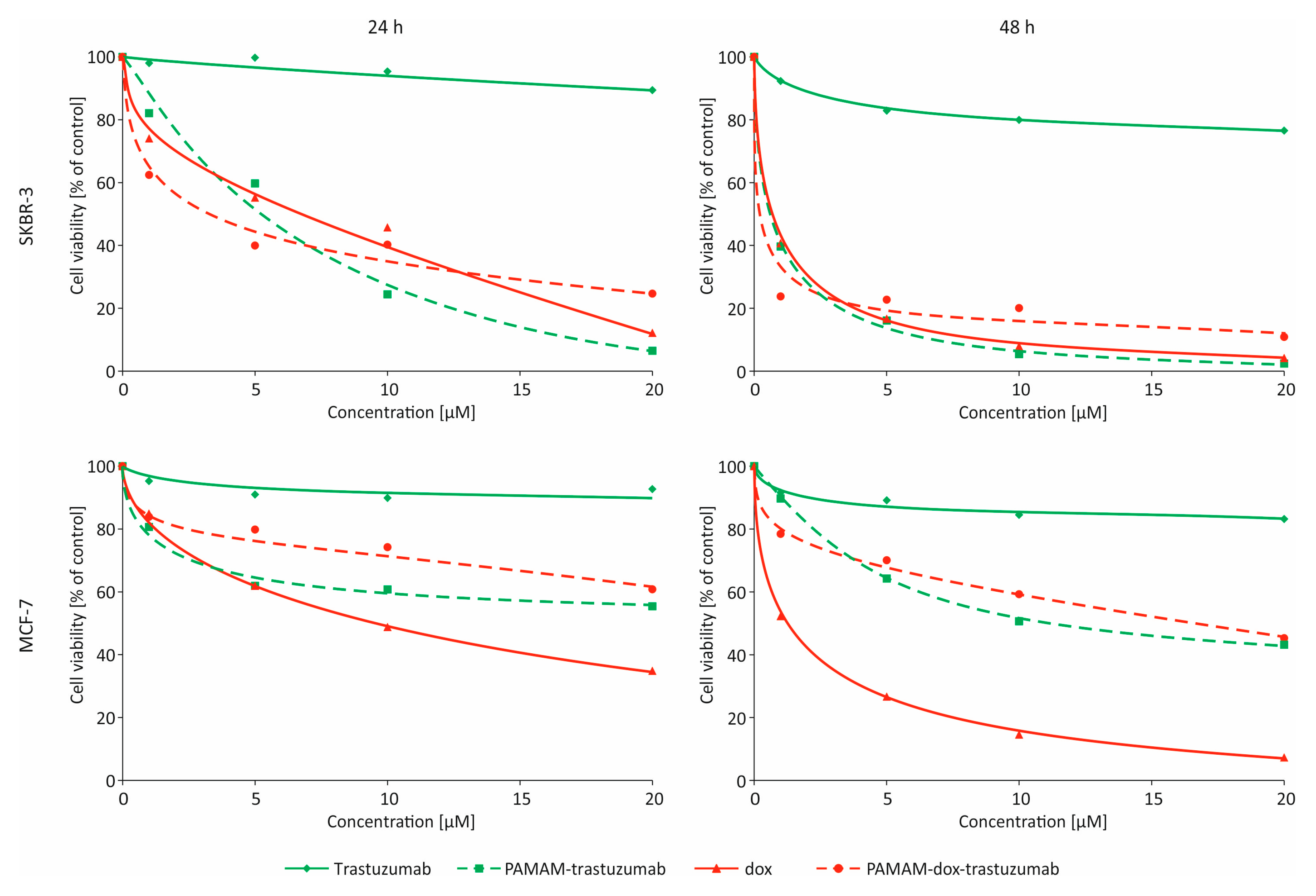
| MCF-7 24 h | MCF-7 24 h–24 h | MCF-7 48 h | SKBR-3 24 h | SKBR-3 24 h–24 h | SKBR-3 48 h | |
|---|---|---|---|---|---|---|
| Trastuzumab | >100 | >100 | >100 | >100 | >100 | >100 |
| PAMAM–trastuzumab | 32.46 ± 4.47 * | 11.31 ± 4.06 * | 11.92 ± 4.08 * | 4.29 ± 0.06 * | 2.81 ± 1.52 * | 0.41 ± 0.06 * |
| Dox | 9.20 ± 1.23 | 1.37 ± 1.61 | 1.10 ± 1.23 | 0.77 ± 0.16 | 0.19 ± 0.08 | 0.34 ± 0.13 |
| PAMAM–dox–trastuzumab | 38.40 ± 5.73 | 8.17 ± 4.85 | 14.86 ± 5.37 | 2.81 ± 0.74 | 0.14 ± 0.04 | 0.003 ± 0.002 * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcinkowska, M.; Sobierajska, E.; Stanczyk, M.; Janaszewska, A.; Chworos, A.; Klajnert-Maculewicz, B. Conjugate of PAMAM Dendrimer, Doxorubicin and Monoclonal Antibody—Trastuzumab: The New Approach of a Well-Known Strategy. Polymers 2018, 10, 187. https://doi.org/10.3390/polym10020187
Marcinkowska M, Sobierajska E, Stanczyk M, Janaszewska A, Chworos A, Klajnert-Maculewicz B. Conjugate of PAMAM Dendrimer, Doxorubicin and Monoclonal Antibody—Trastuzumab: The New Approach of a Well-Known Strategy. Polymers. 2018; 10(2):187. https://doi.org/10.3390/polym10020187
Chicago/Turabian StyleMarcinkowska, Monika, Ewelina Sobierajska, Maciej Stanczyk, Anna Janaszewska, Arkadiusz Chworos, and Barbara Klajnert-Maculewicz. 2018. "Conjugate of PAMAM Dendrimer, Doxorubicin and Monoclonal Antibody—Trastuzumab: The New Approach of a Well-Known Strategy" Polymers 10, no. 2: 187. https://doi.org/10.3390/polym10020187