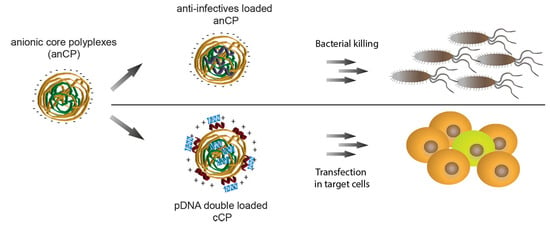
Starch-Chitosan Polyplexes: A Versatile Carrier System for Anti-Infectives and Gene Delivery
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation, Optimization and Characterization of Starch–Chitosan Core Polyplexes
2.2.1. Preparation and Optimization of Starch-Chitosan Core Polyplexes (CP)
2.2.2. Preparation and Optimization of Protasan Coated CP (cCP)
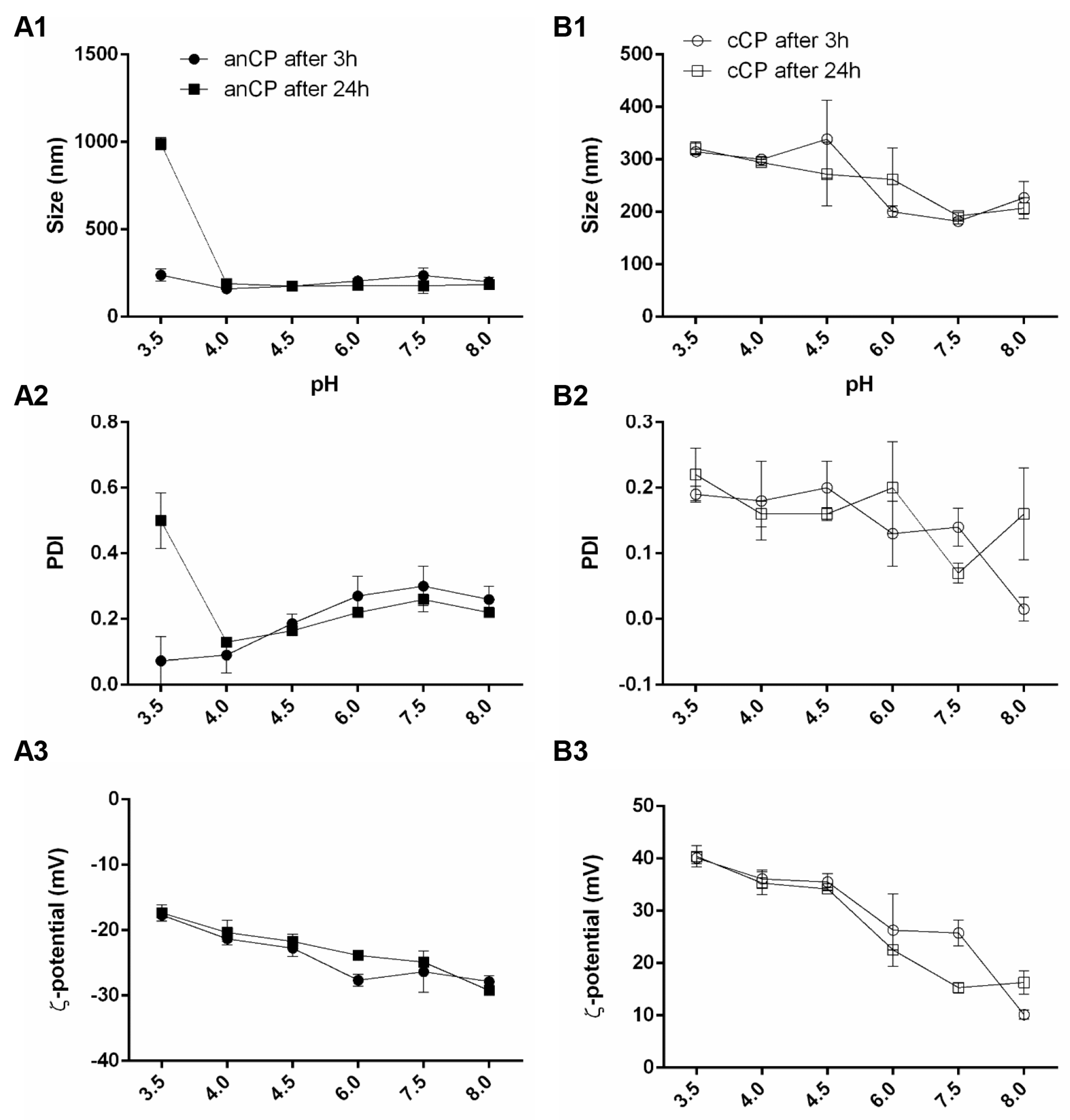
2.2.3. pH-Stability of Drug-Free CP and cCP
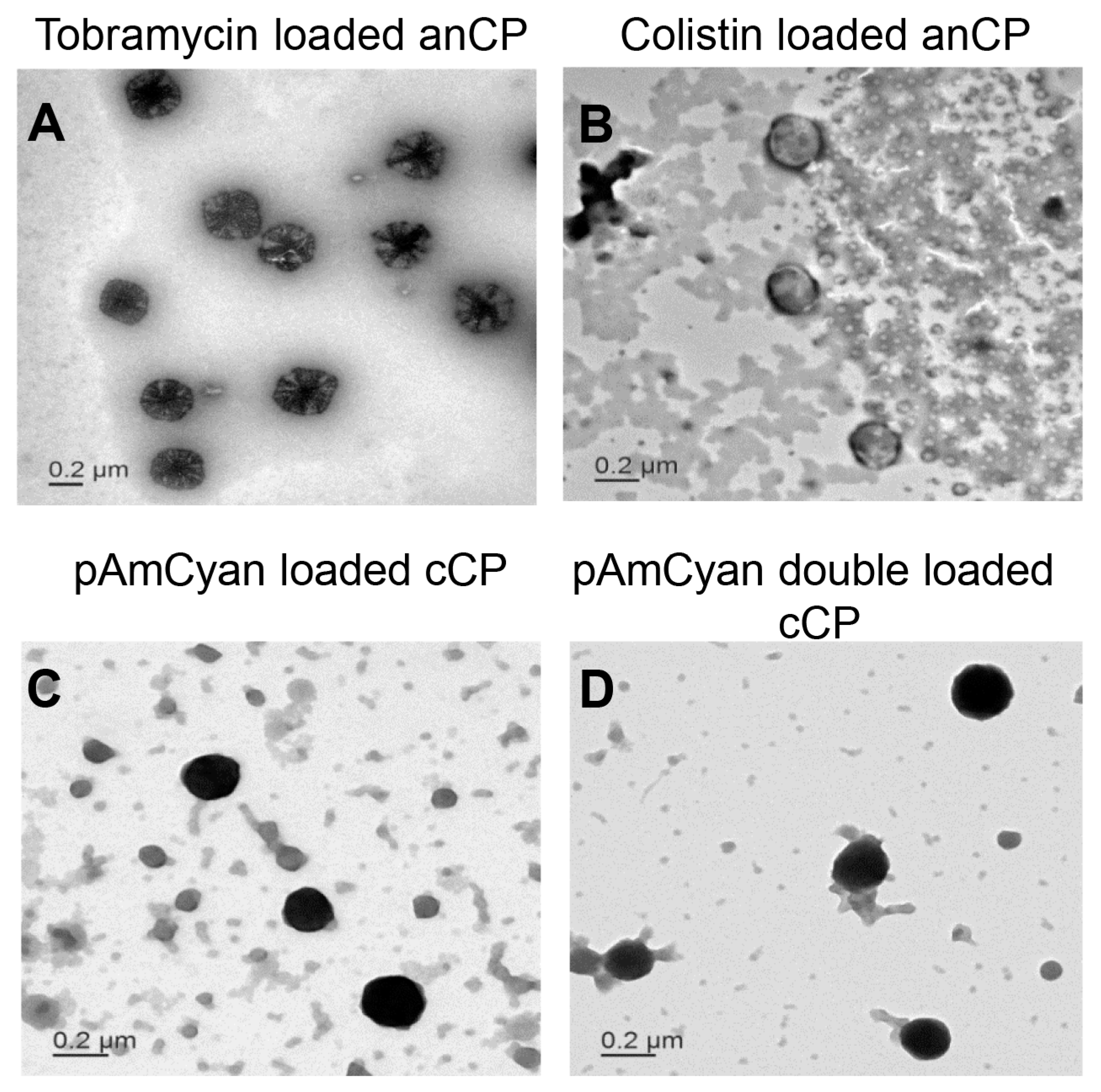
2.2.4. Morphology
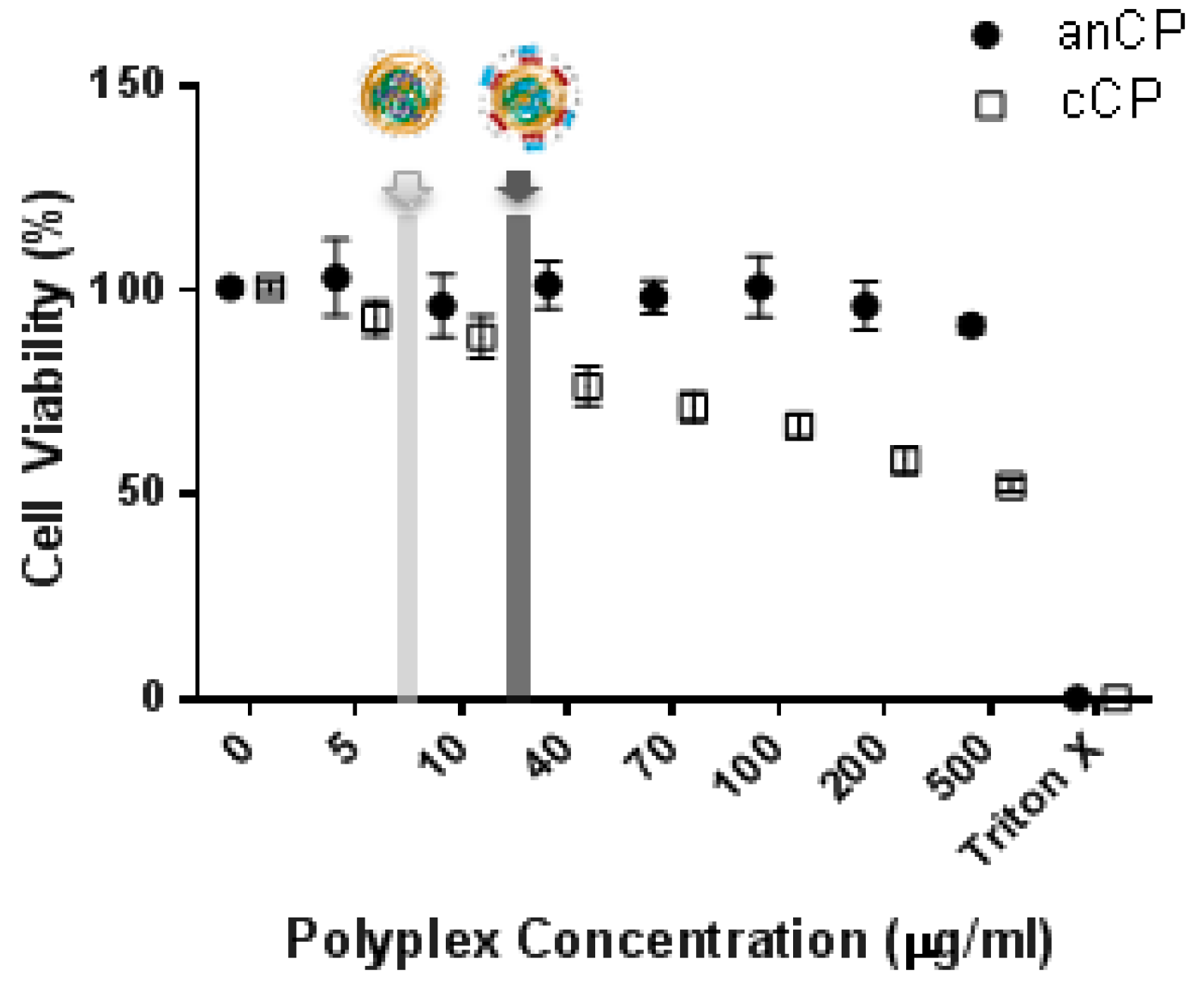
2.2.5. Cytotoxicity Study: MTT Assay
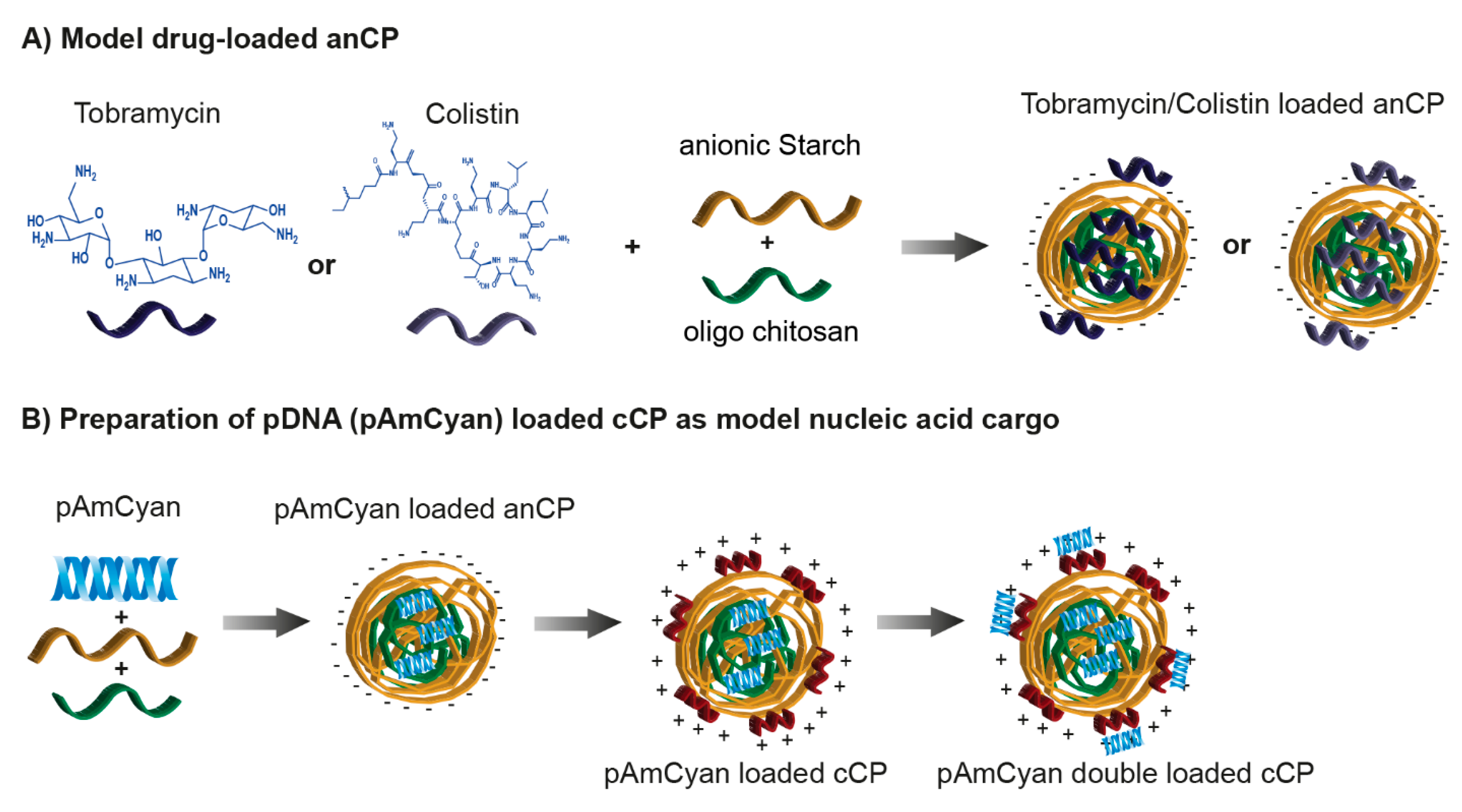
2.3. Cationic Anti-Infective Loaded anCP
2.3.1. Preparation and Optimization of Cationic Anti-Infective Loaded anCP
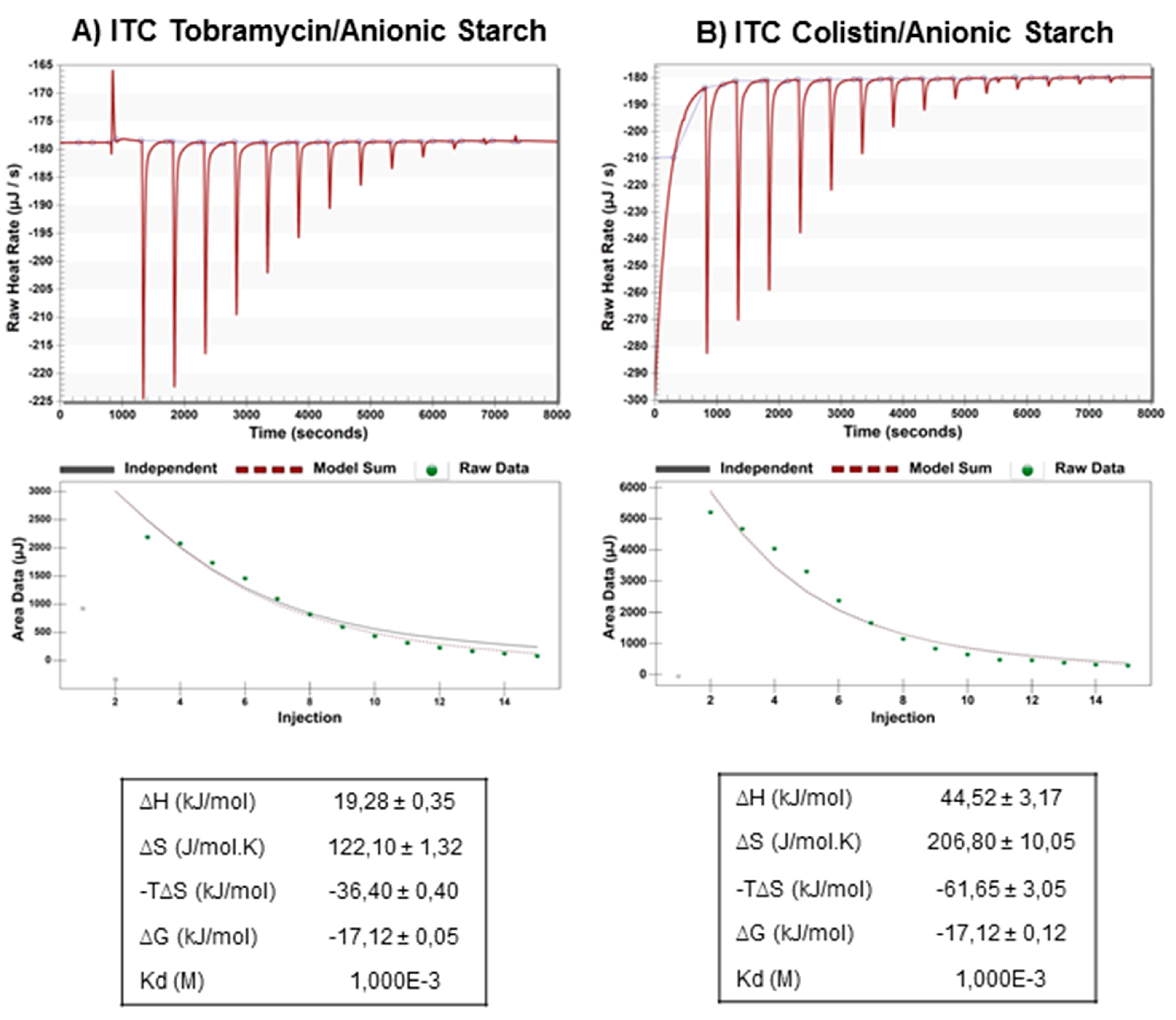
Isothermal Titration Calorimetry
Preparation and Optimization of Cationic Anti-Infective Loaded anCP
Loading Quantification
HPLC Analysis
Aminoglycoside Detection Protocol
Drug Release Study
2.3.2. Minimum Inhibitory Concentration (MIC) Assay
2.4. Preparation of pDNA Loaded cCP
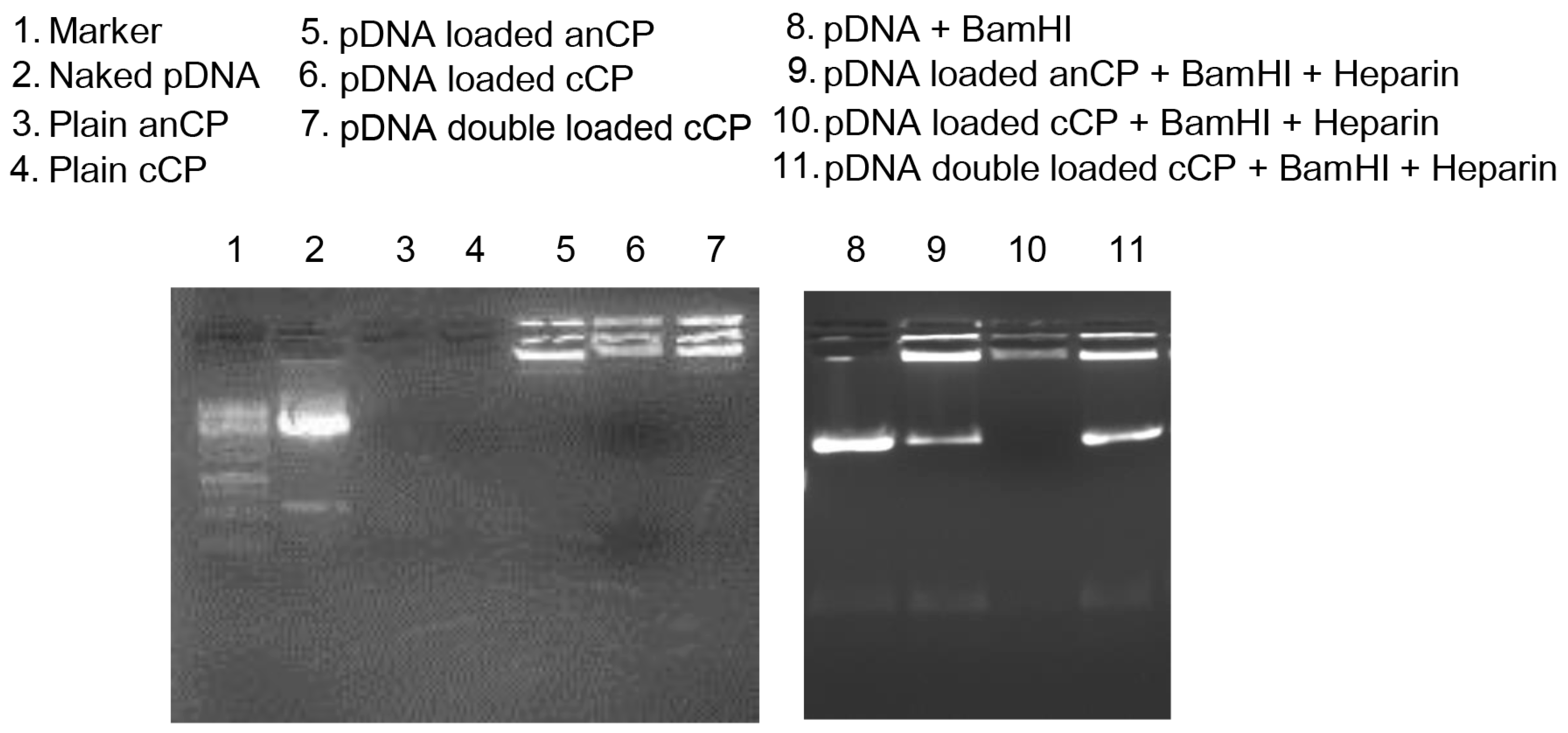
2.4.1. Determination the Complexation of pAmCyan in Starch-Chitosan Polyplexes
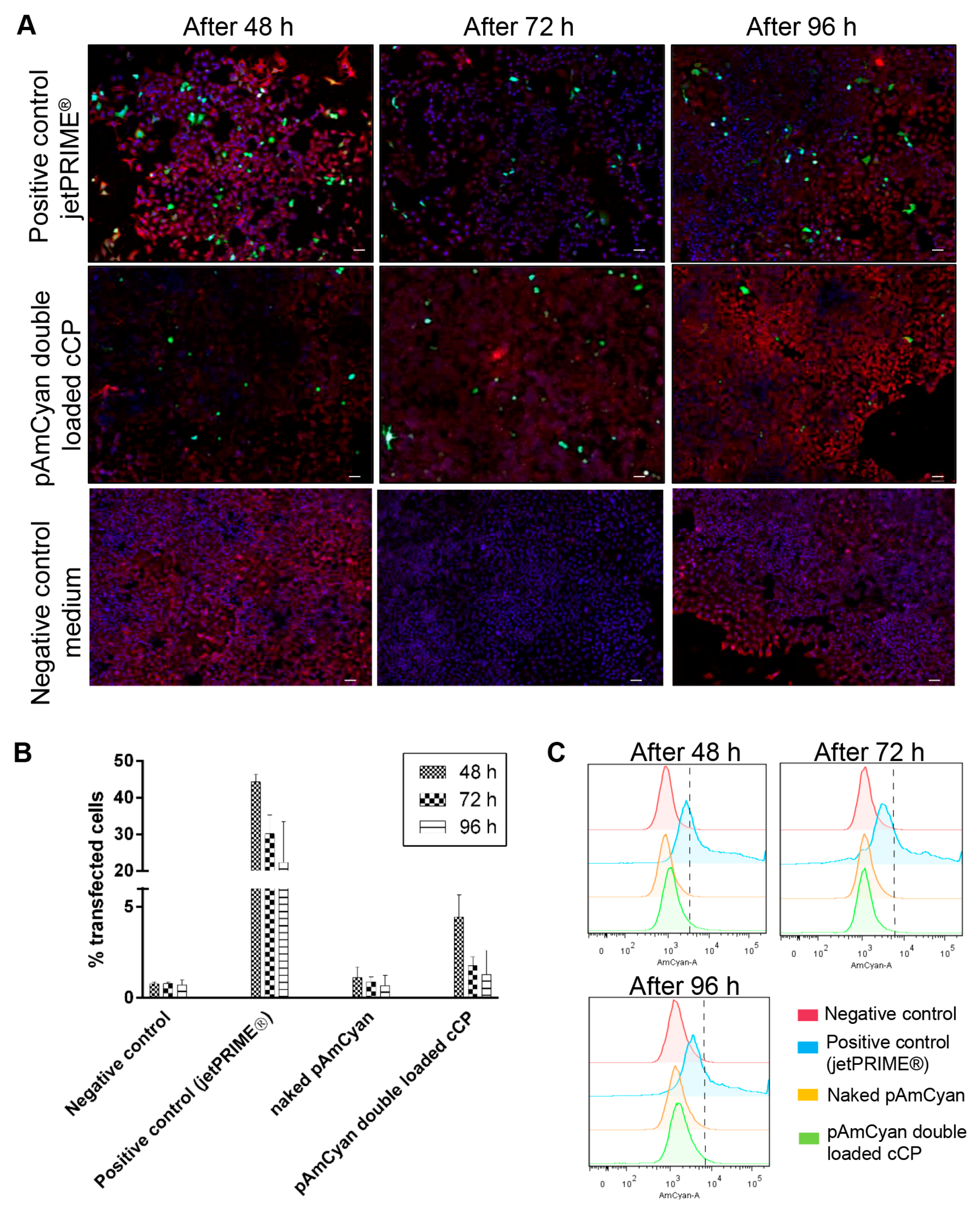
2.4.2. In Vitro Transfection Studies in A549 Cells
3. Results and Discussion
3.1. Preparation and Characterization of Drug-Free Starch-Chitosan Polyplexes
3.1.1. Colloidal Stability of Drug-Free anCP and cCP
3.1.2. Cytotoxicity Assessment
3.2. Loading of anCP with Low-Mw Anti-Infectives
3.2.1. Optimization of the Preparation Process and Drug-Loading
3.2.2. Efficacy of Anti-Infective Loaded anCP
3.3. Loading of anCP with High-Mw pDNA
Potential of Polyplexes for pDNA Delivery
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CP | core polyplexes |
| anCP | anionic core polyplexes |
| cationic CP (or catCP) | cationic core polyplexes |
| cCP | coated polyplexes |
References
- Kang, B.; Opatz, T.; Landfester, K.; Wurm, F.R. Carbohydrate nanocarriers in biomedical applications: Functionalization and construction. Chem. Soc. Rev. 2015, 44, 8301–8325. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, M.F.; Jennings, G.T. Vaccine delivery: A matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 2010, 10, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Abed, N.; Couvreur, P. Nanocarriers for antibiotics: A promising solution to treat intracellular bacterial infections. Int. J. Antimicrob. Agents 2014, 43, 485–496. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, I.; Conte, C.; Miro, A.; Quaglia, F.; Ungaro, F. Pulmonary drug delivery: A role for polymeric nanoparticles? Curr. Top. Med. Chem. 2015, 15, 386–400. [Google Scholar] [CrossRef] [PubMed]
- Mahapatro, A.; Singh, D.K. Biodegradable nanoparticles are excellent vehicle for site directed in-vivo delivery of drugs and vaccines. J. Nanobiotechnol. 2011, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, A.D.; Kiick, K.L. Polysaccharide-modified synthetic polymeric biomaterials. Biopolymers 2010, 94, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Wikström, J.; Elomaa, M.; Syväjärvi, H.; Kuokkanen, J.; Yliperttula, M.; Honkakoski, P.; Urtti, A. Alginate-based microencapsulation of retinal pigment epithelial cell line for cell therapy. Biomaterials 2008, 29, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Hans, M.L.; Lowman, A.M. Biodegradable nanoparticles for drug delivery and targeting. Curr. Opin. Solid State Mater. Sci. 2002, 6, 319–327. [Google Scholar] [CrossRef]
- Azzam, T.; Eliyahu, H.; Shapira, L.; Linial, M.; Barenholz, Y.; Domb, A.J. Polysaccharide? Oligoamine Based Conjugates for Gene Delivery. J. Med. Chem. 2002, 45, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Sim, H.J.; Thambi, T.; Lee, D.S. Heparin-based temperature-sensitive injectable hydrogels for protein delivery. J. Mater. Chem. B 2015, 3, 8892–8901. [Google Scholar] [CrossRef]
- Builders, P.F.; Arhewoh, M.I. Pharmaceutical applications of native starch in conventional drug delivery. Starch Stärke 2016, 68, 864–873. [Google Scholar] [CrossRef]
- Yamada, H.; Loretz, B.; Lehr, C.-M. Design of starch-graft-PEI polymers: An effective and biodegradable gene delivery platform. Biomacromolecules 2014, 15, 1753–1761. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi Najafi, S.H.; Baghaie, M.; Ashori, A. Preparation and characterization of acetylated starch nanoparticles as drug carrier: Ciprofloxacin as a model. Int. J. Biol. Macromol. 2016, 87, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Balmayor, E.R.; Baran, E.T.; Azevedo, H.S.; Reis, R.L. Injectable biodegradable starch/chitosan delivery system for the sustained release of gentamicin to treat bone infections. Carbohydr. Polym. 2012, 87, 32–39. [Google Scholar] [CrossRef]
- Santander-Ortega, M.J.; Stauner, T.; Loretz, B.; Ortega-Vinuesa, J.L.; Bastos-González, D.; Wenz, G.; Schaefer, U.F.; Lehr, C.M. Nanoparticles made from novel starch derivatives for transdermal drug delivery. J. Control. Release 2010, 141, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Bernkop-Schnurch, A.; Dunnhaupt, S. Chitosan-based drug delivery systems. Eur. J. Pharm. Biopharm. 2012, 81, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Grenha, A.; Gomes, M.E.; Rodrigues, M.; Santo, V.E.; Mano, J.F.; Neves, N.M.; Reis, R.L. Development of new chitosan/carrageenan nanoparticles for drug delivery applications. J. Biomed. Mater. Res. Part A 2010, 92, 1265–1272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gan, Q.; Wang, T. Chitosan nanoparticle as protein delivery carrier—Systematic examination of fabrication conditions for efficient loading and release. Colloids Surf. B Biointerfaces 2007, 59, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.-S.; Xu, Y.-L.; Zou, X.-T.; Xu, Z.-R. Chitosan nanoparticles act as an adjuvant to promote both Th1 and Th2 immune responses induced by ovalbumin in mice. Mar. Drugs 2011, 9, 1038–1055. [Google Scholar] [CrossRef] [PubMed]
- De Campos, A.M.; Sánchez, A.; Alonso, M.J. Chitosan nanoparticles: A new vehicle for the improvement of the delivery of drugs to the ocular surface. Application to cyclosporin A. Int. J. Pharm. 2001, 224, 159–168. [Google Scholar] [CrossRef]
- Van der Lubben, I.M.; Verhoef, J.C.; van Aelst, A.C.; Borchard, G.; Junginger, H.E. Chitosan microparticles for oral vaccination: Preparation, characterization and preliminary in vivo uptake studies in murine Peyer’s patches. Biomatrials 2001, 22, 687–694. [Google Scholar] [CrossRef]
- Dodane, V.; Vilivalam, V.D. Pharmaceutical applications of chitosan. Pharm. Sci. Technol. Today 1998, 1, 246–253. [Google Scholar] [CrossRef]
- Onishi, H.; Machida, Y. Biodegradation and distribution of water-soluble chitosan in mice. Biomaterials 1999, 20, 175–182. [Google Scholar] [CrossRef]
- Aspden, T.J.; Mason, J.D.; Jones, N.S.; Lowe, J.; Skaugrud, O.; Illum, L. Chitosan as a nasal delivery system: The effect of chitosan solutions on in vitro and in vivo mucociliary transport rates in human turbinates and volunteers. J. Pharm. Sci. 1997, 86, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Elborn, J.S.; Vataire, A.-L.; Fukushima, A.; Aballea, S.; Khemiri, A.; Moore, C.; Medic, G.; Hemels, M.E.H. Comparison of Inhaled Antibiotics for the Treatment of Chronic Pseudomonas aeruginosa Lung Infection in Patients with Cystic Fibrosis: Systematic Literature Review and Network Meta-analysis. Clin. Ther. 2016, 38, 2204–2226. [Google Scholar] [CrossRef] [PubMed]
- Elborn, S.; Vataire, A.-L.; Fukushima, A.; Aballéa, S.; Khemiri, A.; Moore, C.; Medic, G.; Hemels, M. Efficacy and safety of inhaled antibiotics for chronic pseudomonas infection in cystic fibrosis: Network meta-analysis. Eur. Respir. J. 2016, 48, PA4863. [Google Scholar] [CrossRef]
- Nafee, N.; Schneider, M.; Schaefer, U.F.; Lehr, C.-M. Relevance of the colloidal stability of chitosan/PLGA nanoparticles on their cytotoxicity profile. Int. J. Pharm. 2009, 381, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Benson, J.R.; Hare, P.E. O-phthalaldehyde: Fluorogenic detection of primary amines in the picomole range. Comparison with fluorescamine and ninhydrin. Proc. Natl. Acad. Sci. USA 1975, 72, 619–622. [Google Scholar] [CrossRef] [PubMed]
- Barthold, S.; Kletting, S.; Taffner, J.; de Souza Carvalho-Wodarz, C.; Lepeltier, E.; Loretz, B.; Lehr, C.-M. Preparation of nanosized coacervates of positive and negative starch derivatives intended for pulmonary delivery of proteins. J. Mater. Chem. B 2016, 4, 2377–2386. [Google Scholar] [CrossRef]
- Deacon, J.; Abdelghany, S.M.; Quinn, D.J.; Schmid, D.; Megaw, J.; Donnelly, R.F.; Jones, D.S.; Kissenpfennig, A.; Elborn, J.S.; Gilmore, B.F.; et al. Antimicrobial efficacy of tobramycin polymeric nanoparticles for Pseudomonas aeruginosa infections in cystic fibrosis: Formulation, characterisation and functionalisation with dornase alfa (DNase). J. Control. Release 2015, 198, 55–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dul, M.; Paluch, K.J.; Kelly, H.; Healy, A.M.; Sasse, A.; Tajber, L. Self-assembled carrageenan/protamine polyelectrolyte nanoplexes-Investigation of critical parameters governing their formation and characteristics. Carbohydr. Polym. 2015, 123, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Radovic-Moreno, A.F.; Lu, T.K.; Puscasu, V.A.; Yoon, C.J.; Langer, R.; Farokhzad, O.C. Surface charge-switching polymeric nanoparticles for bacterial cell wall-targeted delivery of antibiotics. ACS Nano 2012, 6, 4279–4287. [Google Scholar] [CrossRef] [PubMed]
- Mandzy, N.; Grulke, E.; Druffel, T. Breakage of TiO2 agglomerates in electrostatically stabilized aqueous dispersions. Powder Technol. 2005, 160, 121–126. [Google Scholar] [CrossRef]
- Jonassen, H.; Kjoniksen, A.-L.; Hiorth, M. Stability of chitosan nanoparticles cross-linked with tripolyphosphate. Biomacromolecules 2012, 13, 3747–3756. [Google Scholar] [CrossRef] [PubMed]
- Honary, S.; Zahir, F. Effect of Zeta Potential on the Properties of Nano-Drug Delivery Systems—A Review (Part 2). Trop. J. Pharm. Res. 2013, 12, 265–273. [Google Scholar] [CrossRef]
- Yoo, M.K.; Sung, Y.K.; Chong, S.C.; Young, M.L. Effect of polymer complex formation on the cloud-point of poly(N-isopropyl acrylamide) (PNIPAAm) in the poly(NIPAAm-co-acrylic acid): Polyelectrolyte complex between poly(acrylic acid) and poly(allylamine). Polymer 1997, 38, 2759–2765. [Google Scholar] [CrossRef]
- Fan, W.; Yan, W.; Xu, Z.; Ni, H. Formation mechanism of monodisperse, low molecular weight chitosan nanoparticles by ionic gelation technique. Colloids Surf. B Biointerfaces 2012, 90, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Zhu, K. The influence of multivalent phosphate structure on the properties of ionically cross-linked chitosan films for controlled drug release. Eur. J. Pharm. Biopharm. 2002, 54, 235–243. [Google Scholar] [CrossRef]
- Ensign, L.M.; Cone, R.; Hanes, J. Nanoparticle-based drug delivery to the vagina: A review. J. Control. Release 2014, 190, 500–514. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.F.; Pye, G.; Bramley, R.; Clark, A.G.; Dyson, T.J.; Hardcastle, J.D. Measurement of gastrointestinal pH profiles in normal ambulant human subjects. Gut 1988, 29, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Melis, G.B.; Ibba, M.T.; Steri, B.; Kotsonis, P.; Matta, V.; Paoletti, A.M. Ruolo del pH come modulatore dell’equilibrio fisiopatologico vaginale. Min. Ginecol. 2000, 52, 111–121. [Google Scholar]
- Fischer, D.; Li, Y.; Ahlemeyer, B.; Krieglstein, J.; Kissel, T. In vitro cytotoxicity testing of polycations: Influence of polymer structure on cell viability and hemolysis. Biomatrials 2003, 24, 1121–1131. [Google Scholar] [CrossRef]
- Frohlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef] [PubMed]
- Maiz, L.; Giron, R.M.; Olveira, C.; Quintana, E.; Lamas, A.; Pastor, D.; Canton, R.; Mensa, J. Inhaled antibiotics for the treatment of chronic bronchopulmonary Pseudomonas aeruginosa infection in cystic fibrosis: Systematic review of randomised controlled trials. Expert Opin. Pharmacother. 2013, 14, 1135–1149. [Google Scholar] [CrossRef] [PubMed]
- Bargoni, A.; Cavalli, R.; Zara, G.P.; Fundaro, A.; Caputo, O.; Gasco, M.R. Transmucosal transport of tobramycin incorporated in solid lipid nanoparticles (SLN) after duodenal administration to rats. Part II—Tissue distribution. Pharmacol. Res. 2001, 43, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Wallace, S.J.; Li, J.; Nation, R.L.; Prankerd, R.J.; Velkov, T.; Boyd, B.J. Self-assembly behavior of colistin and its prodrug colistin methanesulfonate: Implications for solution stability and solubilization. J. Phys. Chem. B 2010, 114, 4836–4840. [Google Scholar] [CrossRef] [PubMed]
- Akinc, A.; Thomas, M.; Klibanov, A.M.; Langer, R. Exploring polyethylenimine-mediated DNA transfection and the proton sponge hypothesis. J. Gene Med. 2005, 7, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Truong, N.P.; Jia, Z.; Burgess, M.; Payne, L.; McMillan, N.A.J.; Monteiro, M.J. Self-catalyzed degradable cationic polymer for release of DNA. Biomacromolecules 2011, 12, 3540–3548. [Google Scholar] [CrossRef] [PubMed]
- Hartono, S.B.; Phuoc, N.T.; Yu, M.; Jia, Z.; Monteiro, M.J.; Qiao, S.; Yu, C. Functionalized large pore mesoporous silica nanoparticles for gene delivery featuring controlled release and co-delivery. J. Mater. Chem. B 2014, 2, 718–726. [Google Scholar] [CrossRef] [Green Version]
- Truong, N.P.; Gu, W.; Prasadam, I.; Jia, Z.; Crawford, R.; Xiao, Y.; Monteiro, M.J. An influenza virus-inspired polymer system for the timed release of siRNA. Nat. Commun. 2013, 4, 1902. [Google Scholar] [CrossRef] [PubMed]
- Sanz, V.; Coley, H.M.; Silva, S.R.P.; McFadden, J. Protamine and Chloroquine Enhance Gene Delivery and Expression Mediated by RNA-Wrapped Single Walled Carbon Nanotubes. J. Nanosci. Nanotechnol. 2012, 12, 1739–1747. [Google Scholar] [CrossRef] [PubMed]
- Kean, T.; Roth, S.; Thanou, M. Trimethylated chitosans as non-viral gene delivery vectors: Cytotoxicity and transfection efficiency. J. Control. Release 2005, 103, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Tang, C.; Yin, C. Exploring advantages/disadvantages and improvements in overcoming gene delivery barriers of amino acid modified trimethylated chitosan. Pharm. Res. 2015, 32, 2038–2050. [Google Scholar] [CrossRef] [PubMed]


| Polyplexes | Size (nm) | PDI | ζ-potential (mV) |
|---|---|---|---|
| Drug-free anCP | 287.9 ± 5.0 | 0.22 ± 0.01 | −29.7 ± 0.4 |
| Drug-free cCP | 205.4 ± 3.9 | 0.14 ± 0.02 | 27.1 ± 1.0 |
| Polyplexes | Size (nm) | PDI | ζ-potential (mV) | %EE | %LR |
|---|---|---|---|---|---|
| Tobramycin loaded anCP | 175.2 ± 2.8 | 0.18 ± 0.00 | −16.8 ± 1.0 | 98.7 ± 0.1 | 2.9 ± 0.0 |
| Colistin loaded anCP | 266.3 ± 6.5 | 0.27 ± 0.01 | −14.6 ± 0.5 | 96.6 ± 0.2 | 17.2 ± 0.1 |
| pAmCyan loaded anCP | 271.8 ± 2.4 | 0.25 ± 0.01 | −29.8 ± 0.6 | 76.6 ± 0.6 | 0.3 ± 0.002 |
| pAmCyan loaded cCP | 214.0 ± 3.5 | 0.17 ± 0.01 | 28.0 ± 0.6 | 67.7 ± 14.1 | 0.2 ± 0.036 |
| pAmCyan double loaded cCP | 204.6 ± 3.5 | 0.16 ± 0.02 | 25.5 ± 0.6 | 93.9 ± 4.5 | 3.3 ± 0.150 |
| Samples | IC90 against E. coli (μg/mL) | IC90 against P. aeruginosa (μg/mL) |
|---|---|---|
| Tobramycin | 0.2–0.3 | 1.56 |
| Tobramycin loaded anCP | 0.2–0.3 * | 1.56 * |
| Colistin | 0.4–0.5 | 3.125 |
| Colistin loaded anCP | 0.5 * | 3.125–6.25 * |
| anCP | >64 | >64 |
| PBS buffer | no inhibition | no inhibition |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasar, H.; Ho, D.-K.; De Rossi, C.; Herrmann, J.; Gordon, S.; Loretz, B.; Lehr, C.-M. Starch-Chitosan Polyplexes: A Versatile Carrier System for Anti-Infectives and Gene Delivery. Polymers 2018, 10, 252. https://doi.org/10.3390/polym10030252
Yasar H, Ho D-K, De Rossi C, Herrmann J, Gordon S, Loretz B, Lehr C-M. Starch-Chitosan Polyplexes: A Versatile Carrier System for Anti-Infectives and Gene Delivery. Polymers. 2018; 10(3):252. https://doi.org/10.3390/polym10030252
Chicago/Turabian StyleYasar, Hanzey, Duy-Khiet Ho, Chiara De Rossi, Jennifer Herrmann, Sarah Gordon, Brigitta Loretz, and Claus-Michael Lehr. 2018. "Starch-Chitosan Polyplexes: A Versatile Carrier System for Anti-Infectives and Gene Delivery" Polymers 10, no. 3: 252. https://doi.org/10.3390/polym10030252