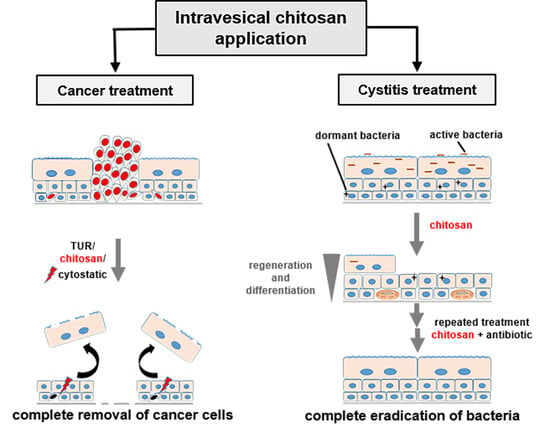
The Use of Polymer Chitosan in Intravesical Treatment of Urinary Bladder Cancer and Infections
Abstract
:1. Urinary Bladder Function and Malfunctions
2. Effect of Chitosan to the Urothelium
3. Chitosan in Treatment of Urinary Bladder Cancer
4. Chitosan in Treatment of Bacterial Infection of the Urinary Bladder
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Khandelwal, P.; Abraham, S.N.; Apodaca, G. Cell biology and physiology of the uroepithelium. Am. J. Physiol.-Ren. Physiol. 2009, 297, F1477–F1501. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.A.; Diamond, J.M. Na+ transport by rabbit urinary bladder, a tight epithelium. J. Membr. Biol. 1976, 28, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Meyers, S.; Liang, F.-X.; Deng, F.-M.; Kachar, B.; Zeidel, M.L.; Sun, T.-T. Role of membrane proteins in permeability barrier function: Uroplakin ablation elevates urothelial permeability. Am. J. Physiol.-Ren. Physiol. 2002, 283, F1200–F1207. [Google Scholar] [CrossRef] [PubMed]
- Kachar, B.; Liang, F.; Lins, U.; Ding, M.; Wu, X.-R.; Stoffler, D.; Aebi, U.; Sun, T.-T. Three-dimensional analysis of the 16 nm urothelial plaque particle: Luminal surface exposure, preferential head-to-head interaction, and hinge formation 1 1Edited by W. Baumeisser. J. Mol. Biol. 1999, 285, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Hurst, R.E.; Rhodes, S.W.; Adamson, P.B.; Parsons, C.L.; Roy, J.B. Functional and structural characteristics of the glycosaminoglycans of the bladder luminal surface. J. Urol. 1987, 138, 433–437. [Google Scholar] [CrossRef]
- Acharya, P.; Beckel, J.; Ruiz, W.G.; Wang, E.; Rojas, R.; Birder, L.; Apodaca, G. Distribution of the tight junction proteins ZO-1, occludin, and claudin-4, -8, and -12 in bladder epithelium. Am. J. Physiol.-Ren. Physiol. 2004, 287, F305–F318. [Google Scholar] [CrossRef] [PubMed]
- Varley, C.L.; Garthwaite, M.A.E.; Cross, W.; Hinley, J.; Trejdosiewicz, L.K.; Southgate, J. PPARγ-regulated tight junction development during human urothelial cytodifferentiation. J. Cell. Physiol. 2006, 208, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Baskin, L.; Meaney, D.; Landsman, A.; Zderic, S.A.; Macarak, E. Bovine bladder compliance increases with normal fetal development. J. Urol. 1994, 152, 692–695, discussion 696–697. [Google Scholar] [CrossRef]
- Truschel, S.T.; Wang, E.; Ruiz, W.G.; Leung, S.-M.; Rojas, R.; Lavelle, J.; Zeidel, M.; Stoffer, D.; Apodaca, G. Stretch-regulated exocytosis/endocytosis in bladder umbrella cells. Mol. Biol. Cell 2002, 13, 830–846. [Google Scholar] [CrossRef] [PubMed]
- Baskin, L.S.; Hayward, S.W.; Young, P.F.; Cunha, G.R. Ontogeny of the rat bladder: Smooth muscle and epithelial differentiation. Acta Anat. (Basel) 1996, 155, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Veranic, P.; Jezernik, K. Trajectorial organisation of cytokeratins within the subapical region of umbrella cells. Cell Motil. Cytoskelet. 2002, 53, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Veranic, P.; Romih, R.; Jezernik, K. What determines differentiation of urothelial umbrella cells? Eur. J. Cell Biol. 2004, 83, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Veranic, P.; Jezernik, K. The response of junctional complexes to induced desquamation in mouse bladder urothelium. Biol. Cell 2000, 92, 105–113. [Google Scholar] [CrossRef]
- Jost, S.P.; Potten, C.S. Urothelial proliferation in growing mice. Cell Tissue Kinet. 1986, 19, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Veranic, P.; Erman, A.; Kerec-Kos, M.; Bogataj, M.; Mrhar, A.; Jezernik, K. Rapid differentiation of superficial urothelial cells after chitosan-induced desquamation. Histochem. Cell Biol. 2009, 131, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Erman, A.; Kerec Kos, M.; Žakelj, S.; Resnik, N.; Romih, R.; Veranič, P. Correlative study of functional and structural regeneration of urothelium after chitosan-induced injury. Histochem. Cell Biol. 2013, 140, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Romih, R.; Koprivec, D.; Martincic, D.S.; Jezernik, K. Restoration of the rat urothelium after cyclophosphamide treatment. Cell Biol. Int. 2001, 25, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Sasaki, J. 12-O-tetradecanoylphorbol-13-acetate induces selective desquamation of superficial cells in rat urinary bladder epithelium. Cell Tissue Res. 1992, 268, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Romih, R.; Jezernik, K.; Masera, A. Uroplakins and cytokeratins in the regenerating rat urothelium after sodium saccharin treatment. Histochem. Cell Biol. 1998, 109, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.; Hindmarsh, J.R.; Ludgate, C.M.; Chisholm, G.D. Observations on the ultrastructure of human urothelium: The response of normal bladder of elderly subjects to hyperthermia. Urol. Res. 1982, 10, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Kos, M.K.; Bogataj, M.; Veranic, P.; Mrhar, A. Permeability of pig urinary bladder wall: Time and concentration dependent effect of chitosan. Biol. Pharm. Bull. 2006, 29, 1685–1691. [Google Scholar] [CrossRef] [PubMed]
- Singla, A.K.; Chawla, M. Chitosan: Some pharmaceutical and biological aspects—An update. J. Pharm. Pharmacol. 2001, 53, 1047–1067. [Google Scholar] [CrossRef] [PubMed]
- Agerkvist, I.; Eriksson, L.; Enfors, S.O. Selective flocculation with chitosan in Escherichia coli disintegrates: Effects of pH and nuclease treatment. Enzym. Microb. Technol. 1990, 12, 584–590. [Google Scholar] [CrossRef]
- Višnjar, T.; Jerman, U.D.; Veranič, P.; Kreft, M.E. Chitosan hydrochloride has no detrimental effect on bladder urothelial cancer cells. Toxicol. In Vitro 2017, 44, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Hsu, L.-W.; Ho, Y.-C.; Chuang, E.-Y.; Chen, C.-T.; Juang, J.-H.; Su, F.-Y.; Hwang, S.-M.; Sung, H.-W. Effects of pH on molecular mechanisms of chitosan-integrin interactions and resulting tight-junction disruptions. Biomaterials 2013, 34, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Pavinatto, F.J.; Pavinatto, A.; Caseli, L.; dos Santos, D.S.; Nobre, T.M.; Zaniquelli, M.E.D.; Oliveira, O.N. Interaction of Chitosan with Cell Membrane Models at the Air−Water Interface. Biomacromolecules 2007, 8, 1633–1640. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Ping, Q.; Jiang, G.; Huang, L.; Tong, Y. Chitosan-coated liposomes: Characterization and interaction with leuprolide. Int. J. Pharm. 2003, 260, 167–173. [Google Scholar] [CrossRef]
- Perugini, P.; Genta, I.; Pavanetto, F.; Conti, B.; Scalia, S.; Baruffini, A. Study on glycolic acid delivery by liposomes and microspheres. Int. J. Pharm. 2000, 196, 51–61. [Google Scholar] [CrossRef]
- Pavinatto, F.J.; Pacholatti, C.P.; Montanha, É.A.; Caseli, L.; Silva, H.S.; Miranda, P.B.; Viitala, T.; Oliveira, O.N. Cholesterol Mediates Chitosan Activity on Phospholipid Monolayers and Langmuir-Blodgett Films. Langmuir 2009, 25, 10051–10061. [Google Scholar] [CrossRef] [PubMed]
- Artursson, P.; Lindmark, T.; Davis, S.S.; Illum, L. Effect of chitosan on the permeability of monolayers of intestinal epithelial cells (Caco-2). Pharm. Res. 1994, 11, 1358–1361. [Google Scholar] [CrossRef] [PubMed]
- Hejazi, R.; Amiji, M. Chitosan-based gastrointestinal delivery systems. J. Control. Release Off. J. Control. Release Soc. 2003, 89, 151–165. [Google Scholar] [CrossRef]
- Min, G.; Wang, H.; Sun, T.-T.; Kong, X.-P. Structural basis for tetraspanin functions as revealed by the cryo-EM structure of uroplakin complexes at 6-A resolution. J. Cell Biol. 2006, 173, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-C.A.; Liang, F.-X.; Zhou, G.; Tu, L.; Tang, C.-H.A.; Zhou, J.; Kreibich, G.; Sun, T.-T. Assembly of urothelial plaques: Tetraspanin function in membrane protein trafficking. Mol. Biol. Cell 2005, 16, 3937–3950. [Google Scholar] [CrossRef] [PubMed]
- Parkin, D.M. The global burden of urinary bladder cancer. Scand. J. Urol. Nephrol. Suppl. 2008, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Ploeg, M.; Aben, K.K.H.; Kiemeney, L.A. The present and future burden of urinary bladder cancer in the world. World J. Urol. 2009, 27, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Habuchi, T.; Marberger, M.; Droller, M.J.; Hemstreet, G.P.; Grossman, H.B.; Schalken, J.A.; Schmitz-Dräger, B.J.; Murphy, W.M.; Bono, A.V.; Goebell, P.; et al. Prognostic markers for bladder cancer: International Consensus Panel on bladder tumor markers. Urology 2005, 66, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Chamie, K.; Litwin, M.S.; Bassett, J.C.; Daskivich, T.J.; Lai, J.; Hanley, J.M.; Konety, B.R.; Saigal, C.S. Urologic Diseases in America Project Recurrence of high-risk bladder cancer: A population-based analysis. Cancer 2013, 119, 3219–3227. [Google Scholar] [CrossRef] [PubMed]
- Nirmal, J.; Chuang, Y.-C.; Tyagi, P.; Chancellor, M.B. Intravesical therapy for lower urinary tract symptoms. Urol. Sci. 2012, 23, 70–77. [Google Scholar] [CrossRef]
- Neutsch, L.; Wambacher, M.; Wirth, E.-M.; Spijker, S.; Kählig, H.; Wirth, M.; Gabor, F. UPEC biomimickry at the urothelial barrier: Lectin-functionalized PLGA microparticles for improved intravesical chemotherapy. Int. J. Pharm. 2013, 450, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Key, J.; Dhawan, D.; Cooper, C.L.; Knapp, D.W.; Kim, K.; Kwon, I.C.; Choi, K.; Park, K.; Decuzzi, P.; Leary, J.F. Multicomponent, peptide-targeted glycol chitosan nanoparticles containing ferrimagnetic iron oxide nanocubes for bladder cancer multimodal imaging. Int. J. Nanomed. 2016, 11, 4141–4155. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Patel, T.R.; Fu, M.; Bertram, J.P.; Saltzman, W.M. Octa-functional PLGA nanoparticles for targeted and efficient siRNA delivery to tumors. Biomaterials 2012, 33, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.T.; Steinbach, J.M.; Liu, J.; Shimizu, S.; Kaimakliotis, H.Z.; Wheeler, M.A.; Hittelman, A.B.; Mark Saltzman, W.; Weiss, R.M. Surface-Modified Nanoparticles Enhance Transurothelial Penetration and Delivery of Survivin siRNA in Treating Bladder Cancer. Mol. Cancer Ther. 2014, 13, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Jerman, U.D.; Kreft, M.E.; Veranič, P. Epithelial-Mesenchymal Interactions in Urinary Bladder and Small Intestine and How to Apply Them in Tissue Engineering. Tissue Eng. Part B 2015, 21, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.G.; Koppolu, B.P.; Ravindranathan, S.; Kurtz, S.L.; Yang, L.; Katz, M.D.; Zaharoff, D.A. Intravesical chitosan/interleukin-12 immunotherapy induces tumor-specific systemic immunity against murine bladder cancer. Cancer Immunol. Immunother. CII 2015, 64, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.G.; Baltz, J.L.; Koppolu, B.P.; Ravindranathan, S.; Nguyen, K.; Zaharoff, D.A. Immunological mechanisms of intravesical chitosan/interleukin-12 immunotherapy against murine bladder cancer. OncoImmunology 2017, 6, e1259050. [Google Scholar] [CrossRef] [PubMed]
- Barber, A.E.; Norton, J.P.; Spivak, A.M.; Mulvey, M.A. Urinary tract infections: Current and emerging management strategies. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2013, 57, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Dielubanza, E.J.; Schaeffer, A.J. Urinary tract infections in women. Med. Clin. N. Am. 2011, 95, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.; Ki, M.; Foxman, B. Acute pyelonephritis among adults: Cost of illness and considerations for the economic evaluation of therapy. Pharmacoeconomics 2005, 23, 1123–1142. [Google Scholar] [CrossRef] [PubMed]
- Bower, J.M.; Eto, D.S.; Mulvey, M.A. Covert Operations of Uropathogenic Escherichia coli within the Urinary Tract. Traffic 2005, 6, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Eto, D.S.; Sundsbak, J.L.; Mulvey, M.A. Actin-gated intracellular growth and resurgence of uropathogenic Escherichia coli. Cell. Microbiol. 2006, 8, 704–717. [Google Scholar] [CrossRef] [PubMed]
- Blango, M.G.; Mulvey, M.A. Persistence of uropathogenic Escherichia coli in the face of multiple antibiotics. Antimicrob. Agents Chemother. 2010, 54, 1855–1863. [Google Scholar] [CrossRef] [PubMed]
- Blango, M.G.; Ott, E.M.; Erman, A.; Veranic, P.; Mulvey, M.A. Forced Resurgence and Targeting of Intracellular Uropathogenic Escherichia coli Reservoirs. PLoS ONE 2014, 9, e93327. [Google Scholar] [CrossRef] [PubMed]
- Erman, A.; Križan Hergouth, V.; Blango, M.G.; Kerec Kos, M.; Mulvey, M.A.; Veranič, P. Repeated treatments with chitosan in combination with antibiotics completely eradicate uropathogenic Escherichia coli from infected mouse urinary bladders. J. Infect. Dis. 2017, 216, 375–381. [Google Scholar] [CrossRef] [PubMed]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erman, A.; Veranič, P. The Use of Polymer Chitosan in Intravesical Treatment of Urinary Bladder Cancer and Infections. Polymers 2018, 10, 265. https://doi.org/10.3390/polym10030265
Erman A, Veranič P. The Use of Polymer Chitosan in Intravesical Treatment of Urinary Bladder Cancer and Infections. Polymers. 2018; 10(3):265. https://doi.org/10.3390/polym10030265
Chicago/Turabian StyleErman, Andreja, and Peter Veranič. 2018. "The Use of Polymer Chitosan in Intravesical Treatment of Urinary Bladder Cancer and Infections" Polymers 10, no. 3: 265. https://doi.org/10.3390/polym10030265
APA StyleErman, A., & Veranič, P. (2018). The Use of Polymer Chitosan in Intravesical Treatment of Urinary Bladder Cancer and Infections. Polymers, 10(3), 265. https://doi.org/10.3390/polym10030265