Mechanocatalytic Solvent-Free Esterification of Sugarcane Bagasse
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
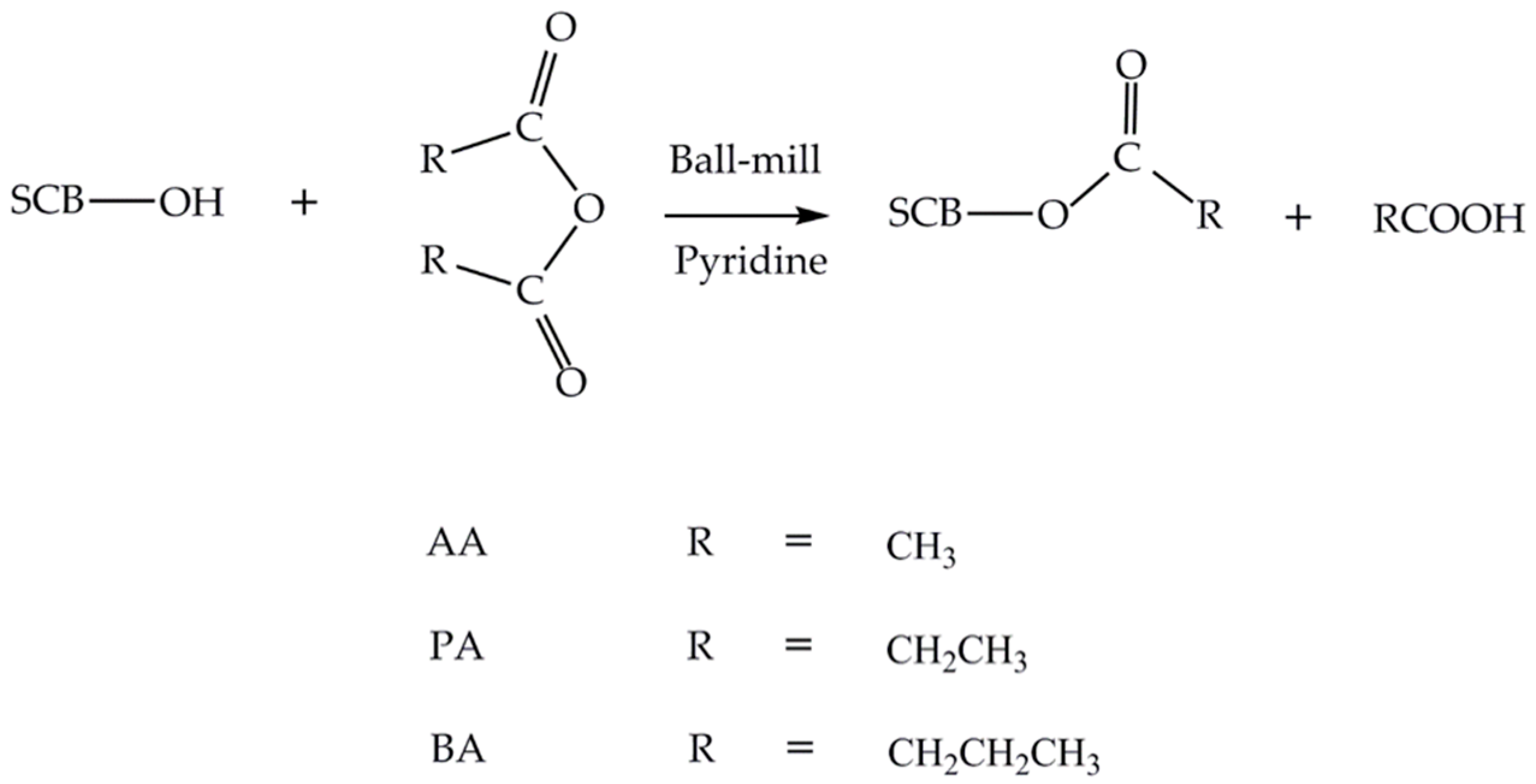
2.2. Mechanocatalytic Solvent-Free Esterification of SCB
2.3. Characterization of the Esterified SCB
3. Results and Discussion
3.1. Preparation of Esterified SCB
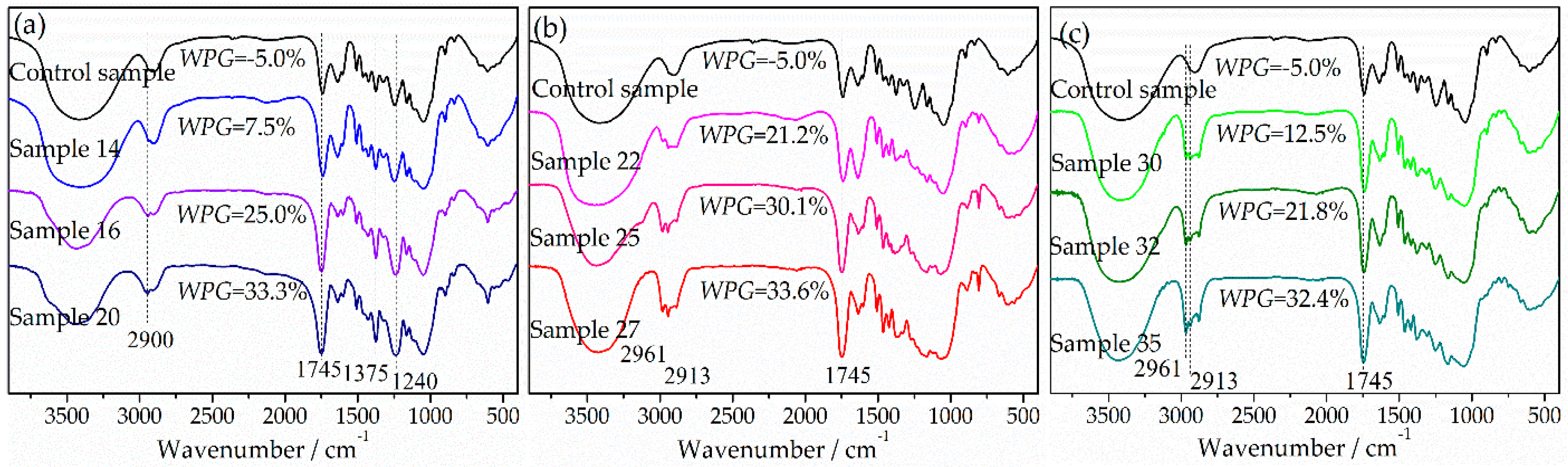
3.2. FT-IR
3.3. CP/MAS 13C-NMR
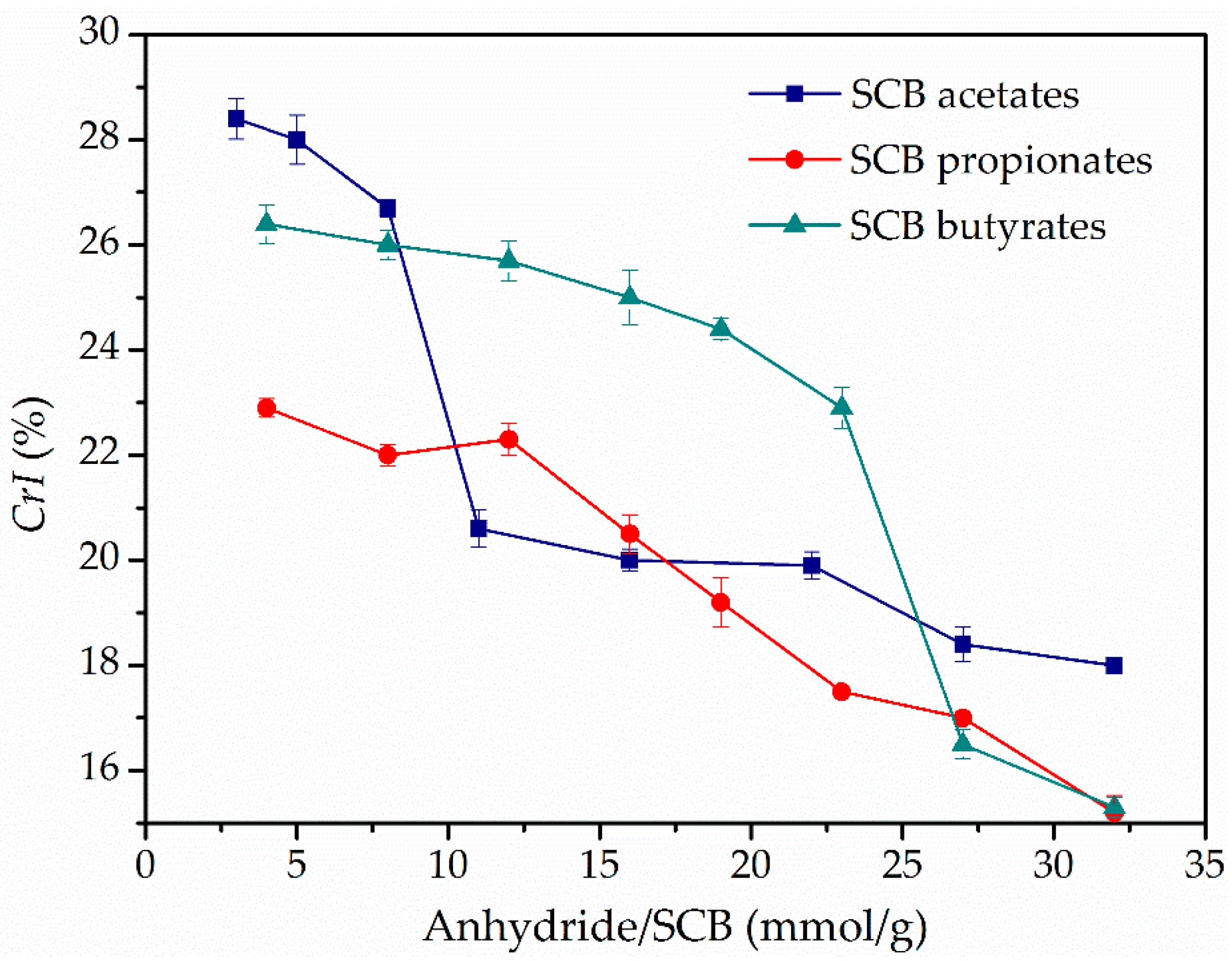
3.4. XRD
3.5. SEM
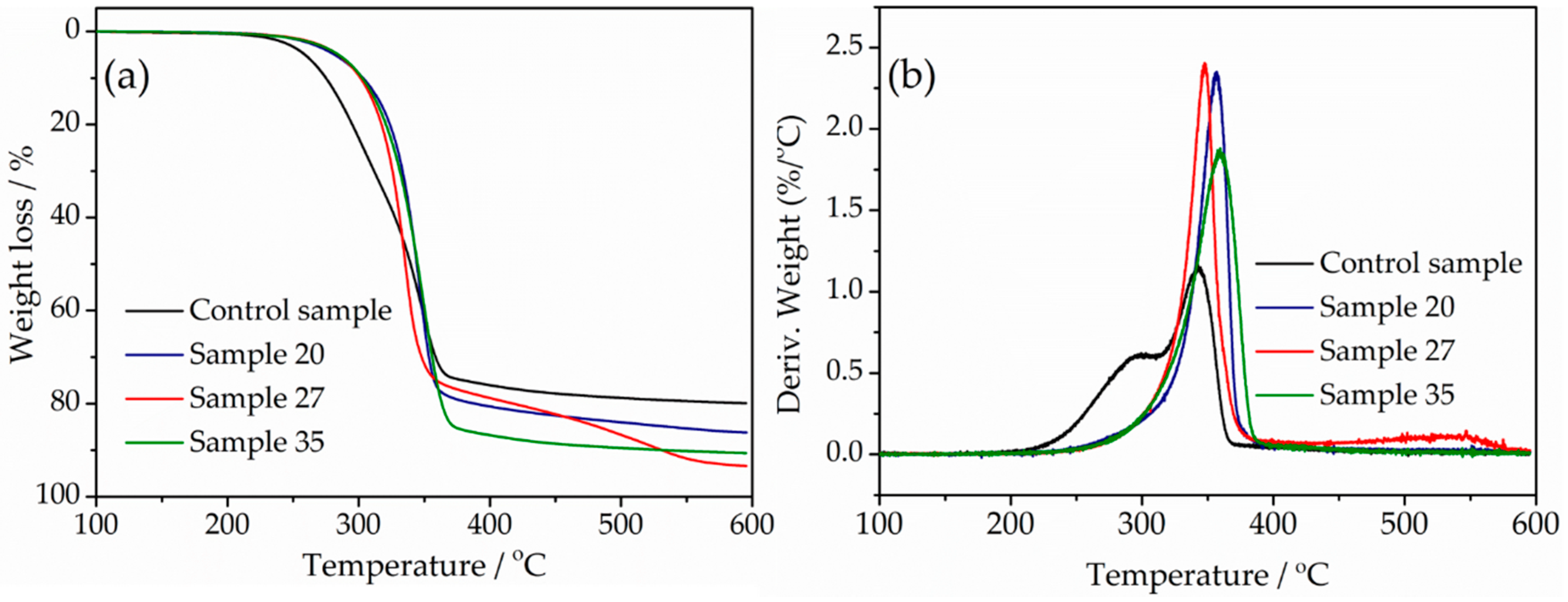
3.6. Thermal Stability
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gallezot, P. Conversion of biomass to selected chemical products. Chem. Soc. Rev. 2012, 41, 1538–1558. [Google Scholar] [CrossRef] [PubMed]
- Ten, E.; Vermerris, W. Functionalized polymers from lignocellulosic biomass: State of the art. Polymers 2013, 5, 600–642. [Google Scholar] [CrossRef]
- Pandey, A.; Soccol, C.R.; Nigam, P.; Soccol, V.T. Biotechnological potential of agro-industrial residues. I: Sugarcane bagasse. Bioresour. Technol. 2000, 74, 69–80. [Google Scholar] [CrossRef]
- Suseno, N.; Sapei, L.; Purwanto, E.; Adiarto, T. Effect of delignification process on physical properties of sugarcane baggase paper. AIP Conf. Proc. 2017, 1840, 080002. [Google Scholar] [CrossRef]
- Rocha, G.J.M.; Martin, C.; Soares, I.B.; Maior, A.M.S.; Baudel, H.M.; Abreu, C.A.M. Dilute mixed-acid pretreatment of sugarcane bagasse for ethanol production. Biomass Bioenergy 2011, 35, 663–670. [Google Scholar] [CrossRef]
- Long, J.; Guo, B.; Teng, J.; Yu, Y.; Wang, L.; Li, X. SO3H-functionalized ionic liquid: Efficient catalyst for bagasse liquefaction. Bioresour. Technol. 2011, 102, 10114–10123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wong, H.; Albertson, P.L.; Doherty, W.O.S.; O’Hara, I.M. Laboratory and pilot scale pretreatment of sugarcane bagasse by acidified aqueous glycerol solutions. Bioresour. Technol. 2013, 138, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, X.; Liu, C.; Sun, R.; Lu, F. Approach to renewable lignocellulosic biomass film directly from bagasse. ACS Sustain. Chem. Eng. 2014, 2, 1164–1168. [Google Scholar] [CrossRef]
- Peng, F.; Ren, J.; Xu, F.; Bian, J.; Peng, P.; Sun, R. Comparative study of hemicelluloses obtained by graded ethanol precipitation from sugarcane bagasse. J. Agric. Food. Chem. 2009, 57, 6305–6317. [Google Scholar] [CrossRef] [PubMed]
- Carpita, N.C.; Gibeaut, D.M. Structural models of primary cell walls in flowering plants: Consistency of molecular structure with the physical properties of the walls during growth. Plant J. 1993, 3, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Fengel, D. Wood and cellulose chemistry. Holz als Roh-und Werkstoff 1992, 50, 340. [Google Scholar] [CrossRef]
- Subair, N.; Jinitha, T.V.; Shaniba, V.; Sreejith, M.P.; Aparna, K.B.; Purushothaman, E. Isolation and characterisation of cellulose nanocrystals from sago seed shells. Carbohydr. Polym. 2018, 180, 13–20. [Google Scholar] [CrossRef]
- Cadete, R.M.; Melo-Cheab, M.A.; Dussán, K.J.; Rodrigues, R.C.L.B.; da Silva, S.S.; Gomes, F.C.O.; Rosa, C.A. Production of bioethanol in sugarcane bagasse hemicellulosic hydrolysate by scheffersomyces parashehatae, scheffersomyces illinoinensis and spathaspora arborariae isolated from brazilian ecosystems. J. Appl. Microbiol. 2017, 123, 1203–1213. [Google Scholar] [CrossRef] [PubMed]
- Marett, J.; Aning, A.; Foster, E.J. The isolation of cellulose nanocrystals from pistachio shells via acid hydrolysis. Ind. Crop. Prod. 2017, 109, 869–874. [Google Scholar] [CrossRef]
- Heinze, T.; Liebert, T. Unconventional methods in cellulose functionalization. Prog. Polym. Sci. 2001, 26, 1689–1762. [Google Scholar] [CrossRef]
- Mizrachi, E.; Mansfield, S.D.; Myburg, A.A. Cellulose factories: Advancing bioenergy production from forest trees. New Phytol. 2012, 194, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Vanholme, R.; Demedts, B.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin biosynthesis and structure. Plant Physiol. 2010, 153, 895. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.L.; Rowell, R.M.; Fadl, N.A.; Yacoub, S.F.; Christainsen, A.W. Thermoplasticization of bagasse. I. Preparation and characterization of esterified bagasse fibers. J. Appl. Polym. Sci. 2000, 76, 561–574. [Google Scholar] [CrossRef]
- Nada, A.A.M.A.; Hassan, M.L. Ion exchange properties of carboxylated bagasse. J. Appl. Polym. Sci. 2006, 102, 1399–1404. [Google Scholar] [CrossRef]
- Sun, X.; Sun, R.; Sun, J. A convenient acetylation of sugarcane bagasse using NBS as a catalyst for the preparation of oil sorption-active materials. J. Mater. Sci. 2003, 38, 3915–3923. [Google Scholar] [CrossRef]
- Chen, M.; Shi, Q. Transforming sugarcane bagasse into bioplastics via homogeneous modification with phthalic anhydride in ionic liquid. ACS Sustain. Chem. Eng. 2015, 3, 2510–2515. [Google Scholar] [CrossRef]
- Chen, M.; Li, R.; Zhang, X.; Feng, J.; Feng, J.; Liu, C.; Shi, Q. Homogeneous transesterification of sugarcane bagasse toward sustainable plastics. ACS Sustain. Chem. Eng. 2017, 5, 360–366. [Google Scholar] [CrossRef]
- Li, M.; Sun, S.; Xu, F.; Sun, R. Mild synthesis of benzylated bamboo in LiCl/DMSO solution. J. Appl. Polym. Sci. 2012, 125, 274–282. [Google Scholar] [CrossRef]
- Xie, H.; King, A.; Kilpelainen, I.; Granstrom, M.; Argyropoulos, D.S. Thorough chemical modification of wood-based lignocellulosic materials in ionic liquids. Biomacromolecules 2007, 8, 3740–3748. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Tan, Y.; Zhang, Y.; Liu, X.; Hu, H.; Qin, Y.; Huang, H. Direct production of cellulose laurate by mechanical activation-strengthened solid phase synthesis. Bioresour. Technol. 2012, 118, 624–627. [Google Scholar] [CrossRef] [PubMed]
- Voronov, A.; Kohut, A.; Synytska, A.; Peukert, W. Mechanochemical modification of silica with poly(1-vinyl-2-pyrrolidone) by grinding in a stirred media mill. J. Appl. Polym. Sci. 2007, 104, 3708–3714. [Google Scholar] [CrossRef]
- Esteban, L.; Carrasco, J. Evaluation of different strategies for pulverization of forest biomasses. Powder Technol. 2006, 166, 139–151. [Google Scholar] [CrossRef]
- Schell, D.; Harwood, C. Milling of lignocellulosic biomass. Appl. Biochem. Biotechnol. 1994, 45–46, 159–168. [Google Scholar] [CrossRef]
- Bitra, V.; Womac, A.R.; Chevanan, N.; Miu, P.; Igathinathane, C.; Sokhansanj, S.; Smith, D.R. Direct mechanical energy measures of hammer mill comminution of switchgrass, wheat straw, and corn stover and analysis of their particle size distributions. Powder Technol. 2009, 193, 32–45. [Google Scholar] [CrossRef]
- Hick, S.M.; Griebel, C.; Restrepo, D.T.; Truitt, J.H.; Buker, E.J.; Bylda, C.; Blair, R.G. Mechanocatalysis for biomass-derived chemicals and fuels. Green Chem. 2010, 12, 468–474. [Google Scholar] [CrossRef]
- Avolio, R.; Bonadies, I.; Capitani, D.; Errico, M.E.; Gentile, G.; Avella, M. A multitechnique approach to assess the effect of ball milling on cellulose. Carbohydr. Polym. 2012, 87, 265–273. [Google Scholar] [CrossRef]
- Peydecastaing, J.; Vaca-Garcia, C.; Borredon, E. Accurate determination of the degree of substitution of long chain cellulose esters. Cellulose 2009, 16, 289. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Hill, C. Chemical modification of wood (I): Acetic anhydride modification. In Wood Modification: Chemical, Thermal and Other Processes, 2nd ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2006; pp. 45–76. [Google Scholar] [CrossRef]
- Shaikh, H.M.; Pandare, K.V.; Nair, G.; Varma, A.J. Utilization of sugarcane bagasse cellulose for producing cellulose acetates: Novel use of residual hemicellulose as plasticizer. Carbohydr. Polym. 2009, 76, 23–29. [Google Scholar] [CrossRef]
- Rowell, R.; Moisuk, R.; Meyer, J.A. Wood-polymer composites: Cell wall grafting with alkylene oxides and lumen treatments with methyl methacrylate. Wood Sci. Technol. 1982, 15, 90–96. [Google Scholar]
- Hauptmann, M.; Gindl-Altmutter, W.; Hansmann, C.; Bacher, M.; Rosenau, T.; Liebner, F.; D’Amico, S.; Schwanninger, M. Wood modification with tricine. Holzforschung 2015, 69, 8. [Google Scholar] [CrossRef]
- Popescu, C.M.; Popescu, M.C.; Vasile, C. Structural analysis of photodegraded lime wood by means of FT-IR and 2D IR correlation spectroscopy. Int. J. Biol. Macromol. 2011, 48, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Hase, Y.; Davanzo, C.U.; Kawai, K.; Sala, O. The vibrational spectra of phthalic anhydride. J. Mol. Struct. 1976, 30, 37–44. [Google Scholar] [CrossRef]
- Chang, S.; Chang, H. Comparisons of the photostability of esterified wood. Polym. Degrad. Stab. 2001, 71, 261–266. [Google Scholar] [CrossRef]
- Tang, L.; Huang, B.; Yang, N.; Li, T.; Lu, Q.; Lin, W.; Chen, X. Organic solvent-free and efficient manufacture of functionalized cellulose nanocrystals via one-pot tandem reactions. Green Chem. 2013, 15, 2369–2373. [Google Scholar] [CrossRef]
- Barud, H.S.; de Araújo Júnior, A.M.; Santos, D.B.; de Assunção, R.M.N.; Meireles, C.S.; Cerqueira, D.A.; Rodrigues Filho, G.; Ribeiro, C.A.; Messaddeq, Y.; Ribeiro, S.J.L. Thermal behavior of cellulose acetate produced from homogeneous acetylation of bacterial cellulose. Thermochim. Acta 2008, 471, 61–69. [Google Scholar] [CrossRef]
- Tosh, B. Thermal analysis of cellulose esters prepared from different molecular weight fractions of high α-cellulose pulp. Indian J. Chem. Technol. 2011, 18, 451–457. [Google Scholar]
- Chen, D.; Zhang, A.P.; Liu, C.F.; Sun, R.C. Modification of sugarcane bagasse with acetic anhydride and butyric anhydride in ionic liquid 1-butyl-3-methylimidazolium chloride. Bioresources 2012, 7, 3476–3487. [Google Scholar]
- Wang, H.; Wen, X.; Zhang, X.; Liu, C. Acetylation of microcrystalline cellulose by transesterification in AmimCl/DMSO cosolvent system. Molecules 2017, 22, 1419. [Google Scholar] [CrossRef] [PubMed]
- Gaan, S.; Mauclaire, L.; Rupper, P.; Salimova, V.; Tran, T.T.; Heuberger, M. Thermal degradation of cellulose acetate in presence of bis-phosphoramidates. J. Anal. Appl. Pyrolysis 2011, 90, 33–41. [Google Scholar] [CrossRef]
- Nasatto, L.P.; Pignon, F.; Silveira, L.J.; Duarte, E.M.; Noseda, D.M.; Rinaudo, M. Methylcellulose, a cellulose derivative with original physical properties and extended applications. Polymers 2015, 7, 777–803. [Google Scholar] [CrossRef]



| No. | Anhydride | Anhydride/SCB (mmol/g) | Pyridine/Anhydride (mol/mol) | Rotation Speed (r/min) | Time (h) | WPG (%) | DS (mmol/g) |
|---|---|---|---|---|---|---|---|
| Control | / | / | / | 800 | 4.0 | −5.0 ± 0.13 | / |
| 1 | AA | 11:1 | 1:1 | 800 | 0.5 | 10.7 ± 0.21 | 4.9 ± 0.51 |
| 2 | AA | 11:1 | 1:1 | 800 | 1.0 | 16.5 ± 0.47 | 5.5 ± 0.34 |
| 3 | AA | 11:1 | 1:1 | 800 | 2.0 | 17.8 ± 0.35 | 5.8 ± 0.41 |
| 4 | AA | 11:1 | 1:1 | 800 | 4.0 | 22.2 ± 0.64 | 7.6 ± 0.39 |
| 5 | AA | 11:1 | 1:1 | 1000 | 4.0 | 24.1 ± 0.32 | 7.8 ± 0.22 |
| 6 | AA | 3:1 | / | 1200 | 4.0 | 2.5 ± 0.21 | 3.0 ± 0.42 |
| 7 | AA | 5:1 | / | 1200 | 4.0 | 4.6 ± 0.34 | 3.5 ± 0.19 |
| 8 | AA | 8:1 | / | 1200 | 4.0 | 6.4 ± 0.27 | 3.7 ± 0.21 |
| 9 | AA | 11:1 | / | 1200 | 4.0 | 5.6 ± 0.49 | 3.8 ± 0.53 |
| 10 | AA | 16:1 | / | 1200 | 4.0 | 1.9 ± 0.21 | 2.7 ± 0.21 |
| 11 | AA | 22:1 | / | 1200 | 4.0 | 3.2 ± 0.52 | 3.5 ± 0.36 |
| 12 | AA | 27:1 | / | 1200 | 4.0 | 4.2 ± 0.46 | 3.6 ± 0.41 |
| 13 | AA | 32:1 | / | 1200 | 4.0 | 5.3 ± 0.39 | 3.9 ± 0.39 |
| 14 | AA | 3:1 | 1:1 | 1200 | 4.0 | 7.5 ± 0.62 | 4.1 ± 0.27 |
| 15 | AA | 5:1 | 1:1 | 1200 | 4.0 | 18.1 ± 0.58 | 6.2 ± 0.32 |
| 16 | AA | 8:1 | 1:1 | 1200 | 4.0 | 25.0 ± 0.47 | 8.1 ± 0.54 |
| 17 | AA | 11:1 | 1:1 | 1200 | 4.0 | 27.3 ± 0.72 | 8.4 ± 0.31 |
| 18 | AA | 16:1 | 1:1 | 1200 | 4.0 | 30.3 ± 0.43 | 8.3 ± 0.48 |
| 19 | AA | 22:1 | 1:1 | 1200 | 4.0 | 30.0 ± 0.67 | 8.6 ± 0.34 |
| 20 | AA | 27:1 | 1:1 | 1200 | 4.0 | 33.3 ± 0.36 | 8.9 ± 0.52 |
| 21 | AA | 32:1 | 1:1 | 1200 | 4.0 | 31.8 ± 0.68 | 8.6 ± 0.29 |
| 22 | PA | 4:1 | 1:1 | 1200 | 4.0 | 21.2 ± 0.52 | 5.3 ± 0.41 |
| 23 | PA | 8:1 | 1:1 | 1200 | 4.0 | 24.2 ± 0.44 | 6.4 ± 0.37 |
| 24 | PA | 12:1 | 1:1 | 1200 | 4.0 | 25.8 ± 0.51 | 6.6 ± 0.16 |
| 25 | PA | 16:1 | 1:1 | 1200 | 4.0 | 30.1 ± 0.82 | 6.8 ± 0.32 |
| 26 | PA | 19:1 | 1:1 | 1200 | 4.0 | 30.2 ± 0.49 | 6.6 ± 0.42 |
| 27 | PA | 23:1 | 1:1 | 1200 | 4.0 | 33.6 ± 0.79 | 7.9 ± 0.22 |
| 28 | PA | 27:1 | 1:1 | 1200 | 4.0 | 31.7 ± 0.41 | 7.2 ± 0.29 |
| 29 | PA | 32:1 | 1:1 | 1200 | 4.0 | 31.3 ± 0.61 | 7.0 ± 0.33 |
| 30 | BA | 4:1 | 1:1 | 1200 | 4.0 | 12.5 ± 0.36 | 2.1 ± 0.29 |
| 31 | BA | 8:1 | 1:1 | 1200 | 4.0 | 17.2 ± 0.52 | 2.4 ± 0.41 |
| 32 | BA | 12:1 | 1:1 | 1200 | 4.0 | 21.8 ± 0.29 | 2.9 ± 0.53 |
| 33 | BA | 16:1 | 1:1 | 1200 | 4.0 | 23.7 ± 0.42 | 3.3 ± 0.17 |
| 34 | BA | 19:1 | 1:1 | 1200 | 4.0 | 27.1 ± 0.51 | 3.9 ± 0.23 |
| 35 | BA | 23:1 | 1:1 | 1200 | 4.0 | 32.4 ± 0.67 | 4.6 ± 0.27 |
| 36 | BA | 27:1 | 1:1 | 1200 | 4.0 | 30.1 ± 0.46 | 4.1 ± 0.42 |
| 37 | BA | 32:1 | 1:1 | 1200 | 4.0 | 30.9 ± 0.51 | 4.3 ± 0.15 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Zhang, X.; Zhu, Z.; Zhang, A.; Zhang, C.; Wang, X.; Liu, C. Mechanocatalytic Solvent-Free Esterification of Sugarcane Bagasse. Polymers 2018, 10, 282. https://doi.org/10.3390/polym10030282
Zhang Q, Zhang X, Zhu Z, Zhang A, Zhang C, Wang X, Liu C. Mechanocatalytic Solvent-Free Esterification of Sugarcane Bagasse. Polymers. 2018; 10(3):282. https://doi.org/10.3390/polym10030282
Chicago/Turabian StyleZhang, Qiang, Xueqin Zhang, Ziyan Zhu, Aiping Zhang, Chunhui Zhang, Xiaoying Wang, and Chuanfu Liu. 2018. "Mechanocatalytic Solvent-Free Esterification of Sugarcane Bagasse" Polymers 10, no. 3: 282. https://doi.org/10.3390/polym10030282








