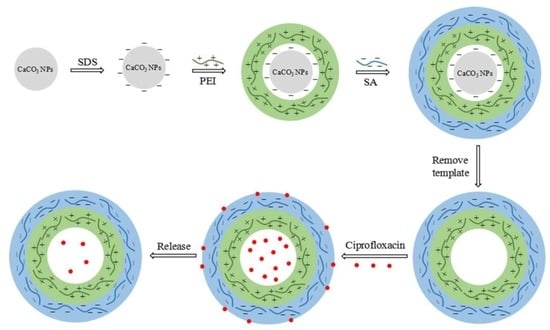
Preparation of Novel Nano-Sized Hydrogel Microcapsules via Layer-By-Layer Assembly as Delivery Vehicles for Drugs onto Hygiene Paper
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of PEI/SA Microcapsules
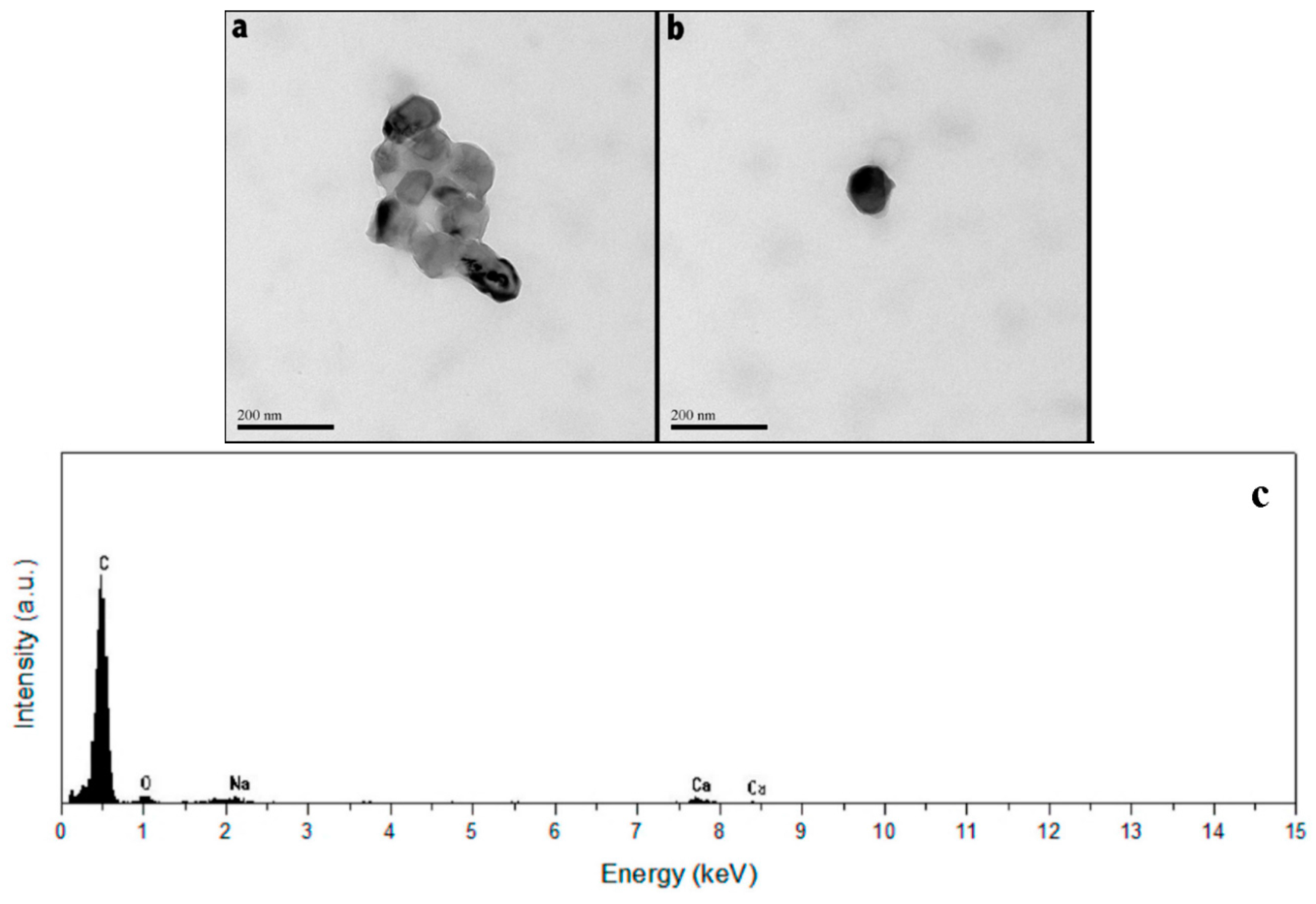
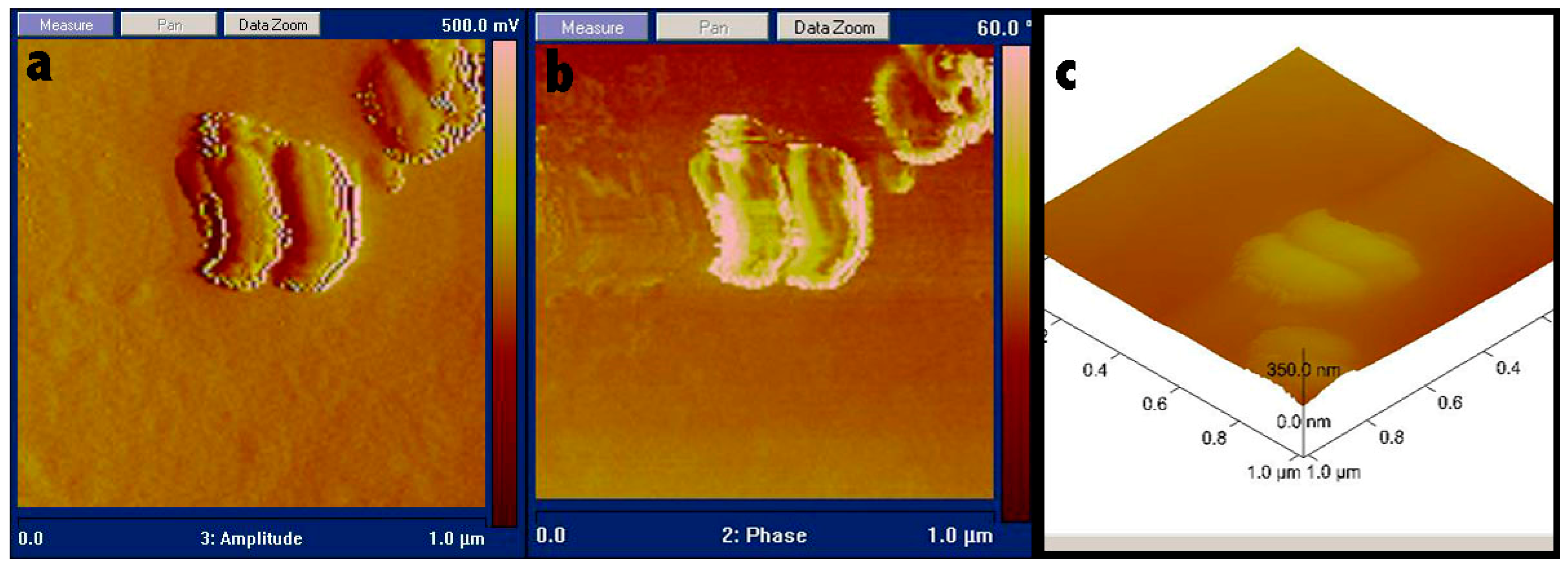
2.3. Characterization of the Hydrogel Microcapsules
2.4. Loading and Release of Ciprofloxacin
2.5. Preparation and Characterization of Hydrogel Microcapsules Absorbed Paper
3. Results
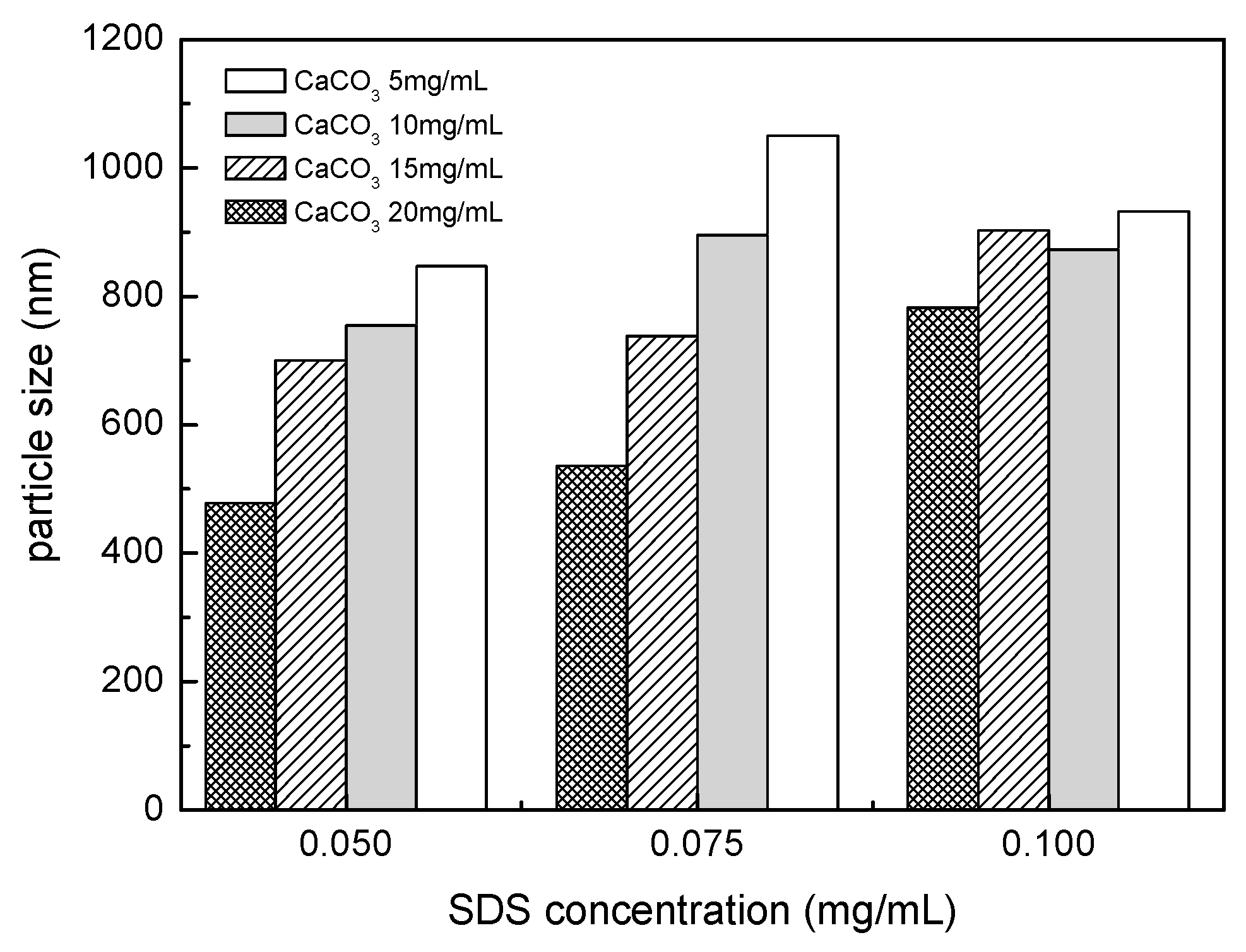
3.1. Dispersion of CaCO3 Nanoparticles
3.2. Fabrication of PEI/SA Hydrogel Microcapsules
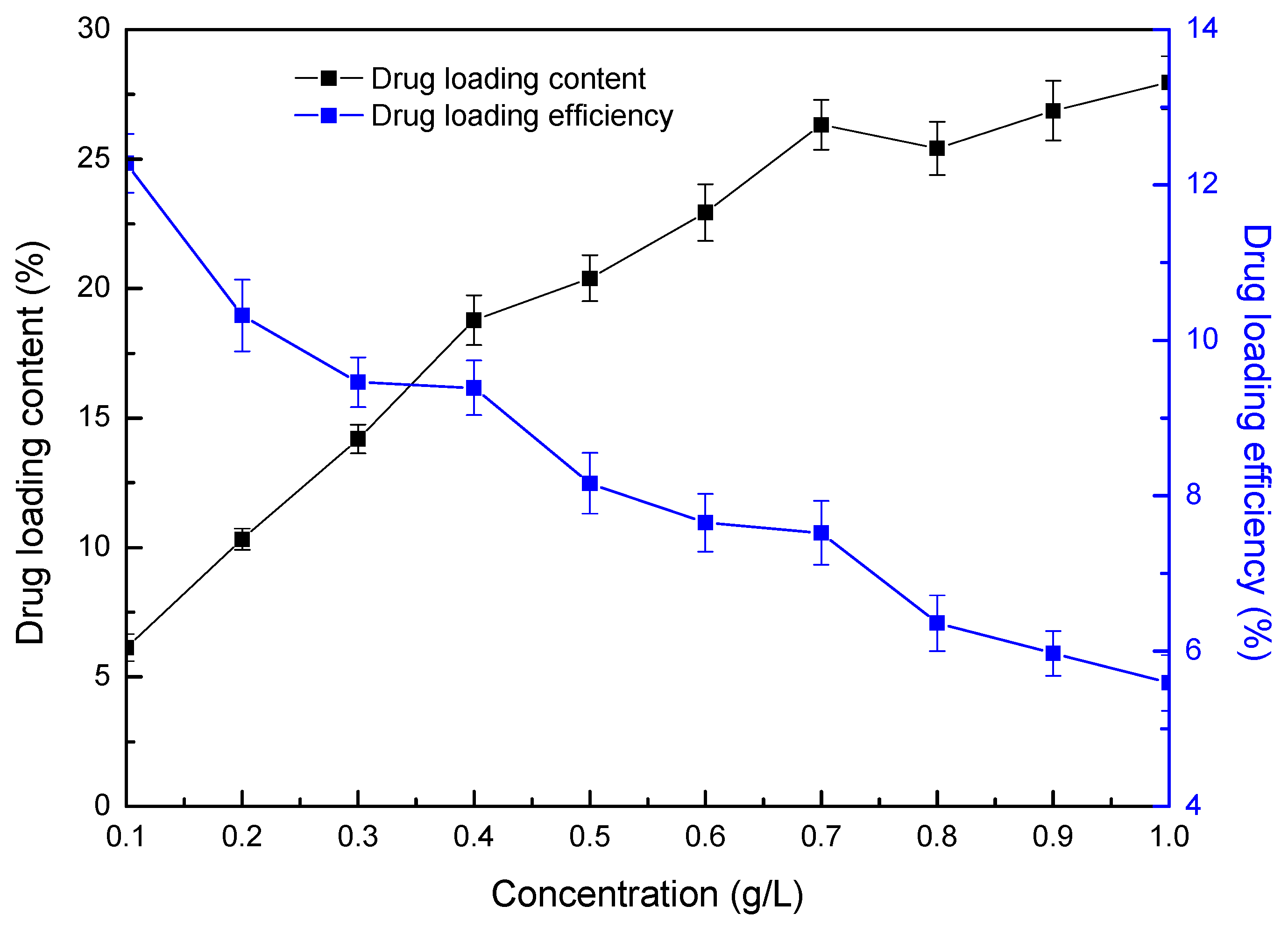
3.3. Loading and Releasing of Ciprofloxacin
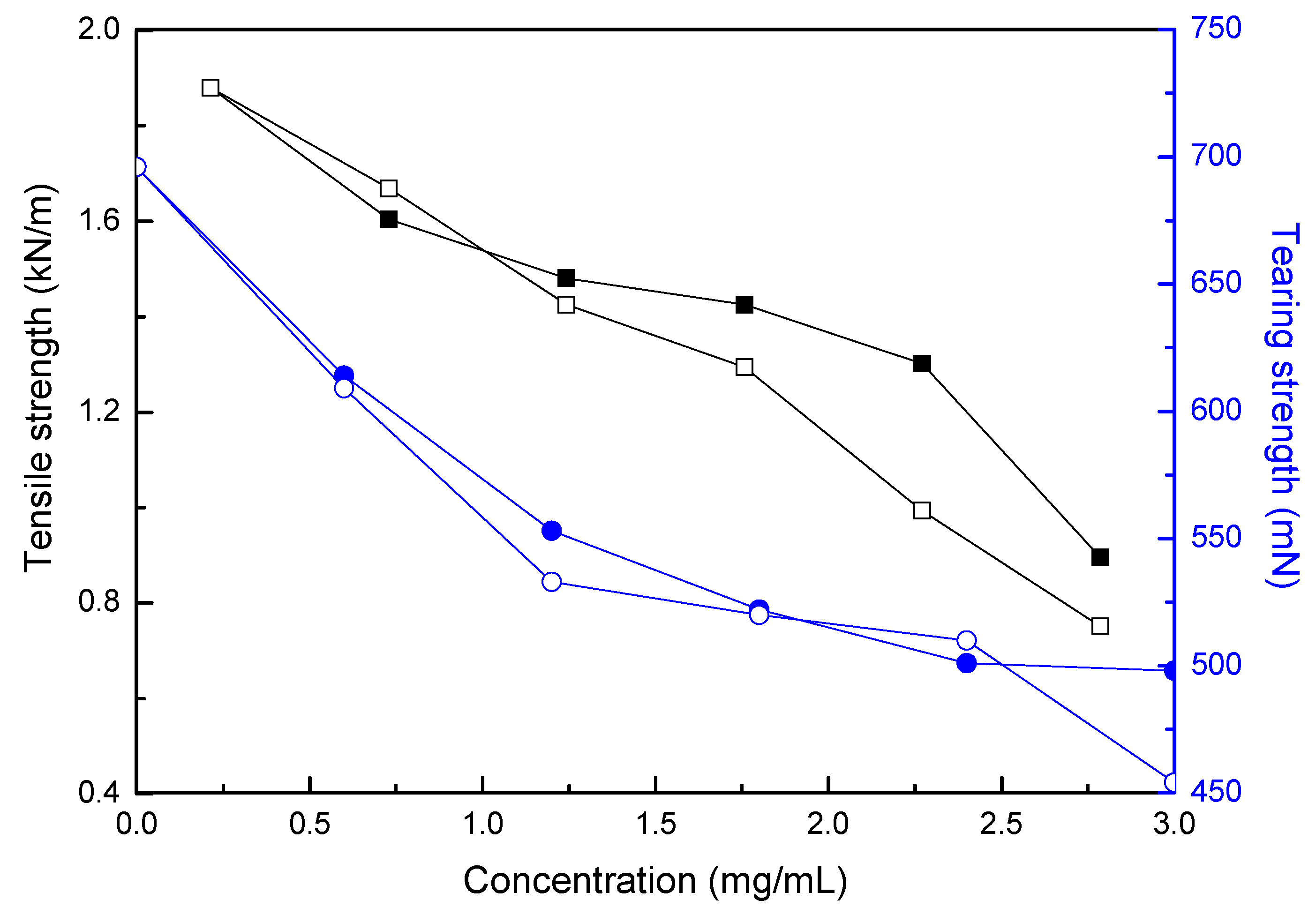
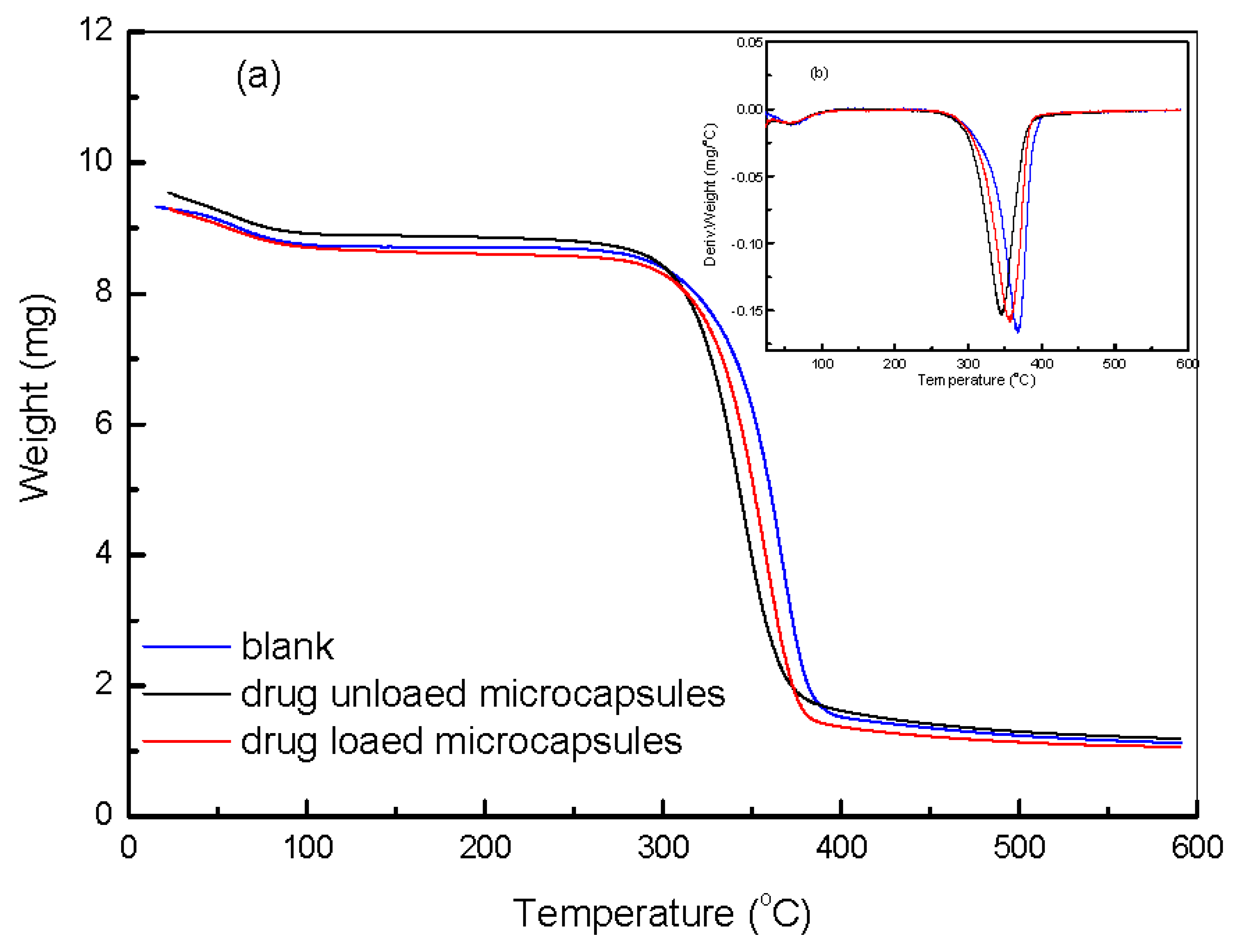
3.4. Incorporation of Hydrogel Microcapsules onto the Paper
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ichikawa, H.; Arimoto, M.; Fukumori, Y. Design of microcapsules with hydrogel as a membrane component and their preparation by a spouted bed. Adv. Powder Technol. 2003, 130, 189–192. [Google Scholar] [CrossRef]
- Zhang, S.H.; Jiang, Z.Y.; Shi, J.F.; Wang, X.Y.; Han, P.P.; Qian, W.L. An efficient, recyclable, and stable immobilized biocatalyst based on bioinspired microcapsules-in-hydrogel scaffolds. ACS Appl. Mater. Interfaces 2016, 8, 25152–25161. [Google Scholar] [CrossRef] [PubMed]
- Siltanen, C.; Diakataou, M.; Lowen, J.; Haque, A.; Rahimian, A.; Stybayeva, G.; Revzin, A. One step fabrication of hydrogel microcapsules with hollow core for assembly and cultivation of hepatocyte spheroids. Acta Biomater. 2017, 50, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Zhao, S.T.; Bielecki, P.; Rao, W.; Choi, J.K.; Zhao, Y.; Yu, J.H.; Zhang, W.J.; He, X.M. One-step microfluidic generation of pre-hatching embryo-like core-shell microcapsules for miniaturized 3D culture of pluripotent stem cells. Lab Chip 2013, 13, 4525–4533. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Y.; Shu, Z.Q.; Gao, D.Y.; Shen, A.Q. Sensing and sensibility: Single-islet-based quality control assay of cryopreserved pancreatic islets with functionalized hydrogel microcapsules. Adv. Healthc. Mater. 2016, 5, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Alessandri, K.; Feyeux, M.; Gurchenkov, B.; Delgado, C.; Trushko, A.; Krause, K.H.; Vignjevic, D.; Nassoy, P.; Roux, A. A 3D printed microfluidic device for production of functionalized hydrogel microcapsules for culture and differentiation of human Neuronal Stem Cells (hNSC). Lab Chip 2016, 16, 1593–1604. [Google Scholar] [CrossRef] [PubMed]
- Byun, A.; Shim, J.; Han, S.W.; Kim, B.; Chae, P.S.; Shin, H.S.; Kim, J.W. One-pot microfluidic fabrication of graphene oxide-patched hollow hydrogel microcapsules with remarkable shell impermeability. Chem. Commun. 2015, 51, 12756–12759. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Bae, C.Y.; Han, J.I.; Park, J.K. In situ analysis of heterogeneity in the lipid content of single green microalgae in alginate hydrogel microcapsules. Anal. Chem. 2013, 85, 8749–8756. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.L.; Zelikin, A.N.; Johnston, A.P.R.; Caruso, F. Tuning the formation and degradation of layer-by-layer assembled polymer hydrogel microcapsules. Langmuir 2009, 25, 14079–14085. [Google Scholar] [CrossRef] [PubMed]
- Kozlovskaya, V.; Kharlampieva, E.; Chang, S.; Muhlbauer, R.; Tsukruk, V.V. pH-responsive layered hydrogel microcapsules as gold nanoreactors. Chem. Mater. 2009, 21, 2158–2167. [Google Scholar] [CrossRef]
- Mak, W.C.; Bai, J.; Chang, X.Y.; Trau, D. Matrix-assisted colloidosome reverse-phase layer-by-layer encapsulating biomolecules in hydrogel microcapsules with extremely high efficiency and retention stability. Langmuir 2009, 25, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.G.; McShane, M.J. Synthesis and functionalization of monodisperse poly(ethylene glycol) hydrogel microspheres within polyelectrolyte multilayer microcapsules. Chem. Commun. 2006, 2, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Guan, Y.; Zhou, S.Q. Single component chitosan hydrogel microcapsule from a layer-by-layer approach. Biomacromolecules 2005, 6, 2365–2369. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.J.; Wang, C.J.; Ning, Q.Y.; Gao, Q.; Gao, C.Y.; Gou, Z.R.; Ye, J. A new strategy to sustained release of ocular drugs by one-step drug-loaded microcapsule manufacturing in hydrogel punctal plugs. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 255, 2173–2184. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.K.; Son, Y.M.; Lee, N.E. Hydrogel encapsulation of cells in core-shell microcapsules for cell delivery. Adv. Healthc. Mater. 2015, 4, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Zaeim, D.; Sarabi-Jamab, M.; Ghorani, B.; Kadkhodaee, R.; Tromp, R.H. Electrospray assisted fabrication of hydrogel microcapsules by single- and double-stage procedures for encapsulation of probiotics. Food Bioprod. Process. 2017, 102, 250–259. [Google Scholar] [CrossRef]
- Wen, H.Y.; Gao, T.; Fu, Z.Z.; Liu, X.; Xu, J.T.; He, Y.S.; Xu, N.X.; Jiao, P.; Fan, A.; Huang, S.P. Enhancement of membrane stability on magnetic responsive hydrogel microcapsules for potential on-demand cell separation. Carbohydr. Polym. 2017, 157, 1451–1460. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.T.; Agarwal, P.; Rao, W.; Huang, H.S.; Zhang, R.L.; Liu, Z.G.; Yu, J.H.; Weisleder, N.; Zhang, W.J.; He, X.M. Coaxial electrospray of liquid core-hydrogel shell microcapsules for encapsulation and miniaturized 3D culture of pluripotent stem cells. Integr. Biol. 2014, 6, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.L.; Chiu, A.; Sahay, G.; Doloff, J.C.; Dholakia, N.; Thakrar, R.; Cohen, J.; Vegas, A.; Chen, D.L.; Bratlie, K.M. Core-shell hydrogel microcapsules for improved islets encapsulation. Adv. Healthc. Mater. 2013, 2, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.S.; Sun, M.R.; Heisler-Taylor, T.; Kiourti, A.; Volakis, J.; Lafyatis, G.; He, X.M. Stiffness-independent highly efficient on-chip extraction of cell-laden hydrogel microcapsules from oil emulsion into aqueous solution by dielectrophoresis. Chem. Mater. 2015, 11, 5369–5374. [Google Scholar] [CrossRef] [PubMed]
- Mytnyk, S.; Ziemecka, I.; Olive, A.G.L.; van der Meer, J.W.M.; Totlani, K.A.; Oldenhof, S.; Kreutzer, M.T.; van Steijn, V.; van Esch, J.H. Microcapsules with a permeable hydrogel shell and an aqueous core continuously produced in a 3D microdevice by all-aqueous microfluidics. RSC Adv. 2017, 7, 11331–11337. [Google Scholar] [CrossRef]
- Zan, X.J.; Garapaty, A.; Champion, J.A. Engineering polyelectrolyte capsules with independently controlled size and shape. Langmuir 2015, 31, 7601–7608. [Google Scholar] [CrossRef] [PubMed]
- Yashchenok, A.; Parakhonskiy, B.; Donatan, S.; Kohler, D.; Skirtach, A.; Mohwald, H. Polyelectrolyte multilayer microcapsules templated on spherical, elliptical and square calcium carbonate particles. J. Mater. Chem. B 2013, 1, 1223–1228. [Google Scholar] [CrossRef]
- Shaillender, M.; Luo, R.C.; Venkatraman, S.S.; Neu, B. Layer-by-layer microcapsules templated on erythrocyte ghost carriers. Int. J. Pharm. 2011, 415, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Rangelov, S.; Petrov, P. Template-assisted approaches for preparation of nano-sized polymer structures. In Nano-Size Polymers: Preparation, Properties, Applications; Fakirov, S., Ed.; Springer: Cham, Switzerland, 2016; pp. 367–396. ISBN 978-3-319-39713-9. [Google Scholar]
- Caruso, F. Hollow capsule processing through colloidal templating and self-assembly. Chem. Eur. J. 2000, 6, 413–419. [Google Scholar] [CrossRef]
- Antipov, A.A.; Shchukin, D.; Fedutik, Y.; Petrov, A.I.; Sukhorukov, G.B.; Mohwald, H. Carbonate microparticles for hollow polyelectrolyte capsules fabrication. Colloids Surf. A Physicochem. Eng. Asp. 2003, 224, 175–183. [Google Scholar] [CrossRef]
- Toncheva, N.; Tsvetanov, C.; Rangelov, S.; Trzebicka, B.; Dworak, A. Hydroxyl end-functionalized poly(2-isopropyl oxazoline)s used as nano-sized colloidal templates for preparation of hollow polymeric nanocapsules. Polymer 2013, 54, 5166–5173. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, X.L.; Zhu, K.X.; He, X.M. Hydrogel encapsulation facilitates rapid-cooling cryopreservation of stem cell-laden core-shell microcapsules as cell-biomaterial constructs. Adv. Healthc. Mater. 2017, 6, 1700988. [Google Scholar] [CrossRef] [PubMed]
- Boontheekul, T.; Kong, H.J.; Mooney, D.J. Controlling alginate gel degradation utilizing partial oxidation and bimodal molecular weight distribution. Biomaterials 2005, 26, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- Sarker, B.; Papageorgiou, D.G.; Silva, R.; Zehnder, T.; Gul-E-Noor, F.; Bertmer, M.; Kaschta, J.; Chrissafis, K.; Detsch, R.; Boccaccini, A.R. Fabrication of alginate-gelatin crosslinked hydrogel microcapsules and evaluation of the microstructure and physico-chemical properties. J. Mater. Chem. B 2014, 2, 1470–1480. [Google Scholar] [CrossRef]
- Zheng, J.N.; Xie, H.G.; Yu, W.T.; Tan, M.Q.; Gong, F.Q.; Liu, X.D.; Wang, F.; Lv, G.J.; Liu, W.F.; Zheng, G.S. Enhancement of surface graft density of MPEG on alginate/chitosan hydrogel microcapsules for protein repellency. Langmuir 2012, 28, 13261–13273. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.N.; Xie, H.G.; Yu, W.T.; Liu, X.D.; Xie, W.Y.; Zhu, J.; Ma, X.J. Chitosan-g-MPEG-modified alginate/chitosan hydrogel microcapsules: A quantitative study of the effect of polymer architecture on the resistance to protein adsorption. Langmuir 2010, 26, 17156–17164. [Google Scholar] [CrossRef] [PubMed]
- Orive, G.; De Castro, M.; Kong, H.J.; Hernandez, R.; Ponce, S.; Mooney, D.J.; Pedraz, J.L. Bioactive cell-hydrogel microcapsules for cell-based drug delivery. J. Control. Release 2009, 135, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.S.; Liu, X.D.; Wang, X.L.; Chen, L.; Xie, H.G.; Wang, F.; Zheng, H.Z.; Yu, W.T.; Ma, X.J. Improving stability and biocompatibility of alginate/chitosan microcapsule by fabricating bi-functional membrane. Macromol. Biosci. 2014, 14, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Tam, S.K.; Bilodeau, S.; Dusseault, J.; Langlois, G.; Halle, J.P.; Yahia, L.H. Biocompatibility and physicochemical characteristics of alginate-polycation microcapsules. Acta Biomater. 2011, 7, 1683–1692. [Google Scholar] [CrossRef] [PubMed]
- Tam, S.K.; Dusseault, J.; Bilodeau, S.; Langlois, G.; Halle, J.P.; Yahia, L. Factors influencing alginate gel biocompatibility. J. Biomed. Mater. Res. A 2011, 98A, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Mak, W.C.; Cheung, K.Y.; Trau, D. Influence of different polyelectrolytes on layer-by-layer microcapsule properties: Encapsulation efficiency and colloidal and temperature stability. Chem. Mater. 2008, 20, 5475–5484. [Google Scholar] [CrossRef]
- Dong, C.; Ye, Y.; Qian, L.Y.; Zhao, G.L.; He, B.H.; Xiao, H.N. Antibacterial modification of cellulose fibers by grafting beta-cyclodextrin and inclusion with ciprofloxacin. Cellulose 2014, 21, 1921–1932. [Google Scholar] [CrossRef]
- Liao, W.C.; Lilienthal, S.; Kahn, J.S.; Riutin, M.; Sohn, Y.S.; Nechushtai, R.; Willner, I. pH- and ligand-induced release of loads from DNA-acrylamide hydrogel microcapsules. Chem. Sci. 2017, 8, 3362–3373. [Google Scholar] [CrossRef] [PubMed]
- Parakhonskiy, B.V.; Yashchenok, A.M.; Konrad, M.; Skirtach, A.G. Colloidal micro- and nano-particles as templates for polyelectrolyte multilayer capsules. Adv. Colloid Interface Sci. 2014, 207, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Volodkin, D.V.; von Klitzing, R.; Mohwald, H. Pure protein microspheres by calcium carbonate templating. Angew. Chem. Int. Ed. 2010, 49, 9258–9261. [Google Scholar] [CrossRef] [PubMed]
- Boyjoo, Y.; Pareek, V.K.; Liu, J. Synthesis of micro and nano-sized calcium carbonate particles and their applications. J. Mater. Chem. A 2014, 2, 14270–14288. [Google Scholar] [CrossRef]
- Kang, S.H.; Hirasawa, I.; Kim, W.S.; Choi, C.K. Morphological control of calcium carbonate crystallized in reverse micelle system with anionic surfactants SDS and AOT. J. Colloid Interface Sci. 2005, 288, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Maniti, O.; Rebaud, S.; Sarkis, J.; Jia, Y.; Zhao, J.; Marcillat, O.; Granjon, T.; Blum, L.; Li, J.B.; Girad-Egrot, A. Carrier-inside-carrier: polyelectrolyte microcapsules as reservoir for drug-loaded liposomes. J. Liposome Res. 2015, 25, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Moulin, P.; Roques, H. Zeta potential measurement of calcium carbonate. J. Colloid Interface Sci. 2003, 261, 115–126. [Google Scholar] [CrossRef]
- Han, B.S.; Shen, B.Y.; Wang, Z.H.; Shi, M.M.; Li, H.W.; Peng, C.H.; Zhao, Q.H. Layered microcapsules for daunorubicin loading and release as well as in vitro and in vivo studies. Polym. Adv. Technol. 2008, 19, 36–46. [Google Scholar] [CrossRef]
- Sukhorukov, G.B.; Rogach, A.L.; Garstka, M.; Springer, S.; Parak, W.J.; Munoz-Javier, A.; Kreft, O.; Skirtach, A.G.; Susha, A.S.; Ramaye, Y. Multifunctionalized polymer microcapsules: Novel tools for biological and pharmacological applications. Small 2007, 3, 944–955. [Google Scholar] [CrossRef] [PubMed]
- Peyratout, C.S.; Dahne, L. Tailor-made polyelectrolyte microcapsules: From multilayers to smart containers. Angew. Chem. Int. Ed. 2004, 43, 3762–3783. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Pandey, P.; Tiwari, G.; Tiwari, R.; Rai, A.K. FTIR spectroscopy: A tool for quantitative analysis of ciprofloxacin in tablets. Indian J. Pharm. Sci. 2012, 74, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.Y.; Donath, E.; Mohwald, H.; Shen, J.C. Spontaneous deposition of water-soluble substances into microcapsules: Phenomenon, mechanism, and application. Angew. Chem. Int. Ed. 2002, 114, 3943–3947. [Google Scholar] [CrossRef]
- Mao, Z.W.; Ma, L.; Gao, C.Y.; Shen, J.C. Preformed microcapsules for loading and sustained release of ciprofloxacin hydrochloride. J. Control. Release 2005, 104, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Yew, P.Y.M.; Chee, P.L.; Cally, O.; Zhang, K.; Liow, S.S.; Loh, X.J. Quarternized short polyethylenimine shows good activity against drug-resistant bacteria. Macromol. Mater. Eng. 2017, 302, 1700186. [Google Scholar] [CrossRef]


| Microcapsule Concentration (mg/mL) | Antimicrobial Efficiency (%) | |
|---|---|---|
| Drug Unloaded Samples | Drug Loaded Samples | |
| 0.5 | 22.1 ± 1.1 | 46.7 ± 2.1 |
| 1.0 | 31.3 ± 1.5 | 58.4 ± 2.6 |
| 1.5 | 37.8 ± 1.7 | 69.8 ± 2.8 |
| 2.0 | 39.6 ± 1.9 | 73.4 ± 3.1 |
| 2.5 | 40.8 ± 1.6 | 82.1 ± 4.1 |
| 3.0 | 40.6 ± 1.5 | 86.3 ± 3.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Zou, J.; Xiao, H.; He, B.; Hou, X.; Qian, L. Preparation of Novel Nano-Sized Hydrogel Microcapsules via Layer-By-Layer Assembly as Delivery Vehicles for Drugs onto Hygiene Paper. Polymers 2018, 10, 335. https://doi.org/10.3390/polym10030335
Li J, Zou J, Xiao H, He B, Hou X, Qian L. Preparation of Novel Nano-Sized Hydrogel Microcapsules via Layer-By-Layer Assembly as Delivery Vehicles for Drugs onto Hygiene Paper. Polymers. 2018; 10(3):335. https://doi.org/10.3390/polym10030335
Chicago/Turabian StyleLi, Junrong, Jing Zou, Huining Xiao, Beihai He, Xiaobang Hou, and Liying Qian. 2018. "Preparation of Novel Nano-Sized Hydrogel Microcapsules via Layer-By-Layer Assembly as Delivery Vehicles for Drugs onto Hygiene Paper" Polymers 10, no. 3: 335. https://doi.org/10.3390/polym10030335