Regulating Dielectric and Ferroelectric Properties of Poly(vinylidene fluoride-trifluoroethylene) with Inner CH=CH Bonds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
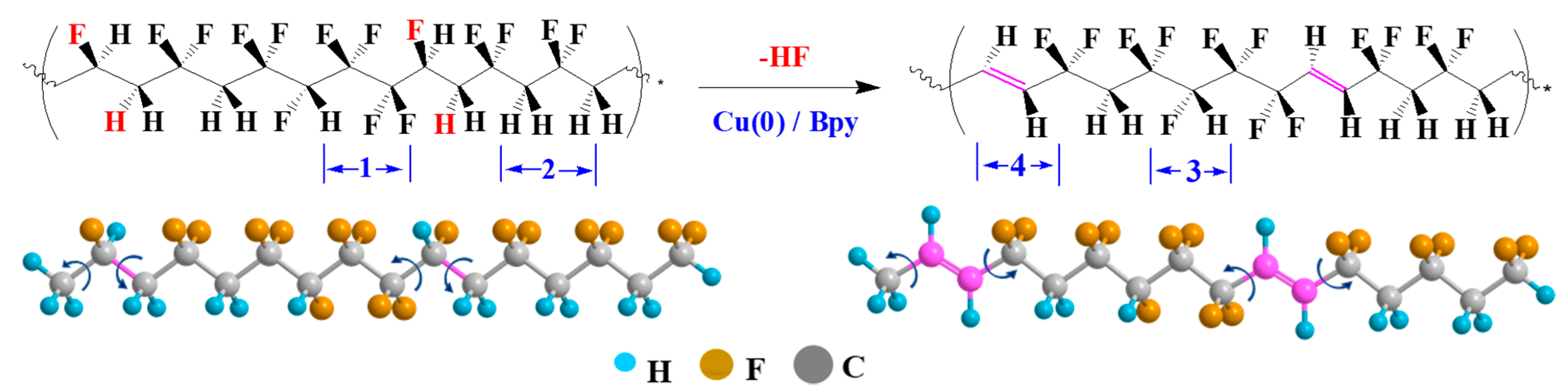
2.2. Synthesis of P(VDF-TrFE-DB) from P(VDF-TrFE) via SET-RE Process
2.3. Film Fabrication under Varied Conditions, Instrumentation, and Characterization
2.4. Instrumentation and Characterization
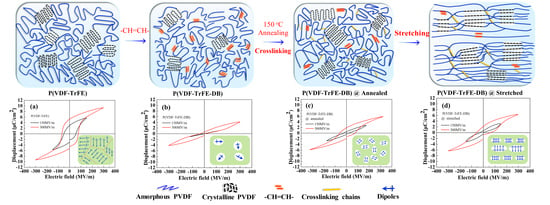
3. Results and Discussion
3.1. Characterization and Chemical Composition of P(VDF-TrFE-DB)s
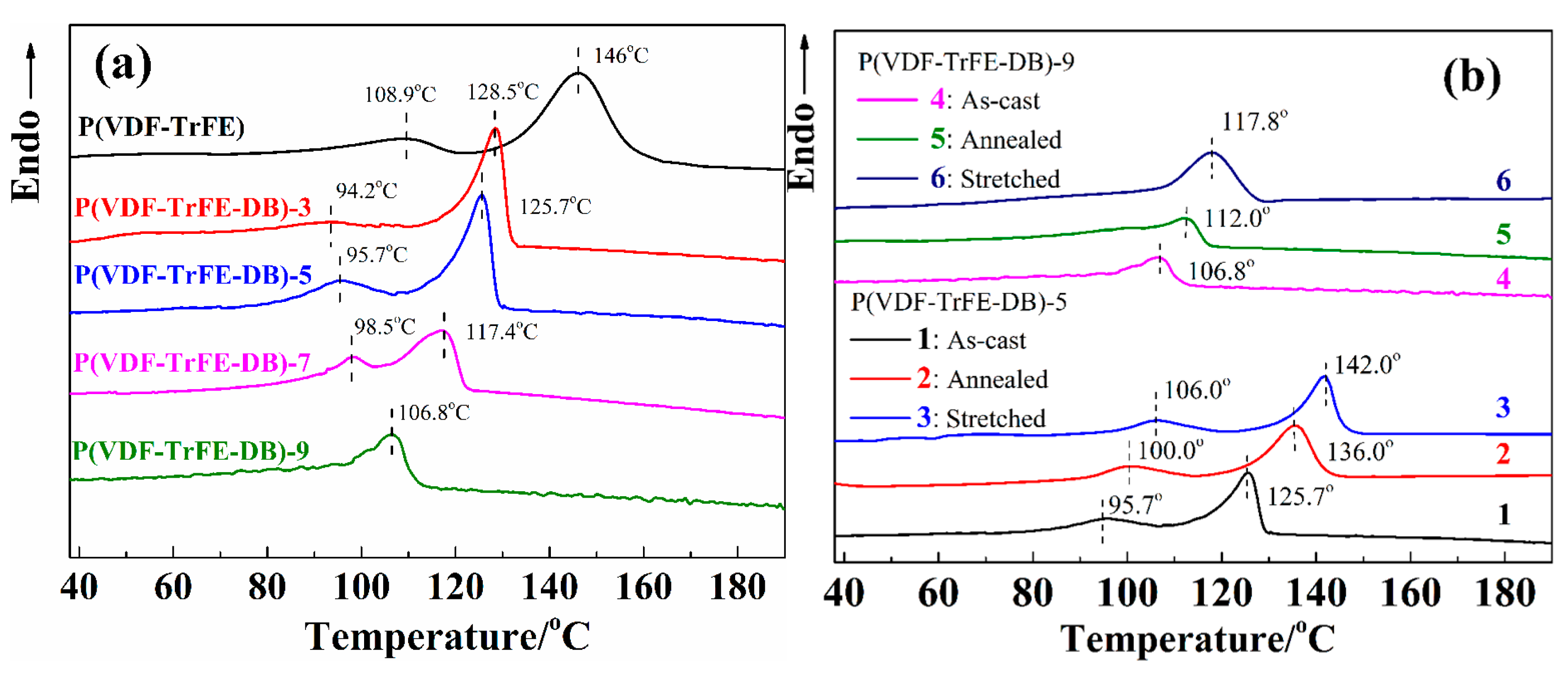
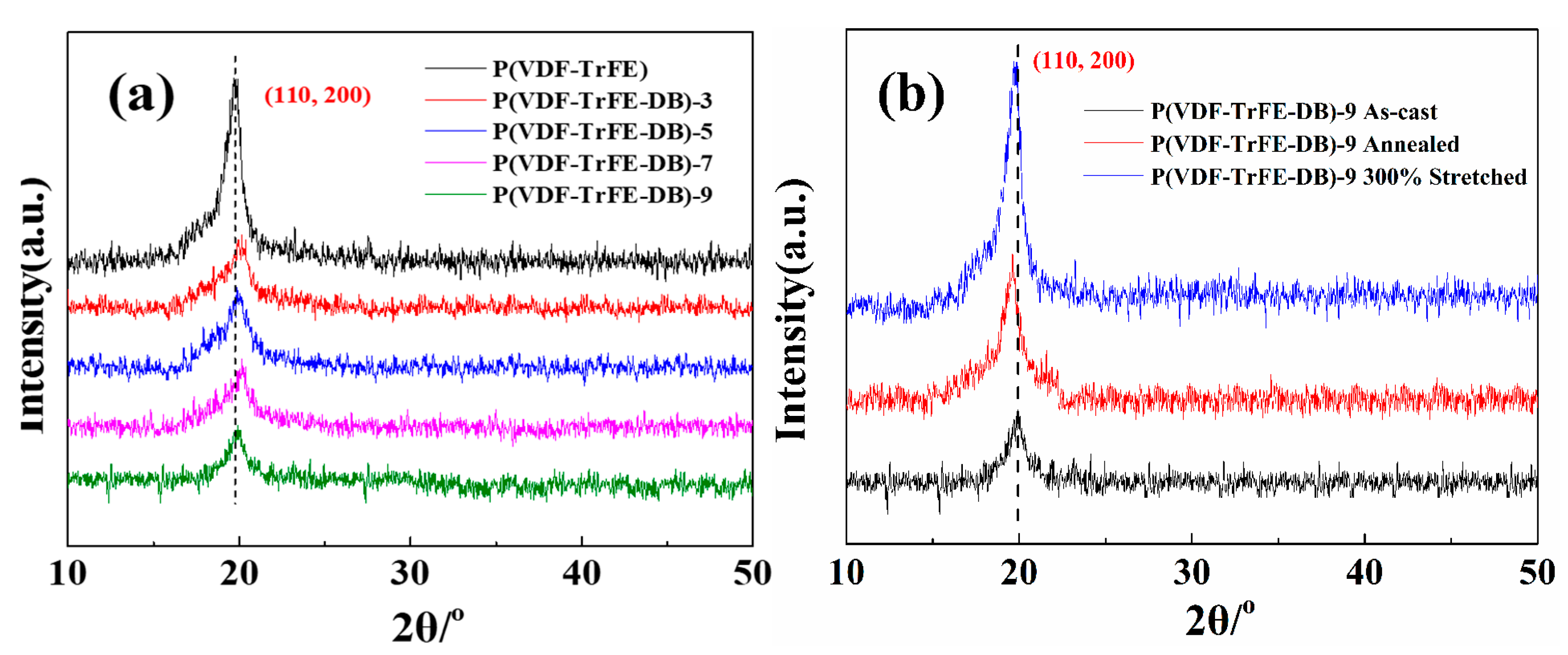
3.2. Crystalline Properties of P(VDF-TrFE-DB) Films Fabricated under Varied Conditions
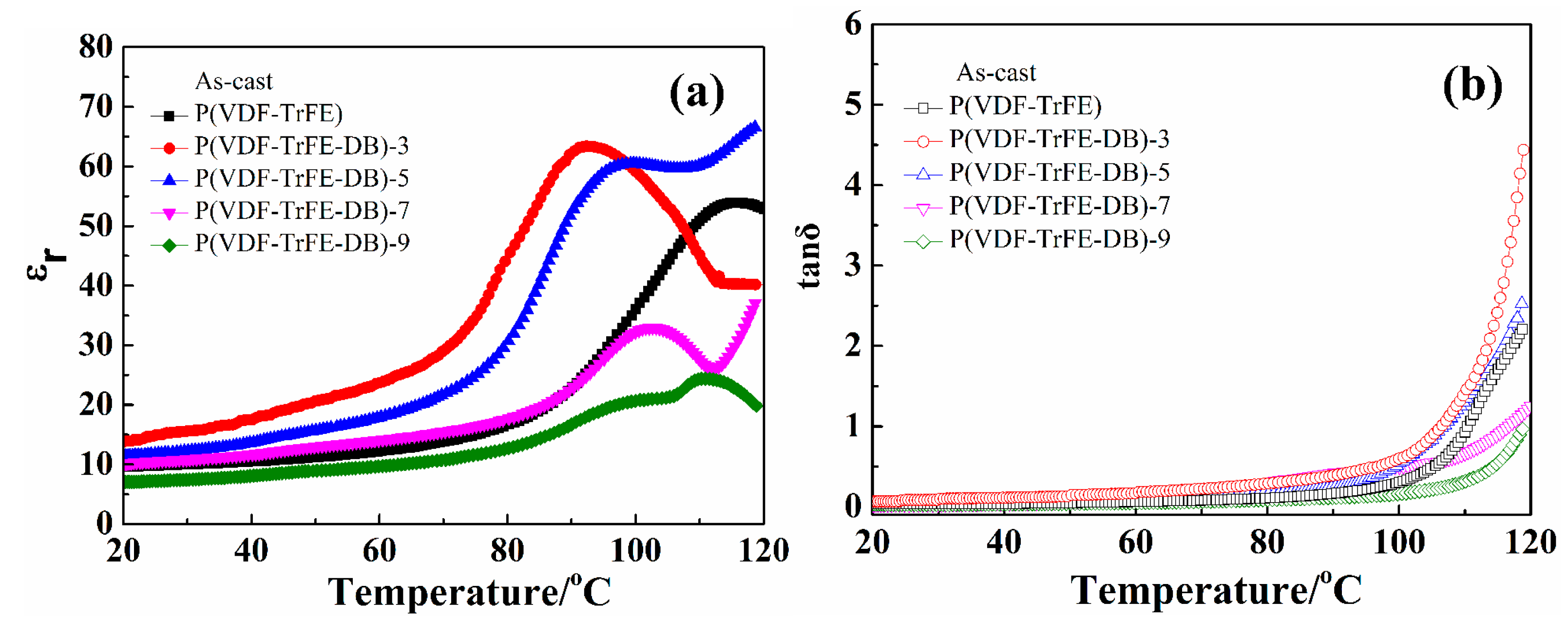
3.3. Dielectric Properties under Low Electric Field
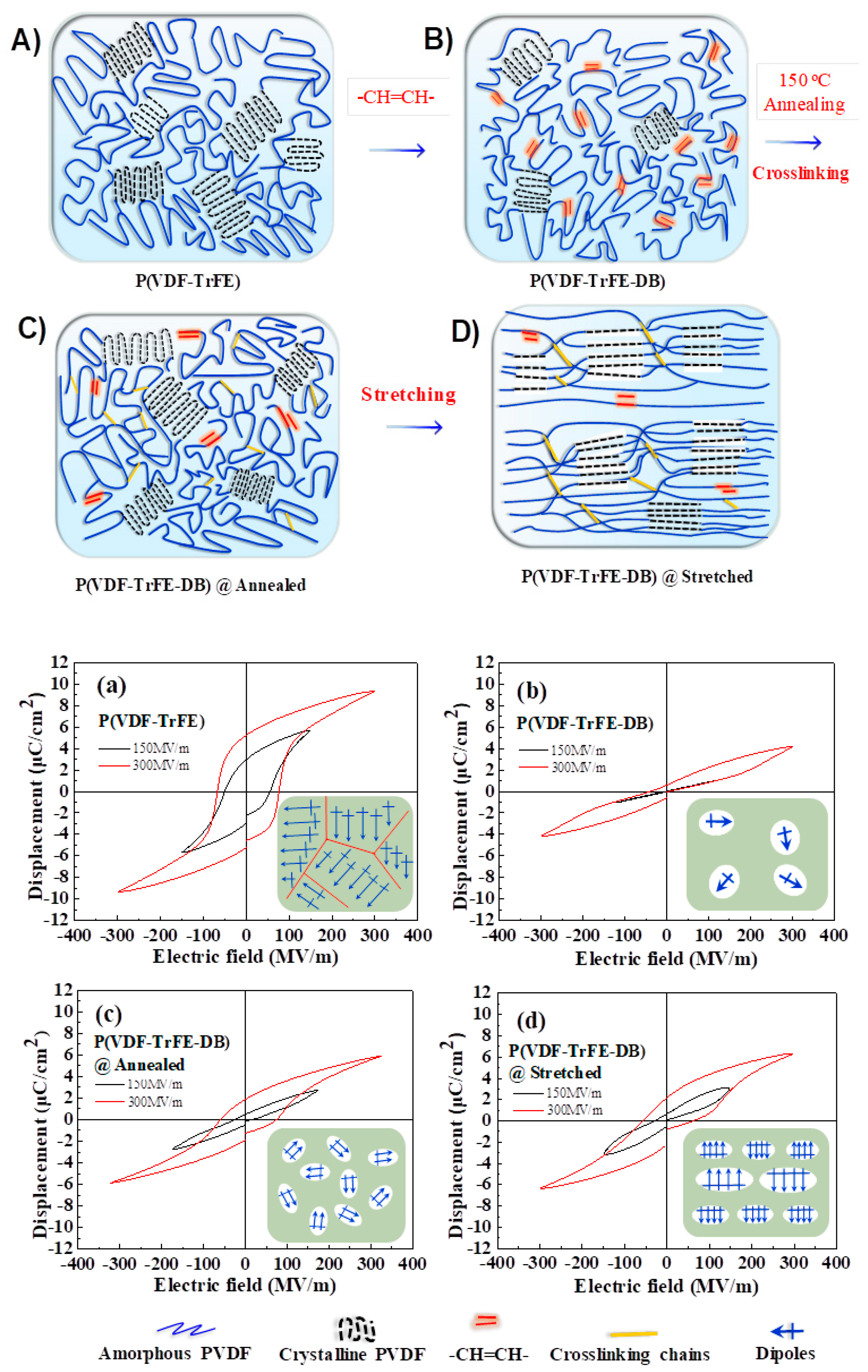
3.4. F-P Transition Behaviors in P(VDF-TrFE-DB)s Copolymers
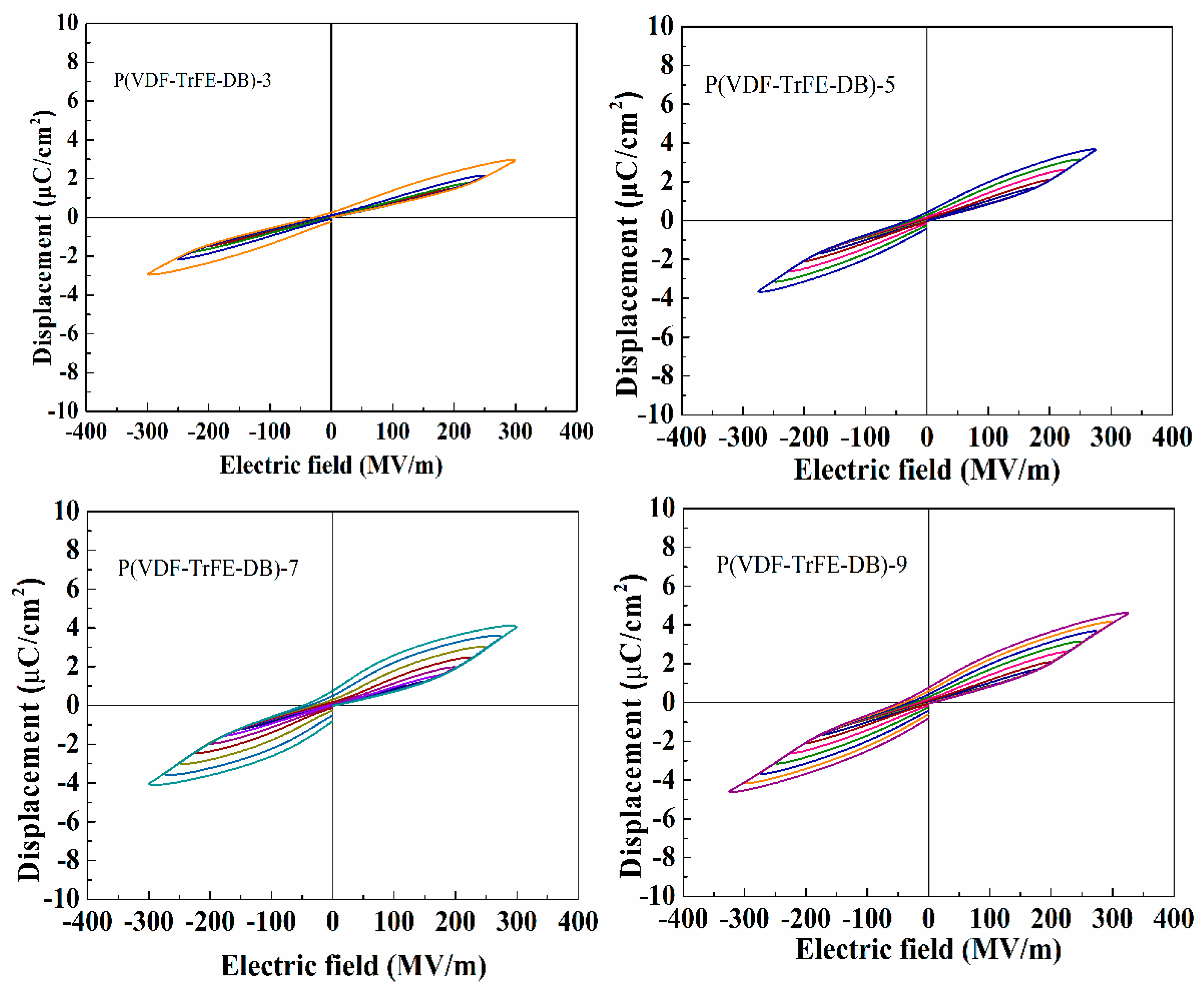
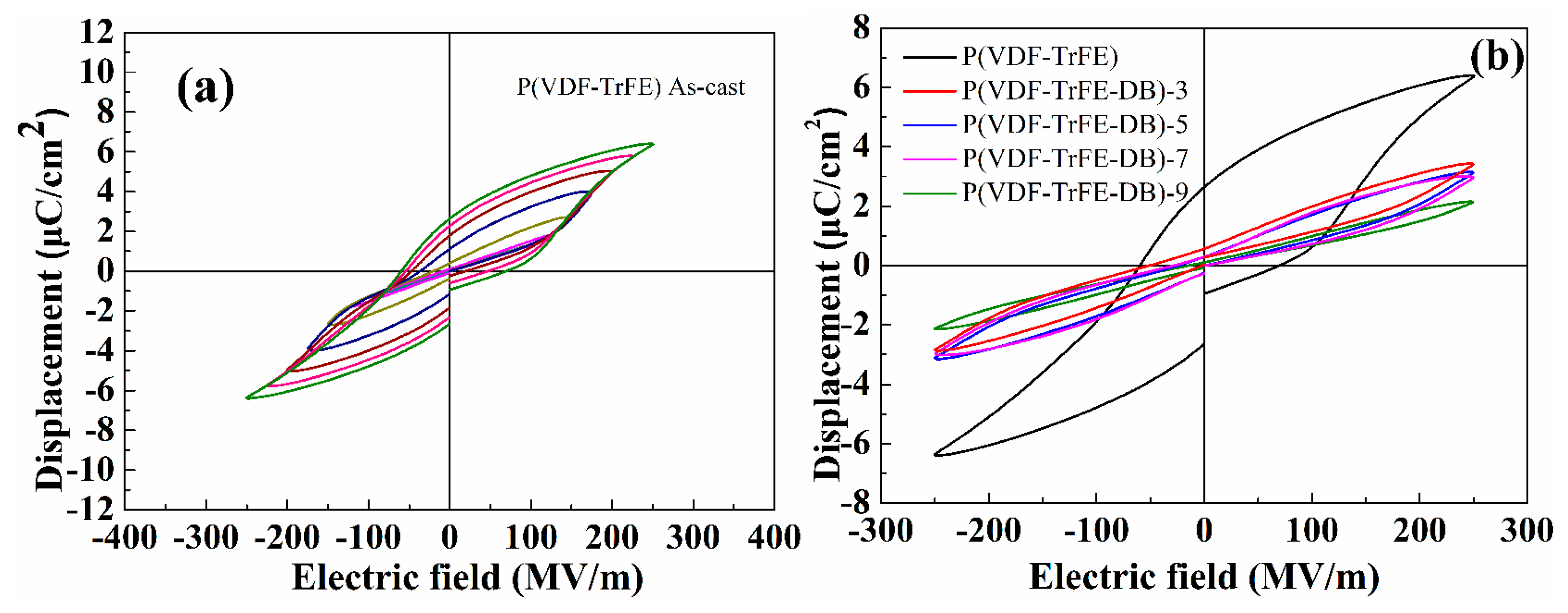
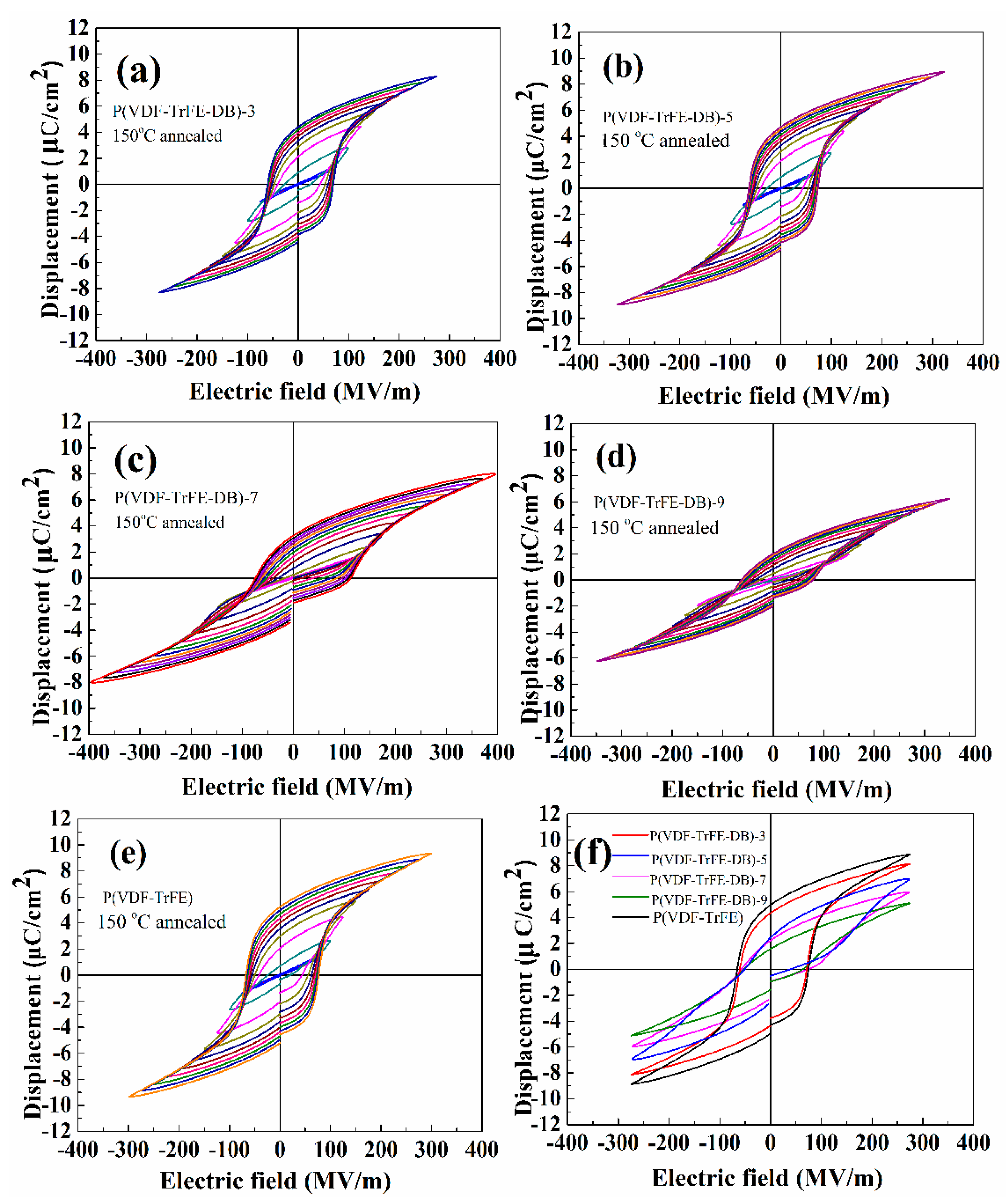
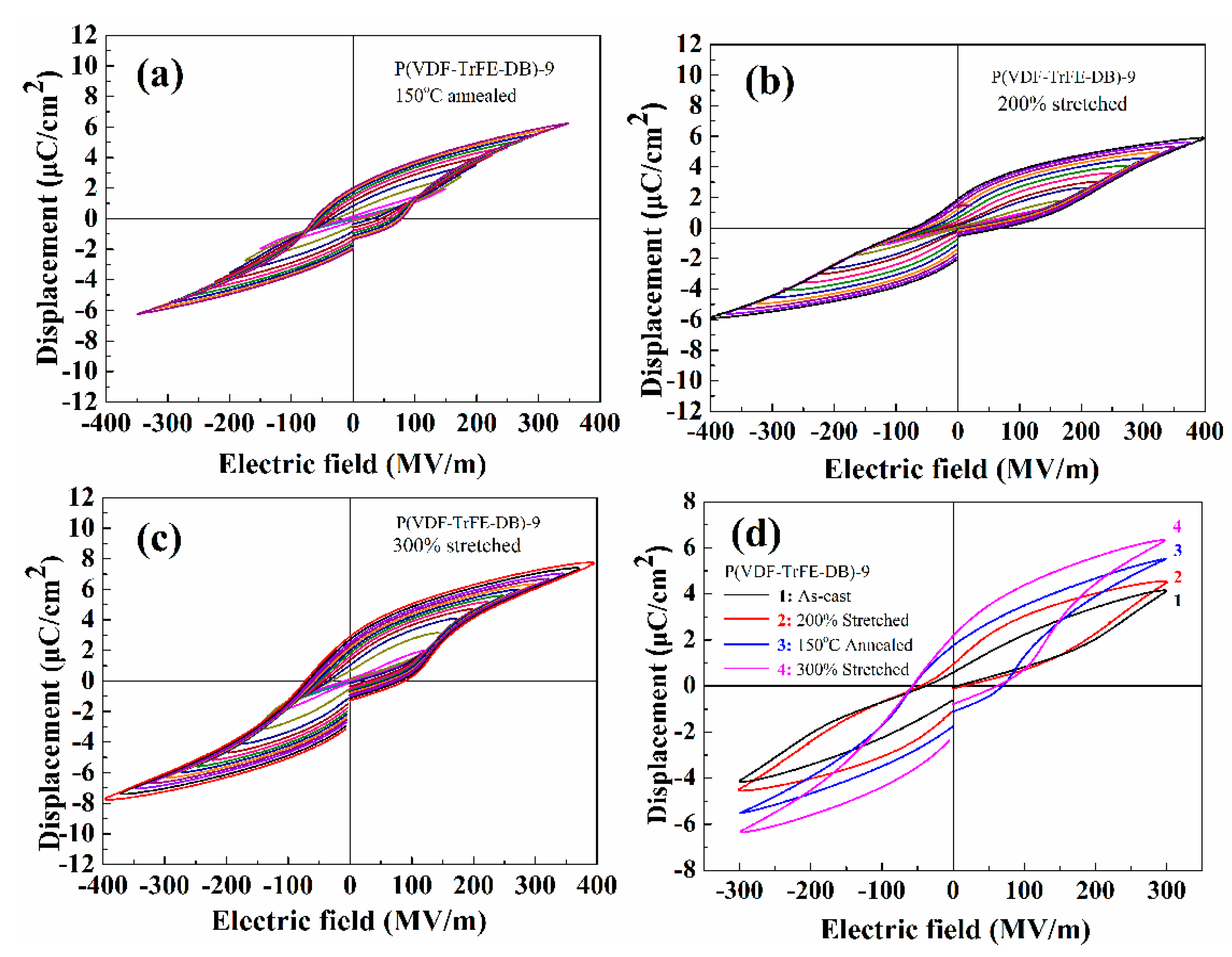
3.5. Ferroelectric Properties under High Electric Fields
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kawai, H. The piezoelectricity of poly (vinylidene fluoride). Jpn. J. Appl. Phys. 1969, 8, 975–976. [Google Scholar] [CrossRef]
- Bergman, J.G.; McFee, J.H.; Crane, G.R. Pyroelectricity and optical second harmonic generation in polyvinylidene fluoride films. Appl. Phys. Lett. 1971, 18, 203–205. [Google Scholar] [CrossRef]
- Wada, Y.; Hayakawa, R. Piezoelectricity and pyroelectricity of polymers. Jpn. J. Appl. Phys. 1976, 15, 2041–2057. [Google Scholar] [CrossRef]
- Lovinger, A.J. Ferroelectric polymers. Science 1983, 220, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.C.; Chung, T.C. Study of VDF/TrFE/CTFE terpolymers for high pulsed capacitor with high energy density and low energy loss. Macromolecules 2007, 40, 783–785. [Google Scholar] [CrossRef]
- Xia, F.F.; Cheng, Z.Y.; Xu, H.S.; Li, H.F.; Zhang, Q.M.; Kavarnos, G.J.; Ting, R.Y.; Abdel-Sadek, G.; Belfield, K.D. High electromechanical responses in a poly(vinylidene fluoride-trifluoroethylene-chlorofluoroethylene) terpolymer. Adv. Mater. 2002, 14, 1574–1577. [Google Scholar] [CrossRef]
- Li, J.; Sun, Z.; Fan, Y. Solution processable low-voltage organic thin film transistors with high-k relaxor ferroelectric polymer as gate insulator. Adv. Mater. 2012, 24, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Luiz, O.F.; Cezar, W.; Roberto, L.M. Co-crystallization in ternary blends of ferroelectric copolymers. J. Polym. Sci. Polym. Phys. 2010, 48, 621–626. [Google Scholar]
- Xu, T.B.; Cheng, Z.Y.; Zhang, Q.M. High-performance micromachined unimorph actuators based on electrostrictive P(vinylidene fluoride-trifluoroethylene) copolymer. Appl. Phys. Lett. 2002, 80, 1082–1084. [Google Scholar] [CrossRef]
- Ameduri, B. From vinylidene fluoride (VDF) to the applications of VDFcontaining polymers and copolymers: Recent developments and future trends. Chem. Rev. 2009, 109, 6632–6685. [Google Scholar] [CrossRef] [PubMed]
- Matko, V.; Milanović, M. Temperature-capacitance-frequency converter with high resolution. Sens. Actuators A 2014, 220, 262–269. [Google Scholar] [CrossRef]
- Matko, V. Next generation AT-cut quartz crystal sensing devices. Sensors 2011, 5, 4474–4482. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.C.; Thomas, E.L. On the α-β transition by deformation of highly oriented poly(vinylidene fluoride). J. Mater. Sci. Lett. 1984, 3, 929–936. [Google Scholar] [CrossRef]
- Yang, D.C.; Chen, Y. β-phase formation of poly(vinylidene fluoride) from the melt induced by quenching. J. Mater. Sci. Lett. 1987, 6, 599–603. [Google Scholar] [CrossRef]
- Vijayakumar, R.P.; Devang, V.K.; Misra, A. Studies on α to β Phase transformations in mechanically deformed PVDF Films. J. Appl. Polym. Sci. 2010, 117, 3491–3497. [Google Scholar]
- Gregorio, R.; Capitao, R.C. Morphology and phase transition of high melt temperature crystallized poly(vinylidene fluoride). J. Mater. Sci. 2000, 35, 299–306. [Google Scholar] [CrossRef]
- Tashiro, K.; Tanaka, R. Structural correlation between crystal lattice and lamellar morphology in the ferroelectric phase transition of vinylidene fluoridetrifluoroethylene copolymers as revealed by the simultaneous measurements of wide-angle and small-angle X-ray scatterings. Polymer 2006, 47, 5433–5444. [Google Scholar] [CrossRef]
- Zhang, Q.M.; Bharti, V.; Zhao, X. Giant electrostriction and relaxor ferroelectric behavior in electron-irradiated poly(vinylidene fluoride-trifluoroethylene) copolymer. Science 1998, 280, 2101–2104. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.C.; Petchsuk, A. Synthesis and properties of ferroelectric fluoroterpolymers with curie transition at ambient temperature. Macromolecules 2002, 35, 7678–7894. [Google Scholar] [CrossRef]
- Chu, B.J.; Zhou, X.; Neese, B.; Zhang, Q.M.; Bauer, F. Relaxor ferroelectric poly(vinylidene fluoride-trifluoroethylene-chlorofluoroethylene) terpolymer for high energy density storage capacitors. IEEE Trans. Dielectr. Electr. Insul. 2006, 13, 1162–1169. [Google Scholar]
- Zhang, Z.C.; Meng, Q.J.; Chung, T.C. Energy storage study of ferroelectric poly(vinylidene fluoride-trifluoroethylene-chlorotrifluoroethylene) terpolymers. Polymer 2009, 50, 707–715. [Google Scholar] [CrossRef]
- Guan, F.X.; Wang, J.; Yang, L.Y.; Tseng, J.K.; Han, K.; Wang, Q.; Zhu, L. Confinement-induced high-field antiferroelectric-like behavior in poly(vinylidene fluoride-co-trifluoroethylene-co-chlorotrifluoroethylene)-graft-polystyrene graft copolymer. Macromolecules 2011, 44, 2190–2199. [Google Scholar] [CrossRef]
- Li, J.J.; Hu, X.; Gao, G.X.; Ding, S.J.; Li, H.Y.; Yang, L.J.; Zhang, Z.C. Tuning phase transition and ferroelectric properties of poly(vinylidene fluoride-cotrifluoroethylene) via grafting with desired poly(methacrylic ester)s as side chains. J. Mater. Chem. C 2013, 1, 1111–1121. [Google Scholar] [CrossRef]
- Li, J.J.; Gong, H.H.; Yang, Q.; Xie, Y.C.; Yang, L.J.; Zhang, Z.C. Linear-like dielectric behavior and low energy loss achieved in poly(ethyl methacrylate) modified poly(vinylidene fluoride-co-trifluoroethylene). Appl. Phys. Lett. 2014, 104, 263901. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Zhao, Y.F.; Tan, S.B.; Zhang, Z.C. Inserting –CH=CH– into P(VDF-TrFE) by C–F activation mediated with Cu(0) in a controlled atom transfer radical elimination process. Polym. Chem. 2017, 8, 1840–1849. [Google Scholar] [CrossRef]
- Lu, Y.Y.; Jason, C.; Bret, N.; Zhang, Q.M.; Wang, Q. A modular approach to ferroelectric polymers with chemically tunable curie temperatures and dielectric constants. J. Am. Chem. Soc. 2006, 128, 8120. [Google Scholar] [CrossRef] [PubMed]
- Murasheva, Y.M.; Shashkov, A.S.; Galil-Ogly, F.A. Analysis of 19F NMR spectra of vinylidene fluoridetrifluorochloroethylene copolymers. Polym. Sci. USSR 1979, 21, 968–974. [Google Scholar] [CrossRef]
- Taguet, A.; Sauguet, L.; Ameduri, B.; Boutevin, B. Fluorinated cotelomers based on vinylidene fluoride (VDF) and hexafluoropropene (HFP): Synthesis, dehydrofluorination and grafting by amine containing an aromatic ring. J. Fluor. Chem. 2007, 128, 619–630. [Google Scholar] [CrossRef]
- Bormashenko, Y.; Pogreb, R.; Stanevsky, O.; Bormashenko, E. Self-organization in thin polycarbonate films and its optical and electro-optical applications. J. Mater. Sci. 2004, 39, 6639–6641. [Google Scholar] [CrossRef]
- Ohigashi, H.; Hattori, T. Improvement of piezoelectric properties of poly(vinylidene fluoride) and its copolymers by crystallization under high pressures. Ferroelectrics 1995, 171, 11–32. [Google Scholar] [CrossRef]
- Hikosaka, M.K.; Sakurai, H.; Ohigashi, A.K. Role of transient metastable hexagonal phase in the formation of extended chain single crystals of vinylidene fluoride and trifluorethylene copolymers. Jpn. J. Appl. Phys. 1994, 33, 214–219. [Google Scholar] [CrossRef]
- Gregorio, R. Determination of the α, β, and γ crystalline phases of poly(vinylidene fluoride) films prepared at different conditions. J. Appl. Polym. Sci. 2010, 100, 3272–3279. [Google Scholar] [CrossRef]
- Lovinger, A.J.; Davis, D.D.; CaisR, E.; Kometani, J.M. The role of molecular defects on the structure and phase transitions of poly(vinylidene fluoride). Polymer 1987, 28, 617–625. [Google Scholar] [CrossRef]
- Logothetis, A.L. Chemistry of fluorocarbon elastomers. Prog. Polym. Sci. 1989, 14, 251. [Google Scholar] [CrossRef]
- Ameduri, B.; Boutevin, B.; Kostov, G. Fluoroelastomers: Synthesis, properties and applications. Prog. Polym. Sci. 2001, 26, 105. [Google Scholar] [CrossRef]
- Tamer, S.A.; Joseph, M.D.; George, W.R. Copolymerization of Vinylidene Fluoride with Hexafluoropropylene in Supercritical Carbon Dioxide. Macromolecules 2006, 40, 3086–3097. [Google Scholar]
- Guan, F.X.; Wang, J.; Pan, J.L.; Wang, Q.; Zhu, L. Crystal Orientation Effect on Electric Energy Storage in Poly(vinylidene fluoride-co-hexafluoropropylene) Copolymers. Macromolecules 2016, 43, 384–392. [Google Scholar] [CrossRef]
- Hahn, B.; Wendorff, J.; Yoon, D.Y. Dielectric relaxation of the crystal-amorphous interphase in poly(vinylidene fluoride) and its blends with poly(methyl methacrylate). Macromolecules 1985, 18, 718–721. [Google Scholar] [CrossRef]
- Gong, H.H.; Miao, B.; Zhang, X.; Lu, J.Y.; Zhang, Z.C. High-field antiferroelectric-like behavior in uniaxially stretched poly(vinylidene fluoridetrifluoroethylene- chlorotrifluoroethylene)-grafted-poly(methyl methacrylate) films with high energy density. RSC Adv. 2016, 6, 1589–1599. [Google Scholar] [CrossRef]
- Tan, S.B.; Hu, X.; Ding, S.J.; Zhang, Z.C.; Li, H.Y. Significantly improving dielectric and energy storage properties via uniaxially stretching crosslinked P(VDF-co-TrFE) films. J. Mater. Chem. A 2013, 1, 10353–10361. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Wang, X.; Yang, Q.; Zhang, Z.C. Ferro- and piezo-electric properties of a poly(vinylfluoride) film with high ferro- to para-electric phase transition temperature. RSC Adv. 2015, 5, 80950–805955. [Google Scholar] [CrossRef]
- Furukawa, T.; Takahashi, Y.; Nakajima, T. Recent advances in ferroelectric polymer thin films for memory applications. Curr. Appl. Phys. 2010, 10, 62–75. [Google Scholar] [CrossRef]
- Ohwada, K.; Tomita, Y. Experiment and Theory of Pb(In1/2Nb1/2)O3: Antiferroelectric, Ferroelectric, or Relaxor State Depending on Perovskite B-Site Randomness. J. Phys. Soc. Jpn. 2010, 79, 54–72. [Google Scholar] [CrossRef]
- Furukawa, T.; Date, M.; Fukada, E. Hysteresis phenomena in poly(vinylidene fluoride) under high electric field. J. Appl. Phys. 1980, 51, 1135–1141. [Google Scholar] [CrossRef]
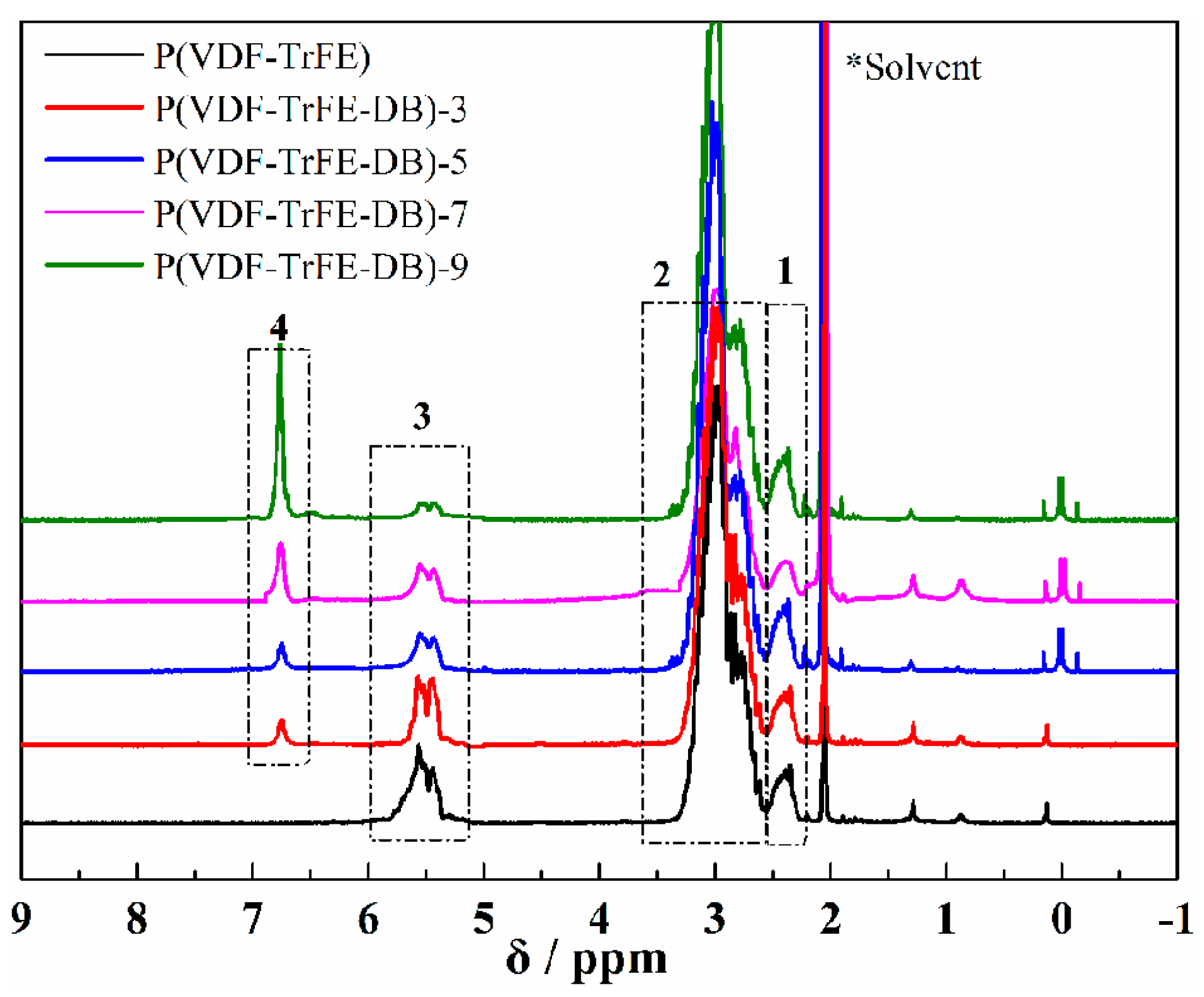



| Abbreviations | Chemical Components (VDF/TrFE/CH=CH in mol %) |
|---|---|
| P(VDF-TrFE-DB)-3 | 77/17/3 |
| P(VDF-TrFE-DB)-5 | 75/15/5 |
| P(VDF-TrFE-DB)-7 | 73/13/7 |
| P(VDF-TrFE-DB)-9 | 71/11/9 |
| Sample | TC (°C) | △Hc (J/g) | Tm (°C) | △Hm (J/g) |
|---|---|---|---|---|
| P(VDF-TrFE-DB)-5 as-cast | 97.5 | 5.82 | 125.7 | 7.31 |
| P(VDF-TrFE-DB)-5 annealed | 100.0 | 5.86 | 136.0 | 7.49 |
| P(VDF-TrFE-DB)-5 stretched | 106.0 | 6.04 | 142.0 | 8.03 |
| P(VDF-TrFE-DB)-9 as-cast | 106.8 | N.A | 106.8 | 4.08 |
| P(VDF-TrFE-DB)-9 annealed | 112.0 | N.A | 112.0 | 7.26 |
| P(VDF-TrFE-DB)-9 stretched | 117.8 | N.A | 117.8 | 10.65 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Tan, S.; Wang, J.; Wang, X.; Zhu, W.; Zhang, Z. Regulating Dielectric and Ferroelectric Properties of Poly(vinylidene fluoride-trifluoroethylene) with Inner CH=CH Bonds. Polymers 2018, 10, 339. https://doi.org/10.3390/polym10030339
Zhang Y, Tan S, Wang J, Wang X, Zhu W, Zhang Z. Regulating Dielectric and Ferroelectric Properties of Poly(vinylidene fluoride-trifluoroethylene) with Inner CH=CH Bonds. Polymers. 2018; 10(3):339. https://doi.org/10.3390/polym10030339
Chicago/Turabian StyleZhang, Yanan, Shaobo Tan, Jian Wang, Xiao Wang, Weiwei Zhu, and Zhicheng Zhang. 2018. "Regulating Dielectric and Ferroelectric Properties of Poly(vinylidene fluoride-trifluoroethylene) with Inner CH=CH Bonds" Polymers 10, no. 3: 339. https://doi.org/10.3390/polym10030339