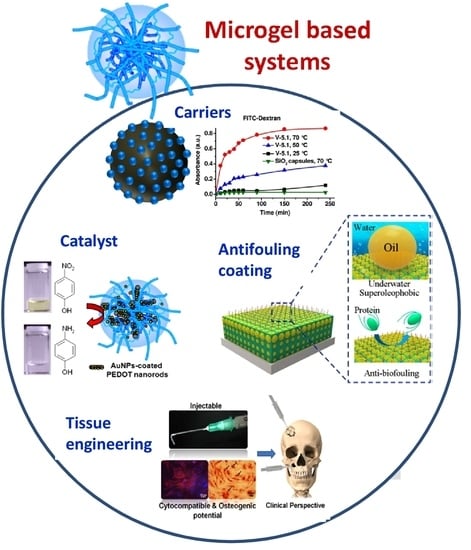
Stimuli-Responsive Microgels and Microgel-Based Systems: Advances in the Exploitation of Microgel Colloidal Properties and Their Interfacial Activity
Abstract
1. Introduction
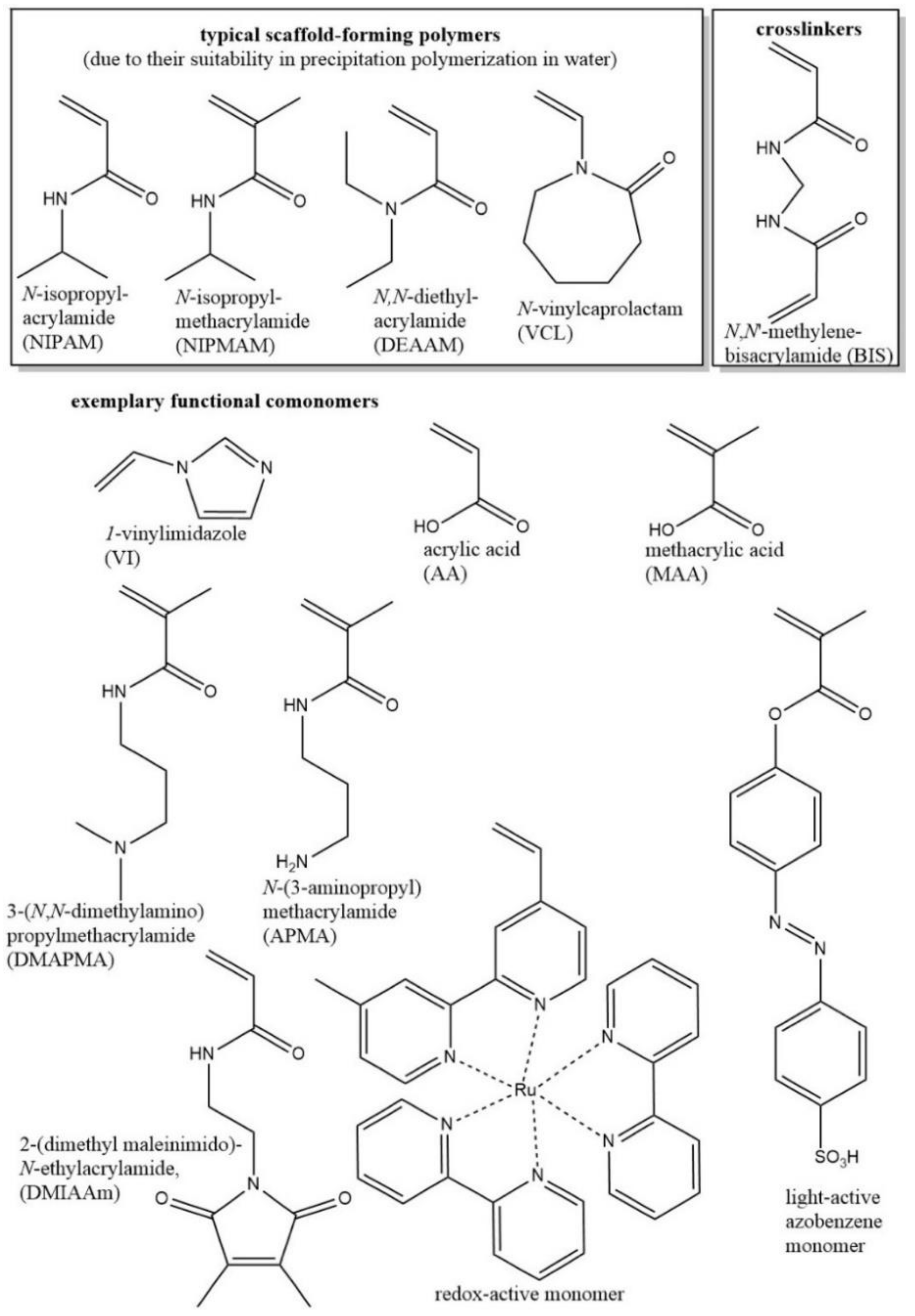
2. Chemical Design of Functional Microgels
3. Deformation of Microgels at Interfaces
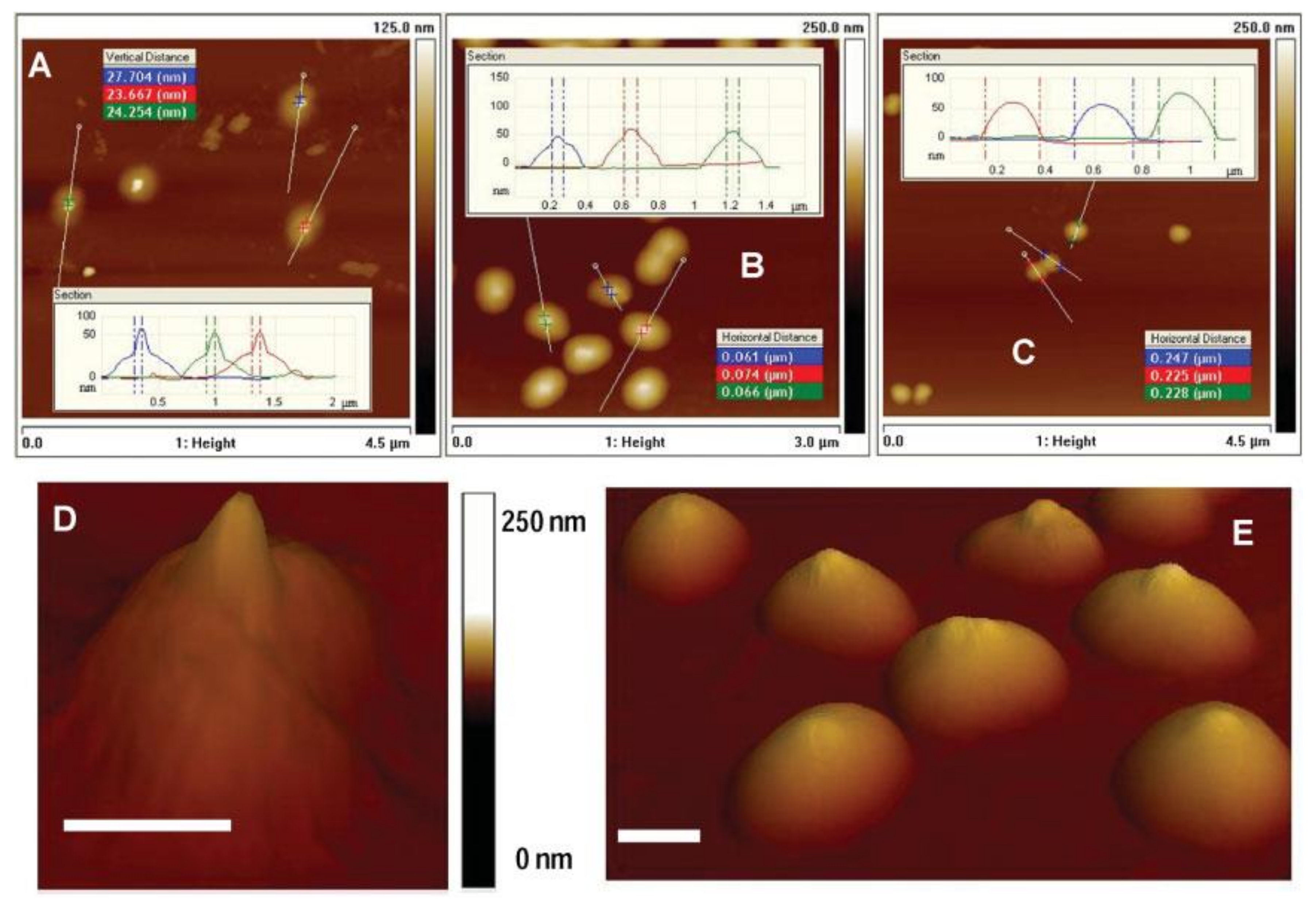
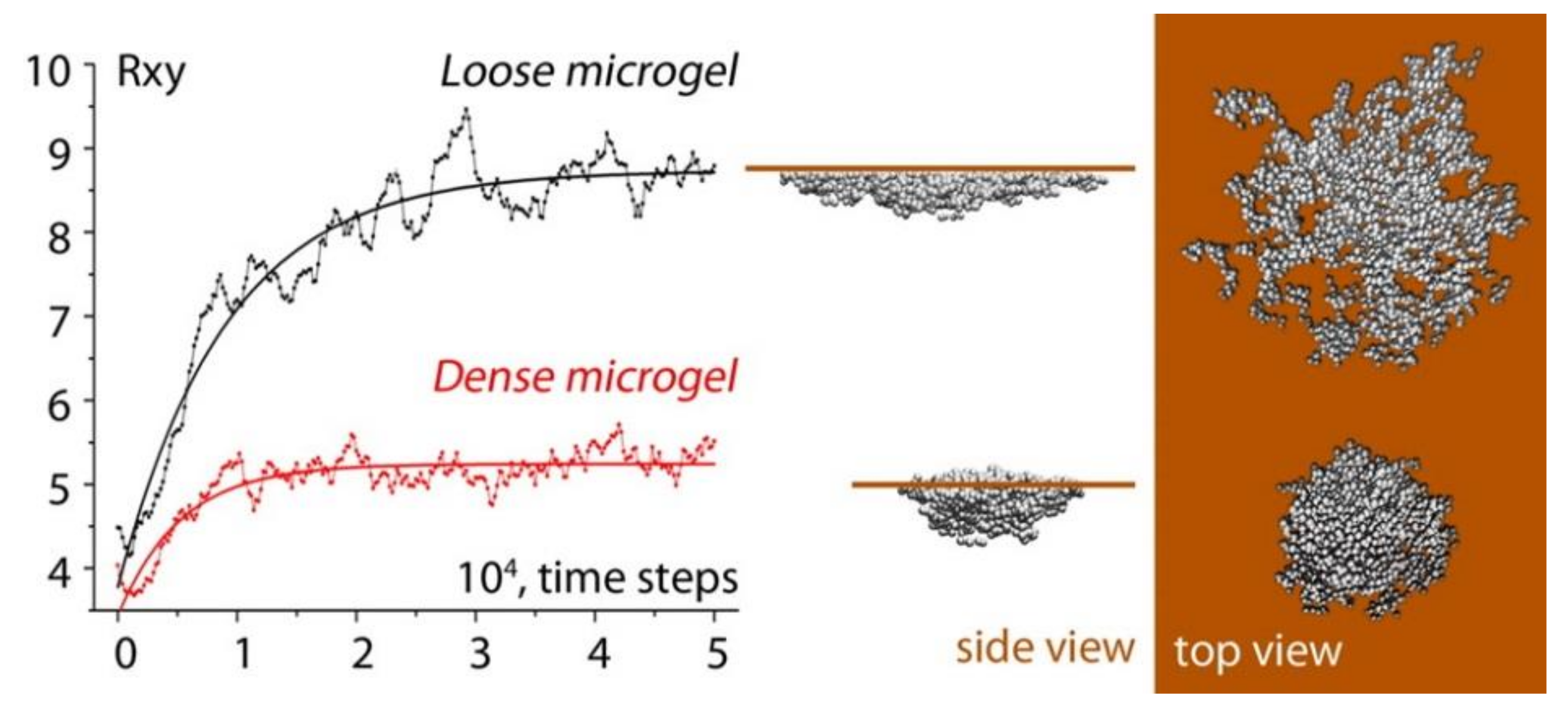
3.1. Microgels at Air-Solid Interfaces
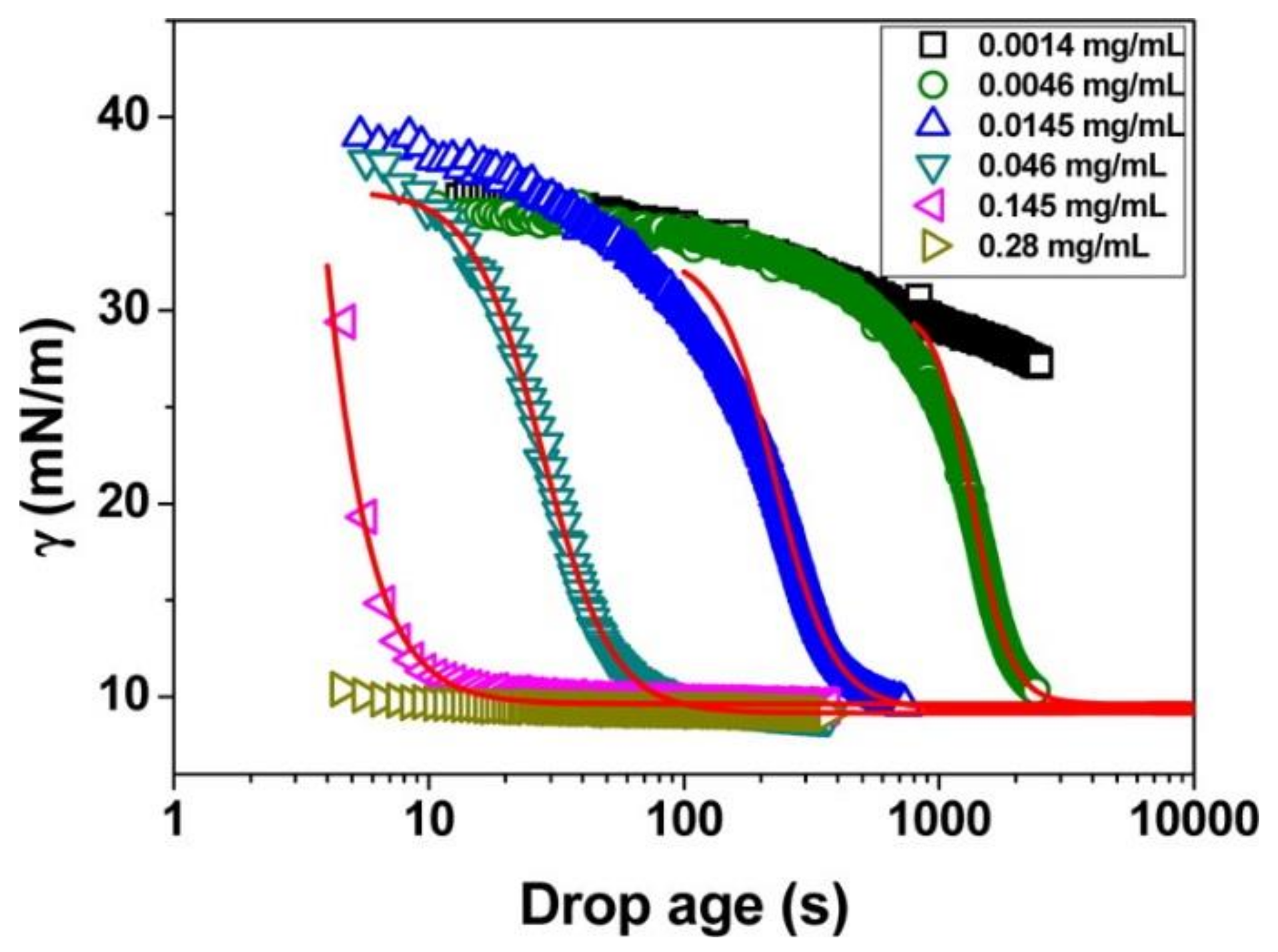
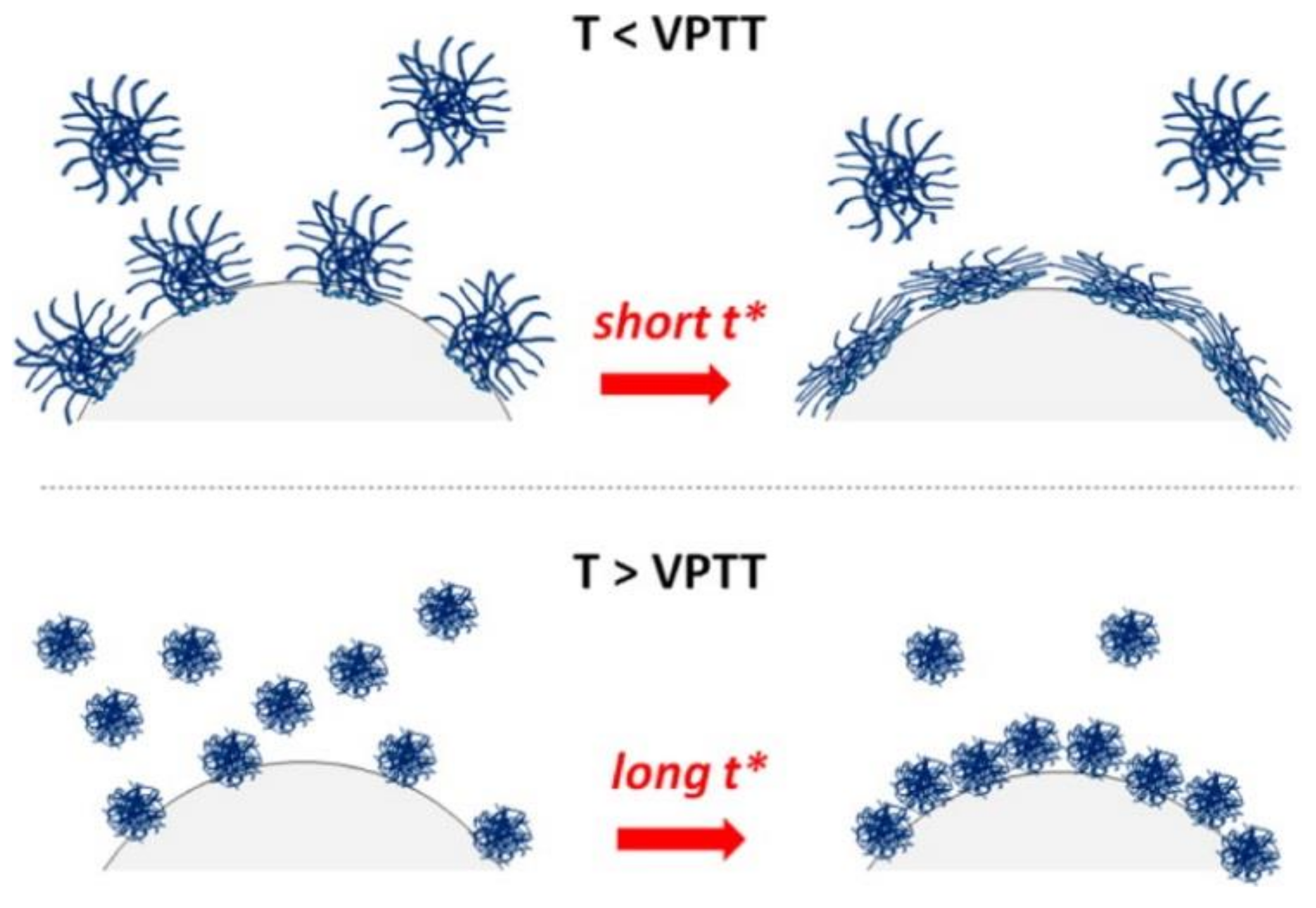
3.2. Microgels at Air-Liquid Interfaces
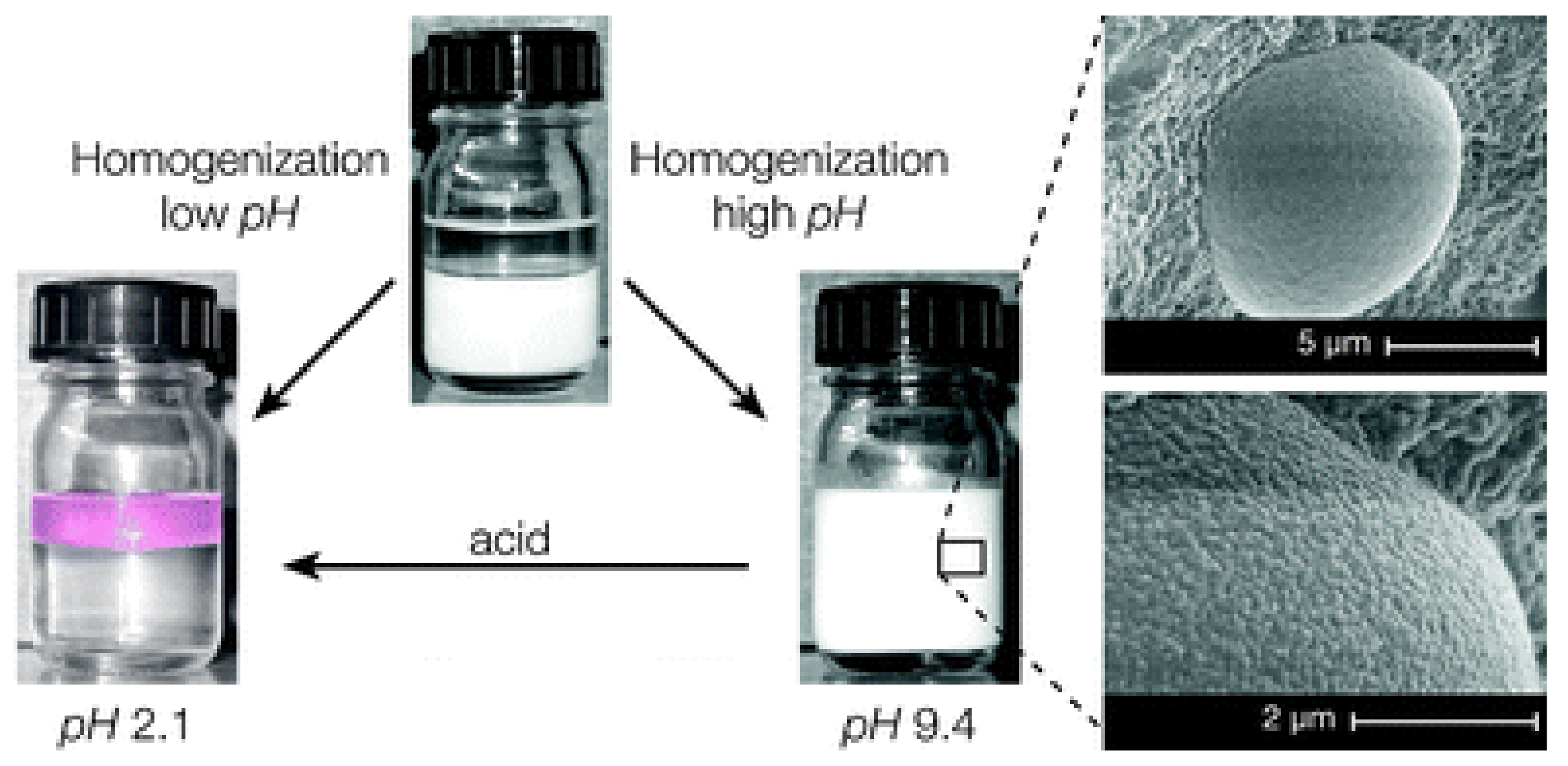
3.3. Microgels at Liquid-Liquid Interfaces
4. Smart Systems Based on Microgel-Stabilized Emulsions
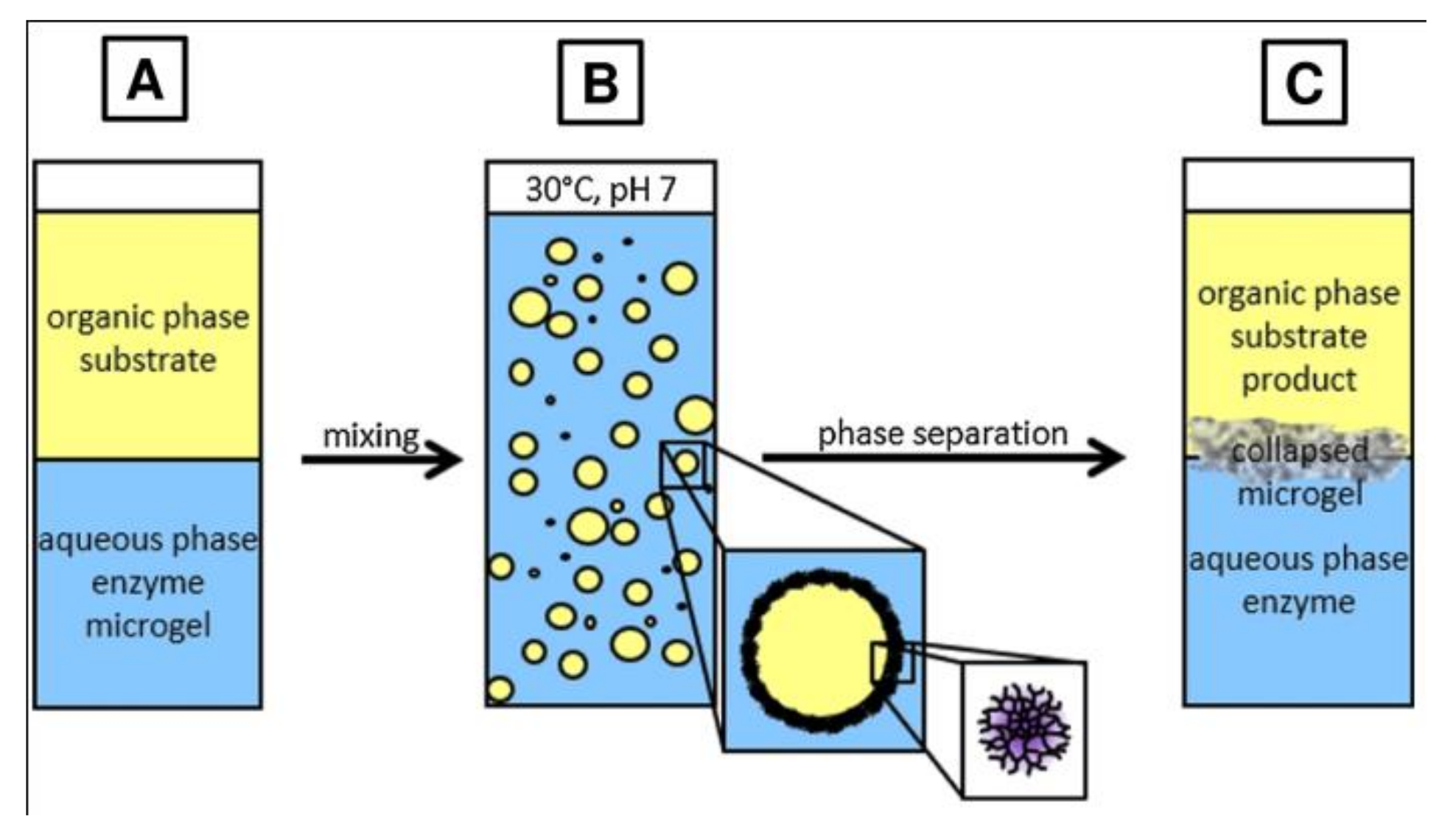
4.1. Microgel-Stabilized Emulsions as Switchable Catalytic Systems
4.2. Microgel-Stabilized Emulsions for Designing Adaptive Capsules
5. Functional Microgel-Based Antifouling Coatings
6. Assembly of Cell-Laden Microgels for Tissue Engineering Applications
7. Conclusions and Future Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Agrawal, G.; Schürings, M.; Zhu, X.; Pich, A. Microgel/SiO2 hybrid colloids prepared using a water soluble silica precursor. Polymer 2012, 53, 1189–1197. [Google Scholar] [CrossRef]
- Pelton, R. Temperature-sensitive aqueous microgels. Adv. Colloid Interface Sci. 2000, 85, 1–33. [Google Scholar] [CrossRef]
- Karg, M.; Hellweg, T. New “smart” poly(NIPAM) microgels and nanoparticle microgel hybrids: Properties and advances in characterisation. Curr. Opin. Colloid Interface Sci. 2009, 14, 438–450. [Google Scholar] [CrossRef]
- Motornov, M.; Roiter, Y.; Tokarev, I.; Minko, S. Stimuli-responsive nanoparticles, nanogels and capsules for integrated multifunctional intelligent systems. Prog. Polym. Sci. 2010, 35, 174–211. [Google Scholar] [CrossRef]
- Wiemer, K.; Dörmbach, K.; Slabu, I.; Agrawal, G.; Schrader, F.; Caumanns, T.; Bourone, S.; Mayer, J.; Steitz, J.; Simon, U. Hydrophobic superparamagnetic FePt nanoparticles in hydrophilic poly(N-vinylcaprolactam) microgels: A new multifunctional hybrid system. J. Mater. Chem. B 2017, 5, 1284–1292. [Google Scholar] [CrossRef]
- Karg, M. Multifunctional inorganic/organic hybrid microgels. Colloid Polym. Sci. 2012, 290, 673–688. [Google Scholar] [CrossRef]
- Nayak, S.; Lyon, L.A. Photoinduced phase transitions in poly(N-isopropylacrylamide) microgels. Chem. Mater. 2004, 16, 2623–2627. [Google Scholar] [CrossRef]
- Jones, C.D.; Lyon, L.A. Synthesis and characterization of multiresponsive core–shell microgels. Macromolecules 2000, 33, 8301–8306. [Google Scholar] [CrossRef]
- Dupin, D.; Fujii, S.; Armes, S.P.; Reeve, P.; Baxter, S.M. Efficient synthesis of sterically stabilized pH-responsive microgels of controllable particle diameter by emulsion polymerization. Langmuir 2006, 22, 3381–3387. [Google Scholar] [CrossRef]
- Klinger, D.; Landfester, K. Stimuli-responsive microgels for the loading and release of functional compounds: Fundamental concepts and applications. Polymer 2012, 53, 5209–5231. [Google Scholar] [CrossRef]
- Pester, C.W.; Konradi, A.; Varnholt, B.; van Rijn, P.; Böker, A. Responsive macroscopic materials from self-assembled cross-linked SiO2-PNIPAAM core/shell structures. Adv. Funct. Mater. 2012, 22, 1724–1731. [Google Scholar] [CrossRef]
- Özlem Nazli, K.; Pester, C.W.; Konradi, A.; Böker, A.; van Rijn, P. Cross-linking density and temperature effects on the self-assembly of SiO2–PNIPAAM core–shell particles at interfaces. Chem. A Eur. J. 2013, 19, 5586–5594. [Google Scholar] [CrossRef] [PubMed]
- Hertle, Y.; Hellweg, T. Thermoresponsive copolymer microgels. J. Mater. Chem. B 2013, 1, 5874–5885. [Google Scholar] [CrossRef]
- Wedel, B.; Hertle, Y.; Wrede, O.; Bookhold, J.; Hellweg, T. Smart homopolymer microgels: Influence of the monomer structure on the particle properties. Polymers 2016, 8, 162. [Google Scholar] [CrossRef]
- Wellert, S.; Richter, M.; Hellweg, T.; von Klitzing, R.; Hertle, Y. Responsive microgels at surfaces and interfaces. Zeitschrift für Physikalische Chemie 2015, 229, 1225–1250. [Google Scholar] [CrossRef]
- Richtering, W.; Saunders, B.R. Gel architectures and their complexity. Soft Matter 2014, 10, 3695–3702. [Google Scholar] [CrossRef]
- Richtering, W.; Pich, A. The special behaviours of responsive core–shell nanogels. Soft Matter 2012, 8, 11423–11430. [Google Scholar] [CrossRef]
- Lu, Y.; Ballauff, M. Thermosensitive core–shell microgels: From colloidal model systems to nanoreactors. Prog. Polym. Sci. 2011, 36, 767–792. [Google Scholar] [CrossRef]
- Pich, A.; Richtering, W. Chemical Design of Responsive Microgels; Springer: Berlin/Heidelberg, Germany, 2010; ISBN 978-3-642-16379-1. [Google Scholar]
- Agrawal, G.; Schürings, M.P.; van Rijn, P.; Pich, A. Formation of catalytically active gold–polymer microgel hybrids via a controlled in situ reductive process. J. Mater. Chem. A 2013, 1, 13244–13251. [Google Scholar] [CrossRef]
- Plamper, F.A.; Richtering, W. Functional microgels and microgel systems. Acc. Chem. Res. 2017, 50, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Mourran, A.; Wu, Y.; Gumerov, R.A.; Rudov, A.A.; Potemkin, I.I.; Pich, A.; Möller, M. When colloidal particles become polymer coils. Langmuir 2016, 32, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Wang, M.; He, J. The behavior of hydrophobic-core/hydrophilic-shell structured microgels at an interface: From mickering emulsion to colloidosomes with dual-level controlled permeability. RSC Adv. 2016, 6, 95067–95072. [Google Scholar] [CrossRef][Green Version]
- Oh, J.K.; Drumright, R.; Siegwart, D.J.; Matyjaszewski, K. The development of microgels/nanogels for drug delivery applications. Prog. Polym. Sci. 2008, 33, 448–477. [Google Scholar] [CrossRef]
- Malmsten, M.; Bysell, H.; Hansson, P. Biomacromolecules in microgels—Opportunities and challenges for drug delivery. Curr. Opin. Colloid Interface Sci. 2010, 15, 435–444. [Google Scholar] [CrossRef]
- Norton, L.; Tegnell, E.; Toporek, S.; Reichert, W. In vitro characterization of vascular endothelial growth factor and dexamethasone releasing hydrogels for implantable probe coatings. Biomaterials 2005, 26, 3285–3297. [Google Scholar] [CrossRef] [PubMed]
- Bouillot, P.; Vincent, B. A comparison of the swelling behaviour of copolymer and interpenetrating network microgel particles. Colloid Polym. Sci. 2000, 278, 74–79. [Google Scholar] [CrossRef]
- Virtanen, O.; Brugnoni, M.; Kather, M.; Pich, A.; Richtering, W. The next step in precipitation polymerization of N-isopropylacrylamide: Particle number density control by monochain globule surface charge modulation. Polym. Chem. 2016, 7, 5123–5131. [Google Scholar] [CrossRef]
- Pich, A.; Richtering, W. Microgels by precipitation polymerization: Synthesis, characterization, and functionalization. In Chemical Design of Responsive Microgels; Pich, A., Richtering, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1–37. ISBN 978-3-642-16379-1. [Google Scholar]
- Grobelny, S.; Hofmann, C.H.; Erlkamp, M.; Plamper, F.A.; Richtering, W.; Winter, R. Conformational changes upon high pressure induced hydration of poly(N-isopropylacrylamide) microgels. Soft Matter 2013, 9, 5862–5866. [Google Scholar] [CrossRef]
- Hofmann, C.H.; Plamper, F.A.; Scherzinger, C.; Hietala, S.; Richtering, W. Cononsolvency revisited: Solvent entrapment by N-isopropylacrylamide and N,N-diethylacrylamide microgels in different water/methanol mixtures. Macromolecules 2012, 46, 523–532. [Google Scholar] [CrossRef]
- Sivakumaran, D.; Mueller, E.; Hoare, T. Temperature-induced assembly of monodisperse, covalently cross-linked, and degradable poly(N-isopropylacrylamide) microgels based on oligomeric precursors. Langmuir 2015, 31, 5767–5778. [Google Scholar] [CrossRef] [PubMed]
- Imaz, A.; Forcada, J. Synthesis strategies to incorporate acrylic acid into N-vinylcaprolactam-based microgels. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 3218–3227. [Google Scholar] [CrossRef]
- Ramli, R.A.; Hashim, S.; Laftah, W.A. Synthesis, characterization, and morphology study of poly(acrylamide-co-acrylic acid)-grafted-poly(styrene-co-methyl methacrylate) “raspberry”-shape like structure microgels by pre-emulsified semi-batch emulsion polymerization. J. Colloid Interface Sci. 2013, 391, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Okhapkin, I.M.; Nasimova, I.R.; Makhaeva, E.E.; Khokhlov, A.R. Effect of complexation of monomer units on pH- and temperature-sensitive properties of poly(N-vinylcaprolactam-co-methacrylic acid). Macromolecules 2003, 36, 8130–8138. [Google Scholar] [CrossRef]
- Birkholz, M.-N.; Agrawal, G.; Bergmann, C.; Schröder, R.; Lechner, S.J.; Pich, A.; Fischer, H. Calcium phosphate/microgel composites for 3D powderbed printing of ceramic materials. Biomed. Eng. Biomed. Tech. 2016, 61, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Eckert, F.; Boyko, V.; Pich, A. Temperature, pH, and magnetic field sensitive hybrid microgels. Small 2007, 3, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Pich, A.; Tessier, A.; Boyko, V.; Lu, Y.; Adler, H.-J.P. Synthesis and characterization of poly(vinylcaprolactam)-based microgels exhibiting temperature and pH-sensitive properties. Macromolecules 2006, 39, 7701–7707. [Google Scholar] [CrossRef]
- Pich, A.; Lu, Y.; Boyko, V.; Arndt, K.-F.; Adler, H.-J.P. Thermo-sensitive poly(N-vinylcaprolactam-co-acetoacetoxyethyl methacrylate) microgels: 2. Incorporation of polypyrrole. Polymer 2003, 44, 7651–7659. [Google Scholar] [CrossRef]
- Lu, Y.; Pich, A.; Adler, H.-J.P.; Wang, G.; Rais, D.; Nešpůrek, S. Composite polypyrrole-containing particles and electrical properties of thin films prepared therefrom. Polymer 2008, 49, 5002–5012. [Google Scholar] [CrossRef]
- Johansson, C.; Hansson, P.; Malmsten, M. Mechanism of lysozyme uptake in poly(acrylic acid) microgels. J. Phys. Chem. B 2009, 113, 6183–6193. [Google Scholar] [CrossRef] [PubMed]
- Pastoriza-Santos, I.; Liz-Marzán, L.M. Binary cooperative complementary nanoscale interfacial materials. Reduction of silver nanoparticles in DMF. Formation of monolayers and stable colloids. Pure Appl. Chem. 2000, 72, 83–90. [Google Scholar] [CrossRef]
- Pich, A.; Karak, A.; Lu, Y.; Ghosh, A.K.; Adler, H.J.P. Preparation of hybrid microgels functionalized by silver nanoparticles. Macromol. Rapid Commun. 2006, 27, 344–350. [Google Scholar] [CrossRef]
- Lu, Y.; Mei, Y.; Drechsler, M.; Ballauff, M. Thermosensitive core–shell particles as carriers for Ag nanoparticles: Modulating the catalytic activity by a phase transition in networks. Angew. Chem. Int. Ed. 2006, 45, 813–816. [Google Scholar] [CrossRef] [PubMed]
- Pich, A.; Karak, A.; Lu, Y.; Ghosh, A.K.; Adler, H.-J.P. Tuneable catalytic properties of hybrid microgels containing gold nanoparticles. J. Nanosci. Nanotechnol. 2006, 6, 3763–3769. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Fu, L.; Su, M.; Vijayamohanan, K.; Dravid, V.P. Novel one-step synthesis of amine-stabilized aqueous colloidal gold nanoparticles. J. Mater. Chem. 2004, 14, 1795–1797. [Google Scholar] [CrossRef]
- Mössmer, S.; Spatz, J.P.; Möller, M.; Aberle, T.; Schmidt, J.; Burchard, W. Solution behavior of poly(styrene)-block-poly(2-vinylpyridine) micelles containing gold nanoparticles. Macromolecules 2000, 33, 4791–4798. [Google Scholar] [CrossRef]
- Contreras-Cáceres, R.; Sánchez-Iglesias, A.; Karg, M.; Pastoriza-Santos, I.; Pérez-Juste, J.; Pacifico, J.; Hellweg, T.; Fernández-Barbero, A.; Liz-Marzán, L.M. Encapsulation and growth of gold nanoparticles in thermoresponsive microgels. Adv. Mater. 2008, 20, 1666–1670. [Google Scholar] [CrossRef]
- Aymonier, C.; Bortzmeyer, D.; Thomann, R.; Mülhaupt, R. Poly(methyl methacrylate)/palladium nanocomposites: Synthesis and characterization of the morphological, thermomechanical, and thermal properties. Chem. Mater. 2003, 15, 4874–4878. [Google Scholar] [CrossRef]
- Lu, Y.; Yuan, J.; Polzer, F.; Drechsler, M.; Preussner, J. In situ growth of catalytic active Au–Pt bimetallic nanorods in thermoresponsive core–shell microgels. ACS Nano 2010, 4, 7078–7086. [Google Scholar] [CrossRef] [PubMed]
- Biffis, A.; Minati, L. Efficient aerobic oxidation of alcohols in water catalysed by microgel-stabilised metal nanoclusters. J. Catal. 2005, 236, 405–409. [Google Scholar] [CrossRef]
- Ma, Y.; Qi, L.; Ma, J.; Cheng, H. Facile synthesis of hollow ZnS nanospheres in block copolymer solutions. Langmuir 2003, 19, 4040–4042. [Google Scholar] [CrossRef]
- Rogach, A.L.; Nagesha, D.; Ostrander, J.W.; Giersig, M.; Kotov, N.A. “Raisin bun”-type composite spheres of silica and semiconductor nanocrystals. Chem. Mater. 2000, 12, 2676–2685. [Google Scholar] [CrossRef]
- Rubio-Retama, J.; Zafeiropoulos, N.E.; Serafinelli, C.; Rojas-Reyna, R.; Voit, B.; Lopez Cabarcos, E.; Stamm, M. Synthesis and characterization of thermosensitive PNIPAM microgels covered with superparamagnetic γ-Fe2O3 nanoparticles. Langmuir 2007, 23, 10280–10285. [Google Scholar] [CrossRef]
- Wilke, P.; Coger, V.; Nachev, M.; Schachschal, S.; Million, N.; Barcikowski, S.; Sures, B.; Reimers, K.; Vogt, P.M.; Pich, A. Biocompatible microgel-modified electrospun fibers for zinc ion release. Polymer 2015, 61, 163–173. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, J.; Paquet, C.; Lin, Y.; Kumacheva, E. From hybrid microgels to photonic crystals. Adv. Funct. Mater. 2003, 13, 468–472. [Google Scholar] [CrossRef]
- Yang, J.; Fang, Y.; Bai, C.; Hu, D.; Zhang, Y. CuS-poly(N-isopropylacrylamide-co-acrylic acid) composite microspheres with patterned surface structures: Preparation and characterization. Chin. Sci. Bull. 2004, 49, 2026–2032. [Google Scholar] [CrossRef]
- Pich, A.; Hain, J.; Lu, Y.; Boyko, V.; Prots, Y.; Adler, H.-J. Hybrid microgels with ZnS inclusions. Macromolecules 2005, 38, 6610–6619. [Google Scholar] [CrossRef]
- Agrawal, M.; Rubio-Retama, J.; Zafeiropoulos, N.; Gaponik, N.; Gupta, S.; Cimrova, V.; Lesnyak, V.; López-Cabarcos, E.; Tzavalas, S.; Rojas-Reyna, R. Switchable photoluminescence of CdTe nanocrystals by temperature-responsive microgels. Langmuir 2008, 24, 9820–9824. [Google Scholar] [CrossRef]
- Wehnert, F.; Pich, A. Fabrication of thermo-responsive hybrid hydrogels by in-situ mineralization and self-assembly of microgel particles. Macromol. Rapid Commun. 2006, 27, 1865–1872. [Google Scholar] [CrossRef]
- Landfester, K.; Willert, M.; Antonietti, M. Preparation of polymer particles in nonaqueous direct and inverse miniemulsions. Macromolecules 2000, 33, 2370–2376. [Google Scholar] [CrossRef]
- Landfester, K. Miniemulsion polymerization and the structure of polymer and hybrid nanoparticles. Angew. Chem. Int. Ed. 2009, 48, 4488–4507. [Google Scholar] [CrossRef] [PubMed]
- Ethirajan, A.; Schoeller, K.; Musyanovych, A.; Ziener, U.; Landfester, K. Synthesis and optimization of gelatin nanoparticles using the miniemulsion process. Biomacromolecules 2008, 9, 2383–2389. [Google Scholar] [CrossRef]
- Wong, J.E.; Gaharwar, A.K.; Müller-Schulte, D.; Bahadur, D.; Richtering, W. Dual-stimuli responsive PNIPAM microgel achieved via layer-by-layer assembly: Magnetic and thermoresponsive. J. Colloid Interface Sci. 2008, 324, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Karg, M.; Pastoriza-Santos, I.; Pérez-Juste, J.; Hellweg, T.; Liz-Marzán, L.M. Nanorod-coated PNIPAM microgels: Thermoresponsive optical properties. Small 2007, 3, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Pinaud, F.; Millereux, R.; Vialar-Trarieux, P.; Catargi, B.; Pinet, S.; Gosse, I.; Sojic, N.; Ravaine, V.R. Differential photoluminescent and electrochemiluminescent behavior for resonance energy transfer processes in thermoresponsive microgels. J. Phys. Chem. B 2015, 119, 12954–12961. [Google Scholar] [CrossRef]
- Suzuki, D.; Yoshida, R. Temporal control of self-oscillation for microgels by cross-linking network structure. Macromolecules 2008, 41, 5830–5838. [Google Scholar] [CrossRef]
- Phua, D.I.; Herman, K.; Balaceanu, A.; Zakrevski, J.; Pich, A. Reversible size modulation of aqueous microgels via orthogonal or combined application of thermo-and phototriggers. Langmuir 2016, 32, 3867–3879. [Google Scholar] [CrossRef] [PubMed]
- Vo, C.D.; Kuckling, D.; Adler, H.-J.; Schönhoff, M. Preparation of thermosensitive nanogels by photo-cross-linking. Colloid Polym. Sci. 2002, 280, 400–409. [Google Scholar] [CrossRef]
- Li, G.; Zhang, G.; Wang, L.; Ge, J. Cationic microgel emulsion with a high solid content by a multistep addition method in inverse microemulsion polymerization. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Hajighasem, A.; Kabiri, K. Novel crosslinking method for preparation of acrylic thickener microgels through inverse emulsion polymerization. Iran. Polym. J. 2015, 24, 1049–1056. [Google Scholar] [CrossRef]
- Tuncer, C.; Samav, Y.; Ülker, D.; Baker, S.B.; Bütün, V. Multi-responsive microgel of a water-soluble monomer via emulsion polymerization. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Landfester, K.; Musyanovych, A. Hydrogels in miniemulsions. In Chemical Design of Responsive Microgels; Pich, A., Richtering, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 39–63. ISBN 978-3-642-16379-1. [Google Scholar]
- Atta, A.M.; Dyab, A.K.; Allohedan, H.A. A novel route to prepare highly surface active nanogel particles based on nonaqueous emulsion polymerization. Polym. Adv. Technol. 2013, 24, 986–996. [Google Scholar] [CrossRef]
- Platen, M.; Mathieu, E.; Lück, S.; Schubel, R.; Jordan, R.; Pautot, S. Poly(2-oxazoline)-based microgel particles for neuronal cell culture. Biomacromolecules 2015, 16, 1516–1524. [Google Scholar] [CrossRef] [PubMed]
- Cortez-Lemus, N.A.; Licea-Claverie, A. Poly(N-vinylcaprolactam), a comprehensive review on a thermoresponsive polymer becoming popular. Prog. Polym. Sci. 2016, 53, 1–51. [Google Scholar] [CrossRef]
- Utech, S.; Prodanovic, R.; Mao, A.S.; Ostafe, R.; Mooney, D.J.; Weitz, D.A. Microfluidic generation of monodisperse, structurally homogeneous alginate microgels for cell encapsulation and 3D cell culture. Adv. Healthc. Mater. 2015, 4, 1628–1633. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Weitz, D.A. Nonspherical colloidosomes with multiple compartments from double emulsions. Small 2009, 5, 1932–1935. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Wang, W.; Ju, X.-J.; Xie, R.; Liu, Z.; Yu, H.-R.; Zhang, C.; Chu, L.-Y. Ultrasensitive microchip based on smart microgel for real-time online detection of trace threat analytes. Proc. Natl. Acad. Sci. USA 2016, 113, 2023–2028. [Google Scholar] [CrossRef] [PubMed]
- Gokmen, M.T.; De Geest, B.G.; Hennink, W.E.; Du Prez, F.E. “Giant” hollow multilayer capsules by microfluidic templating. ACS Appl. Mater. Interfaces 2009, 1, 1196–1202. [Google Scholar] [CrossRef]
- Jeong, W.J.; Kim, J.Y.; Choo, J.; Lee, E.K.; Han, C.S.; Beebe, D.J.; Seong, G.H.; Lee, S.H. Continuous fabrication of biocatalyst immobilized microparticles using photopolymerization and immiscible liquids in microfluidic systems. Langmuir 2005, 21, 3738–3741. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Q.; Wang, J.; Zhu, J.; Wang, H.; Yang, Y. Shape controllable microgel particles prepared by microfluidic combining external ionic crosslinking. Biomicrofluidics 2012, 6, 026502. [Google Scholar] [CrossRef] [PubMed]
- Headen, D.M.; Aubry, G.; Lu, H.; García, A.J. Microfluidic-based generation of size-controlled, biofunctionalized synthetic polymer microgels for cell encapsulation. Adv. Mater. 2014, 26, 3003–3008. [Google Scholar] [CrossRef]
- Seiffert, S.; Weitz, D.A. Microfluidic fabrication of smart microgels from macromolecular precursors. Polymer 2010, 51, 5883–5889. [Google Scholar] [CrossRef][Green Version]
- Chen, C.-H.; Shah, R.K.; Abate, A.R.; Weitz, D.A. Janus particles templated from double emulsion droplets generated using microfluidics. Langmuir 2009, 25, 4320–4323. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.; Li, W.; Seo, M.; Xu, S.; Kumacheva, E. Janus and ternary particles generated by microfluidic synthesis: Design, synthesis, and self-assembly. J. Am. Chem. Soc. 2006, 128, 9408–9412. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Stöver, H.D. Hydrogel microspheres formed by complex coacervation of partially mpeg-grafted poly(styrene-alt-maleic anhydride) with PDADMAC and cross-linking with polyamines. Macromolecules 2003, 36, 8773–8779. [Google Scholar] [CrossRef]
- Maeda, T.; Akasaki, Y.; Yamamoto, K.; Aoyagi, T. Stimuli-responsive coacervate induced in binary functionalized poly(N-isopropylacrylamide) aqueous system and novel method for preparing semi-IPN microgel using the coacervate. Langmuir 2009, 25, 9510–9517. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.-C.; McClements, D.J. Microgels formed by electrostatic complexation of gelatin and OSA starch: Potential fat or starch mimetics. Food Hydrocoll. 2015, 47, 87–93. [Google Scholar] [CrossRef]
- Fettis, M.M.; Wei, Y.; Restuccia, A.; Kurian, J.J.; Wallet, S.M.; Hudalla, G.A. Microgels with tunable affinity-controlled protein release via desolvation of self-assembled peptide nanofibers. J. Mater. Chem. B 2016, 4, 3054–3064. [Google Scholar] [CrossRef]
- Rolland, J.P.; Maynor, B.W.; Euliss, L.E.; Exner, A.E.; Denison, G.M.; DeSimone, J.M. Direct fabrication and harvesting of monodisperse, shape-specific nanobiomaterials. J. Am. Chem. Soc. 2005, 127, 10096–10100. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Byrne, J.D.; Napier, M.E.; DeSimone, J.M. Engineering nanomedicines using stimuli-responsive biomaterials. Adv. Drug Deliv. Rev. 2012, 64, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Reuter, K.G.; Perry, J.L.; Kim, D.; Luft, J.C.; Liu, R.; DeSimone, J.M. Targeted print hydrogels: The role of nanoparticle size and ligand density on cell association, biodistribution, and tumor accumulation. Nano Lett. 2015, 15, 6371–6378. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wong, D.H.; Byrne, J.D.; Chen, K.; Bowerman, C.; DeSimone, J.M. Future of the particle replication in nonwetting templates (PRINT) technology. Angew. Chem. Int. Ed. 2013, 52, 6580–6589. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, G.R.; Lyon, L.A. Microgel translocation through pores under confinement. Angew. Chem. Int. Ed. 2010, 49, 2193–2197. [Google Scholar] [CrossRef] [PubMed]
- Scotti, A.; Gasser, U.; Herman, E.S.; Pelaez-Fernandez, M.; Han, J.; Menzel, A.; Lyon, L.A.; Fernández-Nieves, A. The role of ions in the self-healing behavior of soft particle suspensions. Proc. Natl. Acad. Sci. USA 2016, 113, 5576–5581. [Google Scholar] [CrossRef] [PubMed]
- Sheiko, S.S.; Sun, F.C.; Randall, A.; Shirvanyants, D.; Rubinstein, M.; Lee, H.-I.; Matyjaszewski, K. Adsorption-induced scission of carbon–carbon bonds. Nature 2006, 440, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Lebedeva, N.V.; Sun, F.C.; Lee, H.-I.; Matyjaszewski, K.; Sheiko, S.S. “Fatal adsorption” of brushlike macromolecules: High sensitivity of C–C bond cleavage rates to substrate surface energy. J. Am. Chem. Soc. 2008, 130, 4228–4229. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wiese, S.; Balaceanu, A.; Richtering, W.; Pich, A. Behavior of temperature-responsive copolymer microgels at the oil/water interface. Langmuir 2014, 30, 7660–7669. [Google Scholar] [CrossRef] [PubMed]
- Richtering, W. Responsive emulsions stabilized by stimuli-sensitive microgels: Emulsions with special non-pickering properties. Langmuir 2012, 28, 17218–17229. [Google Scholar] [CrossRef]
- Destribats, M.; Lapeyre, V.; Wolfs, M.; Sellier, E.; Leal-Calderon, F.; Ravaine, V.; Schmitt, V. Soft microgels as pickering emulsion stabilisers: Role of particle deformability. Soft Matter 2011, 7, 7689–7698. [Google Scholar] [CrossRef]
- Zhang, J.; Pelton, R. Poly(N-isopropylacrylamide) microgels at the air–water interface. Langmuir 1999, 15, 8032–8036. [Google Scholar] [CrossRef]
- Brugger, B.; Rütten, S.; Phan, K.H.; Möller, M.; Richtering, W. The colloidal suprastructure of smart microgels at oil–water interfaces. Angew. Chem. Int. Ed. 2009, 48, 3978–3981. [Google Scholar] [CrossRef]
- Geisel, K.; Isa, L.; Richtering, W. Unraveling the 3D localization and deformation of responsive microgels at oil/water interfaces: A step forward in understanding soft emulsion stabilizers. Langmuir 2012, 28, 15770–15776. [Google Scholar] [CrossRef]
- Stieger, M.; Richtering, W.; Pedersen, J.S.; Lindner, P. Small-angle neutron scattering study of structural changes in temperature sensitive microgel colloids. J. Chem. Phys. 2004, 120, 6197–6206. [Google Scholar] [CrossRef]
- Eckert, T.; Richtering, W. Thermodynamic and hydrodynamic interaction in concentrated microgel suspensions: Hard or soft sphere behavior? J. Chem. Phys. 2008, 129, 124902. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Zeiser, M.; Hellweg, T.; Duschl, C.; Fery, A.; Möhwald, H. Adhesion and mechanical properties of PNIPAM microgel films and their potential use as switchable cell culture substrates. Adv. Funct. Mater. 2010, 20, 3235–3243. [Google Scholar] [CrossRef]
- Mason, T.G.; Lin, M.Y. Density profiles of temperature-sensitive microgel particles. Phys. Rev. E 2005, 71, 040801. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Li, Y.; Ngai, T. Tailor-made microgel particles: Synthesis and characterization. Colloids Surf. A Physicochem. Eng. Asp. 2016, 489, 122–127. [Google Scholar] [CrossRef]
- Contreras-Cáceres, R.; Pacifico, J.; Pastoriza-Santos, I.; Pérez-Juste, J.; Fernández-Barbero, A.; Liz-Marzán, L.M. Au@ PNIPAM thermosensitive nanostructures: Control over shell cross-linking, overall dimensions, and core growth. Adv. Funct. Mater. 2009, 19, 3070–3076. [Google Scholar] [CrossRef]
- Schmidt, S.; Motschmann, H.; Hellweg, T.; von Klitzing, R. Thermoresponsive surfaces by spin-coating of PNIPAM-co-PAA microgels: A combined AFM and ellipsometry study. Polymer 2008, 49, 749–756. [Google Scholar] [CrossRef]
- Wellert, S.; Hertle, Y.; Richter, M.; Medebach, M.; Magerl, D.; Wang, W.; Demé, B.; Radulescu, A.; Müller-Buschbaum, P.; Hellweg, T. Inner structure of adsorbed ionic microgel particles. Langmuir 2014, 30, 7168–7176. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Heine, E.; Mourran, A.; Richtering, W.; Keul, H.; Moller, M. Waterborne physically crosslinked antimicrobial nanogels. Polym. Chem. 2016, 7, 364–369. [Google Scholar] [CrossRef]
- Cao, B.H.; Kim, M.W. Molecular weight dependence of the surface tension of aqueous poly(ethylene oxide) solutions. Faraday Discuss. 1994, 98, 245–252. [Google Scholar] [CrossRef]
- Zhang, J.; Pelton, R. Poly(N-isopropylacrylamide) at the air/water interface. Langmuir 1996, 12, 2611–2612. [Google Scholar] [CrossRef][Green Version]
- Kutuzov, S.; He, J.; Tangirala, R.; Emrick, T.; Russell, T.; Böker, A. On the kinetics of nanoparticle self-assembly at liquid/liquid interfaces. Phys. Chem. Chem. Phys. 2007, 9, 6351–6358. [Google Scholar] [CrossRef] [PubMed]
- Beverung, C.; Radke, C.; Blanch, H. Protein adsorption at the oil/water interface: Characterization of adsorption kinetics by dynamic interfacial tension measurements. Biophys. Chem. 1999, 81, 59–80. [Google Scholar] [CrossRef]
- Schmidt, S.; Liu, T.; Rütten, S.; Phan, K.-H.; Möller, M.; Richtering, W. Influence of microgel architecture and oil polarity on stabilization of emulsions by stimuli-sensitive core–shell poly(N-isopropylacrylamide-co-methacrylic acid) microgels: Mickering versus pickering behavior? Langmuir 2011, 27, 9801–9806. [Google Scholar] [CrossRef] [PubMed]
- Stratford, K.; Adhikari, R.; Pagonabarraga, I.; Desplat, J.-C.; Cates, M.E. Colloidal jamming at interfaces: A route to fluid-bicontinuous gels. Science 2005, 309, 2198–2201. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, A.B.; Abkarian, M.; Stone, H.A. Controlled assembly of jammed colloidal shells on fluid droplets. Nat. Mater. 2005, 4, 553–556. [Google Scholar] [CrossRef] [PubMed]
- Herzig, E.; White, K.; Schofield, A.; Poon, W.; Clegg, P. Bicontinuous emulsions stabilized solely by colloidal particles. Nat. Mater. 2007, 6, 966–971. [Google Scholar] [CrossRef]
- Brugger, B.; Rosen, B.A.; Richtering, W. Microgels as stimuli-responsive stabilizers for emulsions. Langmuir 2008, 24, 12202–12208. [Google Scholar] [CrossRef] [PubMed]
- Ngai, T.; Auweter, H.; Behrens, S.H. Environmental responsiveness of microgel particles and particle-stabilized emulsions. Macromolecules 2006, 39, 8171–8177. [Google Scholar] [CrossRef]
- Ngai, T.; Behrens, S.H.; Auweter, H. Novel emulsions stabilized by pH and temperature sensitive microgels. Chem. Commun. 2005, 331–333. [Google Scholar] [CrossRef]
- Agrawal, G.; Ülpenich, A.; Zhu, X.; Möller, M.; Pich, A. Microgel-based adaptive hybrid capsules with tunable shell permeability. Chem. Mater. 2014, 26, 5882–5891. [Google Scholar] [CrossRef]
- Monteux, C.; Marliere, C.; Paris, P.; Pantoustier, N.; Sanson, N.; Perrin, P. Poly(N-isopropylacrylamide) microgels at the oil–water interface: Interfacial properties as a function of temperature. Langmuir 2010, 26, 13839–13846. [Google Scholar] [CrossRef] [PubMed]
- Bishti, S.; Tuna, T.; Agrawal, G.; Pich, A.; Wolfart, S. Modified glass ionomer cement with “remove on demand” properties: An in vitro study. Dent. J. 2017, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ngai, T. Microgel particles at the fluid–fluid interfaces. Nanoscale 2013, 5, 1399–1410. [Google Scholar] [CrossRef] [PubMed]
- Destribats, M.; Eyharts, M.; Lapeyre, V.; Sellier, E.; Varga, I.; Ravaine, V.; Schmitt, V. Impact of PNIPAM microgel size on its ability to stabilize pickering emulsions. Langmuir 2014, 30, 1768–1777. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Geisel, K.; Richtering, W.; Ngai, T. Poly(N-isopropylacrylamide) microgels at the oil–water interface: Adsorption kinetics. Soft Matter 2013, 9, 9939–9946. [Google Scholar] [CrossRef]
- Silverstein, M.S. Emulsion-templated porous polymers: A retrospective perspective. Polymer 2014, 55, 304–320. [Google Scholar] [CrossRef]
- Viswanathan, P.; Chirasatitsin, S.; Ngamkham, K.; Engler, A.J.; Battaglia, G. Cell instructive microporous scaffolds through interface engineering. J. Am. Chem. Soc. 2012, 134, 20103–20109. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Wen, J.; Yao, J.; Chen, X.; Ni, Y.; Shao, Z. Facile fabrication of the porous three-dimensional regenerated silk fibroin scaffolds. Mater. Sci. Eng. C 2013, 33, 3522–3529. [Google Scholar] [CrossRef] [PubMed]
- Nandagiri, V.K.; Gentile, P.; Chiono, V.; Tonda-Turo, C.; Matsiko, A.; Ramtoola, Z.; Montevecchi, F.M.; Ciardelli, G. Incorporation of PLGA nanoparticles into porous chitosan–gelatin scaffolds: Influence on the physical properties and cell behavior. J. Mech. Behav. Biomed. Mater. 2011, 4, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Ye, C.; Zhu, Y.; Qi, Y.; Gou, Z.; Gao, C. Fabrication of poly(lactide-co-glycolide) scaffold embedded spatially with hydroxyapatite particles on pore walls for bone tissue engineering. Polym. Adv. Technol. 2012, 23, 1446–1453. [Google Scholar] [CrossRef]
- Koh, H.-D.; Kang, N.-G.; Lee, J.-S. Fabrication of an open Au/nanoporous film by water-in-oil emulsion-induced block copolymer micelles. Langmuir 2007, 23, 12817–12820. [Google Scholar] [CrossRef] [PubMed]
- Dinsmore, A.; Hsu, M.F.; Nikolaides, M.; Marquez, M.; Bausch, A.; Weitz, D. Colloidosomes: Selectively permeable capsules composed of colloidal particles. Science 2002, 298, 1006–1009. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Lu, Y.; Polzer, F.; Ballauff, M.; Drechsler, M. Catalytic activity of palladium nanoparticles encapsulated in spherical polyelectrolyte brushes and core–shell microgels. Chem. Mater. 2007, 19, 1062–1069. [Google Scholar] [CrossRef]
- Lu, Y.; Yu, M.; Drechsler, M.; Ballauff, M. Ag Nanocomposite Particles: Preparation, Characterization and Application; Macromolecular Symposia, 2007; Wiley Online Library: Hoboken, NJ, USA, 2007; pp. 97–102. [Google Scholar]
- Welsch, N.; Wittemann, A.; Ballauff, M. Enhanced activity of enzymes immobilized in thermoresponsive core–shell microgels. J. Phys. Chem. B 2009, 113, 16039–16045. [Google Scholar] [CrossRef] [PubMed]
- Hain, J.; Schrinner, M.; Lu, Y.; Pich, A. Design of multicomponent microgels by selective deposition of nanomaterials. Small 2008, 4, 2016–2024. [Google Scholar] [CrossRef] [PubMed]
- Biffis, A.; Cunial, S.; Spontoni, P.; Prati, L. Microgel-stabilized gold nanoclusters: Powerful “quasi-homogeneous” catalysts for the aerobic oxidation of alcohols in water. J. Catal. 2007, 251, 1–6. [Google Scholar] [CrossRef]
- Monteillet, H.; Workamp, M.; Li, X.; Schuur, B.; Kleijn, J.M.; Leermakers, F.A.; Sprakel, J. Multi-responsive ionic liquid emulsions stabilized by microgels. Chem. Commun. 2014, 50, 12197–12200. [Google Scholar] [CrossRef] [PubMed]
- Wiese, S.; Tsvetkova, Y.; Daleiden, N.J.E.; Spieß, A.C.; Richtering, W. Microgel stabilized emulsions: Breaking on demand. Colloids Surf. A Physicochem. Eng. Asp. 2016, 495, 193–199. [Google Scholar] [CrossRef]
- Wiese, S.; Spiess, A.C.; Richtering, W. Microgel-stabilized smart emulsions for biocatalysis. Angew. Chem. 2013, 125, 604–607. [Google Scholar] [CrossRef]
- Brugger, B.; Richtering, W. Magnetic, thermosensitive microgels as stimuli-responsive emulsifiers allowing for remote control of separability and stability of oil in water-emulsions. Adv. Mater. 2007, 19, 2973–2978. [Google Scholar] [CrossRef]
- Lawrence, D.B.; Cai, T.; Hu, Z.; Marquez, M.; Dinsmore, A. Temperature-responsive semipermeable capsules composed of colloidal microgel spheres. Langmuir 2007, 23, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.; Zhang, H.; Pich, A. Microgel-based stimuli-responsive capsules. Adv. Funct. Mater. 2009, 19, 554–559. [Google Scholar] [CrossRef]
- Wang, W.; Milani, A.H.; Cui, Z.; Zhu, M.; Saunders, B.R. Pickering emulsions stabilized by pH-responsive microgels and their scalable transformation to robust submicrometer colloidoisomes with selective permeability. Langmuir 2017, 33, 8192–8200. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Milani, A.H.; Carney, L.; Yan, J.; Cui, Z.; Thaiboonrod, S.; Saunders, B.R. Doubly crosslinked microgel-colloidosomes: A versatile method for pH-responsive capsule assembly using microgels as macro-crosslinkers. Chem. Commun. 2015, 51, 3854–3857. [Google Scholar] [CrossRef] [PubMed]
- Guerzoni, L.P.B.; Bohl, J.; Jans, A.; Rose, J.C.; Koehler, J.; Kuehne, A.J.C.; De Laporte, L. Microfluidic fabrication of polyethylene glycol microgel capsules with tailored properties for the delivery of biomolecules. Biomater. Sci. 2017, 5, 1549–1557. [Google Scholar] [CrossRef] [PubMed]
- Ratner, B.D.; Bryant, S.J. Biomaterials: Where we have been and where we are going. Annu. Rev. Biomed. Eng. 2004, 6, 41–75. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cao, Z.; Bai, T.; Carr, L.; Ella-Menye, J.-R.; Irvin, C.; Ratner, B.D.; Jiang, S. Zwitterionic hydrogels implanted in mice resist the foreign-body reaction. Nat. Biotechnol. 2013, 31, 553–556. [Google Scholar] [CrossRef] [PubMed]
- Bryers, J.D. Medical biofilms. Biotechnol. Bioeng. 2008, 100, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Cao, Z. Ultralow-fouling, functionalizable, and hydrolyzable zwitterionic materials and their derivatives for biological applications. Adv. Mater. 2010, 22, 920–932. [Google Scholar] [CrossRef]
- Vaisocherova, H.; Yang, W.; Zhang, Z.; Cao, Z.; Cheng, G.; Piliarik, M.; Homola, J.; Jiang, S. Ultralow fouling and functionalizable surface chemistry based on a zwitterionic polymer enabling sensitive and specific protein detection in undiluted blood plasma. Anal. Chem. 2008, 80, 7894–7901. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Lalani, R.; Cheng, F.; Liu, Q.; Liu, L. Dual-functional electrospun poly(2-hydroxyethyl methacrylate). J. Biomed. Mater. Res. Part A 2011, 99, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Finlay, J.A.; Wang, L.; Gao, Y.; Callow, J.A.; Callow, M.E.; Jiang, S. Polysulfobetaine-grafted surfaces as environmentally benign ultralow fouling marine coatings. Langmuir 2009, 25, 13516–13521. [Google Scholar] [CrossRef]
- McAllister, K.; Sazani, P.; Adam, M.; Cho, M.J.; Rubinstein, M.; Samulski, R.J.; DeSimone, J.M. Polymeric nanogels produced via inverse microemulsion polymerization as potential gene and antisense delivery agents. J. Am. Chem. Soc. 2002, 124, 15198–15207. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Li, G.; Xue, H.; Chen, S.; Bryers, J.D.; Jiang, S. Zwitterionic carboxybetaine polymer surfaces and their resistance to long-term biofilm formation. Biomaterials 2009, 30, 5234–5240. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-C.; Zhao, C.; Hsieh, I.F.; Subramanian, S.; Liu, L.; Cheng, G.; Li, L.; Cheng, S.Z.D.; Zheng, J. Strong resistance of poly(ethylene glycol) based l-tyrosine polyurethanes to protein adsorption and cell adhesion. Polym. Int. 2012, 61, 616–621. [Google Scholar] [CrossRef]
- Shen, M.; Wagner, M.S.; Castner, D.G.; Ratner, B.D.; Horbett, T.A. Multivariate surface analysis of plasma-deposited tetraglyme for reduction of protein adsorption and monocyte adhesion. Langmuir 2003, 19, 1692–1699. [Google Scholar] [CrossRef]
- Zhang, M.; Horbett, T.A. Tetraglyme coatings reduce fibrinogen and von willebrand factor adsorption and platelet adhesion under both static and flow conditions. J. Biomed. Mater. Res. Part A 2009, 89, 791–803. [Google Scholar] [CrossRef]
- Martwiset, S.; Koh, A.E.; Chen, W. Nonfouling characteristics of dextran-containing surfaces. Langmuir 2006, 22, 8192–8196. [Google Scholar] [CrossRef] [PubMed]
- Holland, N.B.; Qiu, Y.; Ruegsegger, M.; Marchant, R.E. Biomimetic engineering of non-adhesive glycocalyx-like surfaces using oligosaccharide surfactant polymers. Nature 1998, 392, 799–801. [Google Scholar] [CrossRef] [PubMed]
- Wyszogrodzka, M.; Haag, R. Synthesis and characterization of glycerol dendrons, self-assembled monolayers on gold: A detailed study of their protein resistance. Biomacromolecules 2009, 10, 1043–1054. [Google Scholar] [CrossRef] [PubMed]
- Luk, Y.-Y.; Kato, M.; Mrksich, M. Self-assembled monolayers of alkanethiolates presenting mannitol groups are inert to protein adsorption and cell attachment. Langmuir 2000, 16, 9604–9608. [Google Scholar] [CrossRef]
- Yang, W.; Chen, S.; Cheng, G.; Vaisocherova, H.; Xue, H.; Li, W.; Zhang, J.; Jiang, S. Film thickness dependence of protein adsorption from blood serum and plasma onto poly(sulfobetaine)-grafted surfaces. Langmuir 2008, 24, 9211–9214. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, P.; Bruin, A.; Klijnstra, J.W.; Nyström, A.M.; Johansson, M.; Malkoch, M.; Hult, A. Poly(ethylene glycol)-based thiol-ene hydrogel coatings-curing chemistry, aqueous stability, and potential marine antifouling applications. ACS Appl. Mater. Interfaces 2010, 2, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Mi, L.; Cao, Z.; Xue, H.; Yu, Q.; Carr, L.; Jiang, S. Functionalizable and ultrastable zwitterionic nanogels. Langmuir 2010, 26, 6883–6886. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, Q.; Patel, K.; Li, L.; Li, X.; Wang, Q.; Zhang, G.; Zheng, J. Synthesis and characterization of pH-sensitive poly(N-2-hydroxyethyl acrylamide)–acrylic acid (poly(HEAA/AA)) nanogels with antifouling protection for controlled release. Soft Matter 2012, 8, 7848–7857. [Google Scholar] [CrossRef]
- Bridges, A.W.; Singh, N.; Burns, K.L.; Babensee, J.E.; Andrew Lyon, L.; García, A.J. Reduced acute inflammatory responses to microgel conformal coatings. Biomaterials 2008, 29, 4605–4615. [Google Scholar] [CrossRef]
- Bridges, A.W.; Whitmire, R.E.; Singh, N.; Templeman, K.L.; Babensee, J.E.; Lyon, L.A.; García, A.J. Chronic inflammatory responses to microgel-based implant coatings. J. Biomed. Mater. Res. Part A 2010, 94, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.; Zhou, H.; Li, T.; Li, C.; Duan, Y.Y. Polyethylene glycol-containing polyurethane hydrogel coatings for improving the biocompatibility of neural electrodes. Acta Biomater. 2012, 8, 2233–2242. [Google Scholar] [CrossRef]
- Chen, K.; Zhou, S.; Wu, L. Self-healing underwater superoleophobic and antibiofouling coatings based on the assembly of hierarchical microgel spheres. ACS Nano 2016, 10, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Heris, H.K.; Daoud, J.; Sheibani, S.; Vali, H.; Tabrizian, M.; Mongeau, L. Vocal fold tissue regeneration: Investigation of the viability, adhesion, and migration of human fibroblasts in a hyaluronic acid/gelatin microgel-reinforced composite hydrogel for vocal fold tissue regeneration. Adv. Healthc. Mater. 2016, 5, 188. [Google Scholar] [CrossRef]
- Bae, H.; Chu, H.; Edalat, F.; Cha, J.M.; Sant, S.; Kashyap, A.; Ahari, A.F.; Kwon, C.H.; Nichol, J.W.; Manoucheri, S. Development of functional biomaterials with micro-and nanoscale technologies for tissue engineering and drug delivery applications. J. Tissue Eng. Regen. Med. 2014, 8, 1–14. [Google Scholar] [CrossRef]
- El-Sherbiny, I.M.; Yacoub, M.H. Hydrogel scaffolds for tissue engineering: Progress and challenges. Glob. Cardiol. Sci. Pract. 2013, 2013, 316–342. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Chen, J.; Deng, C.; Suuronen, E.J.; Zhong, Z. Click hydrogels, microgels and nanogels: Emerging platforms for drug delivery and tissue engineering. Biomaterials 2014, 35, 4969–4985. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Xiao, W.; Sun, J.; Zhong, M.; Guo, L.; Fan, H.; Zhang, X. A biocompatible hydrogel with improved stiffness and hydrophilicity for modular tissue engineering assembly. J. Mater. Chem. B 2015, 3, 2753–2763. [Google Scholar] [CrossRef]
- Steinhilber, D.; Rossow, T.; Wedepohl, S.; Paulus, F.; Seiffert, S.; Haag, R. A microgel construction kit for bioorthogonal encapsulation and pH-controlled release of living cells. Angew. Chem. Int. Ed. 2013, 52, 13538–13543. [Google Scholar] [CrossRef] [PubMed]
- Zamanian, B.; Masaeli, M.; Nichol, J.W.; Khabiry, M.; Hancock, M.J.; Bae, H.; Khademhosseini, A. Interface-directed self-assembly of cell-laden microgels. Small 2010, 6, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Guven, S.; Chen, P.; Inci, F.; Tasoglu, S.; Erkmen, B.; Demirci, U. Multiscale assembly for tissue engineering and regenerative medicine. Trends Biotechnol. 2015, 33, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.R.; Aghaei-Ghareh-Bolagh, B.; Dang, T.T.; Topkaya, S.N.; Gao, X.; Yang, S.Y.; Jung, S.M.; Oh, J.H.; Dokmeci, M.R.; Tang, X.S. Cell-laden microengineered and mechanically tunable hybrid hydrogels of gelatin and graphene oxide. Adv. Mater. 2013, 25, 6385–6391. [Google Scholar] [CrossRef] [PubMed]
- Panda, P.; Ali, S.; Lo, E.; Chung, B.G.; Hatton, T.A.; Khademhosseini, A.; Doyle, P.S. Stop-flow lithography to generate cell-laden microgel particles. Lab Chip 2008, 8, 1056–1061. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, K.; Zhang, H.; Pang, B.; Choi, C.-H.; Mao, A.S.; Liao, H.; Utech, S.; Mooney, D.J.; Wang, H.; et al. Microfluidic templated multicompartment microgels for 3D encapsulation and pairing of single cells. Small 2018. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Gao, X.; Dong, H.; He, H.; Cao, X. High-throughput generation of hyaluronic acid microgels via microfluidics-assisted enzymatic crosslinking and/or diels–alder click chemistry for cell encapsulation and delivery. Appl. Mater. Today 2017, 9, 49–59. [Google Scholar] [CrossRef]
- Xia, P.; Zhang, K.; Gong, Y.; Li, G.; Yan, S.; Yin, J. Injectable stem cell laden open porous microgels that favor adipogenesis: In vitro and in vivo evaluation. ACS Appl. Mater. Interfaces 2017, 9, 34751–34761. [Google Scholar] [CrossRef]
- Uhlig, K.; Wegener, T.; He, J.; Zeiser, M.; Bookhold, J.; Dewald, I.; Godino, N.; Jaeger, M.; Hellweg, T.; Fery, A. Patterned thermoresponsive microgel coatings for noninvasive processing of adherent cells. Biomacromolecules 2016, 17, 1110–1116. [Google Scholar] [CrossRef] [PubMed]
- Arun Kumar, R.; Sivashanmugam, A.; Deepthi, S.; Iseki, S.; Chennazhi, K.; Nair, S.V.; Jayakumar, R. Injectable chitin-poly(ε-caprolactone)/nanohydroxyapatite composite microgels prepared by simple regeneration technique for bone tissue engineering. ACS Appl. Mater. Interfaces 2015, 7, 9399–9409. [Google Scholar] [CrossRef] [PubMed]
- Tasoglu, S.; Kavaz, D.; Gurkan, U.A.; Guven, S.; Chen, P.; Zheng, R.; Demirci, U. Paramagnetic levitational assembly of hydrogels. Adv. Mater. 2013, 25, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Wu, C.A.M.; Rengarajan, V.; Finley, T.D.; Keles, H.O.; Sung, Y.; Li, B.; Gurkan, U.A.; Demirci, U. Three-dimensional magnetic assembly of microscale hydrogels. Adv. Mater. 2011, 23, 4254–4260. [Google Scholar] [CrossRef] [PubMed]
- Tasoglu, S.; Yu, C.H.; Gungordu, H.I.; Guven, S.; Vural, T.; Demirci, U. Guided and magnetic self-assembly of tunable magnetoceptive gels. Nat. Commun. 2014, 5, 4702. [Google Scholar] [CrossRef] [PubMed]


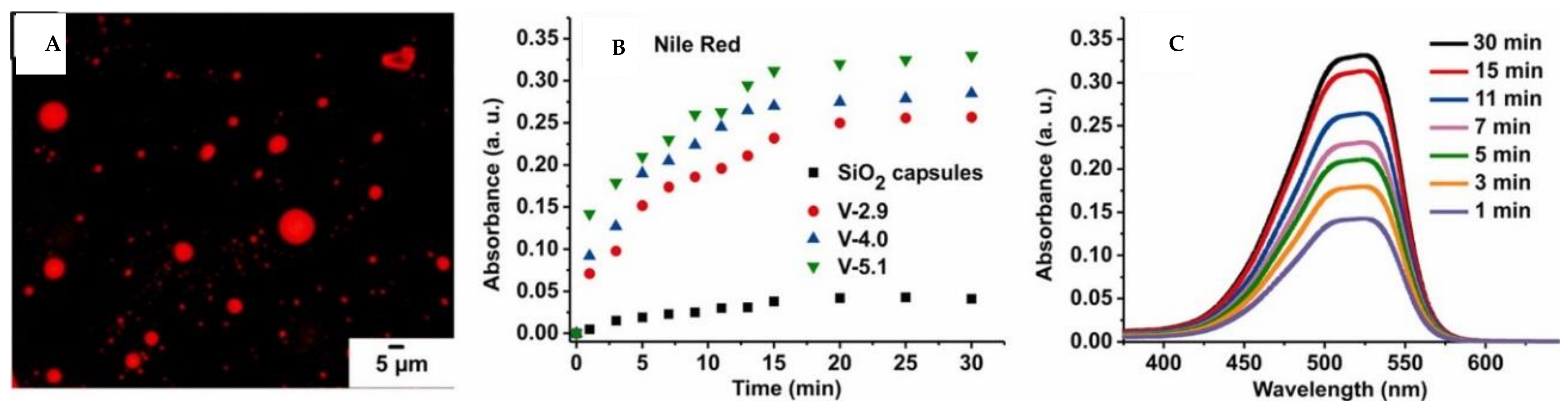
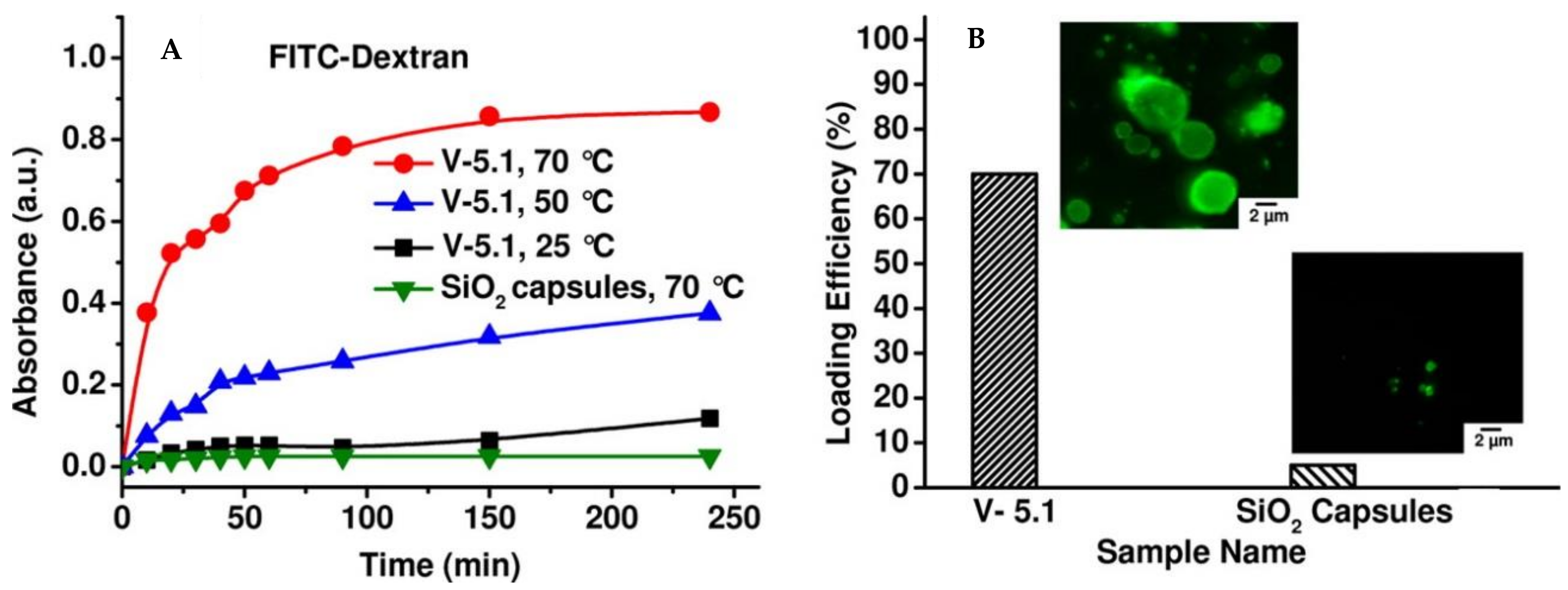

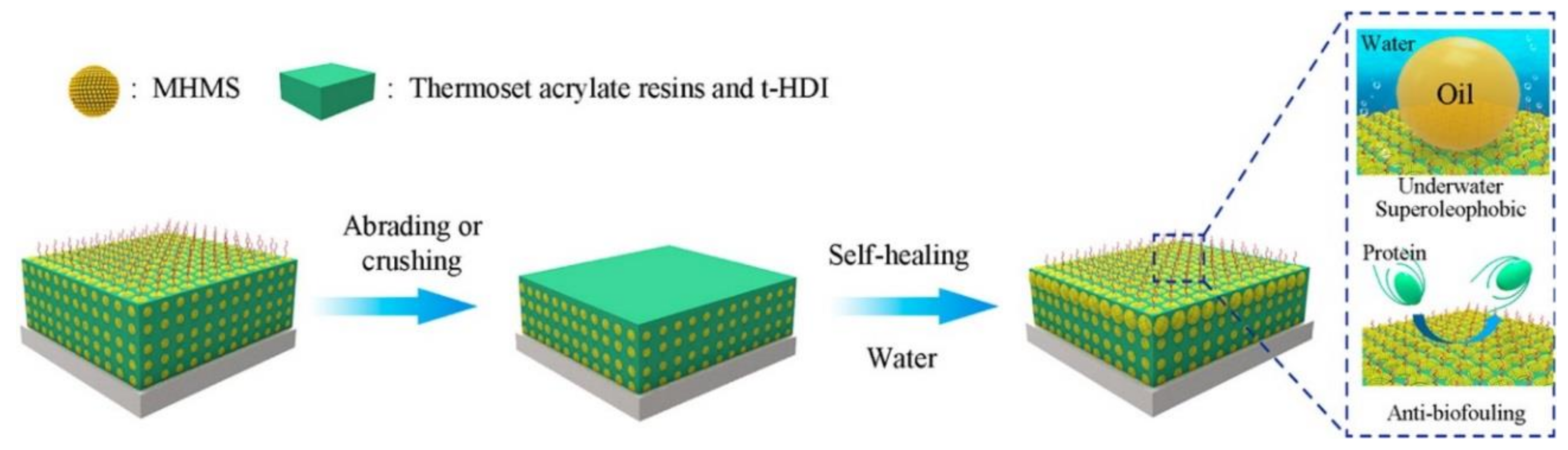

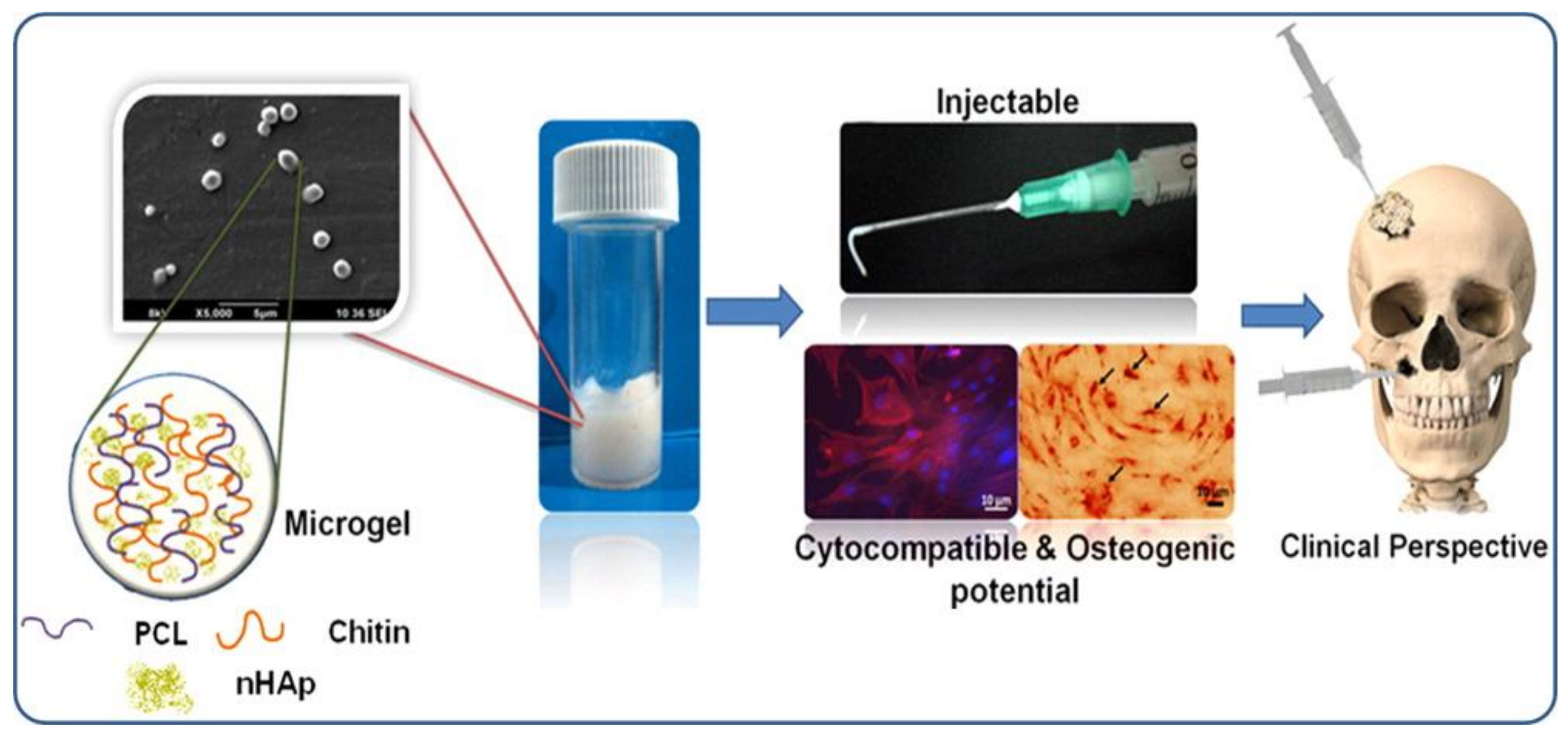
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agrawal, G.; Agrawal, R. Stimuli-Responsive Microgels and Microgel-Based Systems: Advances in the Exploitation of Microgel Colloidal Properties and Their Interfacial Activity. Polymers 2018, 10, 418. https://doi.org/10.3390/polym10040418
Agrawal G, Agrawal R. Stimuli-Responsive Microgels and Microgel-Based Systems: Advances in the Exploitation of Microgel Colloidal Properties and Their Interfacial Activity. Polymers. 2018; 10(4):418. https://doi.org/10.3390/polym10040418
Chicago/Turabian StyleAgrawal, Garima, and Rahul Agrawal. 2018. "Stimuli-Responsive Microgels and Microgel-Based Systems: Advances in the Exploitation of Microgel Colloidal Properties and Their Interfacial Activity" Polymers 10, no. 4: 418. https://doi.org/10.3390/polym10040418
APA StyleAgrawal, G., & Agrawal, R. (2018). Stimuli-Responsive Microgels and Microgel-Based Systems: Advances in the Exploitation of Microgel Colloidal Properties and Their Interfacial Activity. Polymers, 10(4), 418. https://doi.org/10.3390/polym10040418