The Influence of Accelerated UV-A and Q-SUN Irradiation on the Antibacterial Properties of Hydrophobic Coatings Containing Eucomis comosa Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extract Preparation
2.3. Coating Preparation and Antimicrobial Properties Analysis
- (1)
- MHPC (2 g) was introduced into 48 g of water, this was mixed for 1 h using a magnetic stirrer (Ika, Staufen im Breisgau, Germany) at 40 °C at 1500 rpm. Cocoa butter (10 g) was heated to 40 °C. Then, 40 g of MHPC was mixed with 10 g of cocoa butter and homogenized (1000 rpm) (Heidolph, Sigma-Aldrich, Poznań, Poland). The hydrophobic mixture was used to cover the PLA films to obtain coatings devoid of any active substances.
- (2)
- E. comosa extract (12.5 g) was mixed with 25.5 g of water. Next, 2 g of MHPC was introduced into 38 g of E. comosa solution. The mixture was mixed for 1 h using a magnetic stirrer (Ika, Staufen im Breisgau, Germany) at 40 °C at 1000 rpm. Cocoa butter (10 g) was heated to 40 °C and 40 g of MHPC containing E. comosa extract was then mixed with the cocoa butter and homogenized (1000 rpm) (Heidolph, Sigma-Aldrich, Poznań, Poland). The hydrophobic mixture was used to cover the PLA films to obtain 25% active substance coatings.
- (3)
- MHPC (2 g) was introduced into 48 g of water. The mixture was mixed for 1 h using a magnetic stirrer (Ika, Staufen im Breisgau, Germany) at 40 °C at 1500 rpm. The hydrophilic MHPC was used to cover PLA films to obtain coatings devoid of any active substances and hydrophobic substances. The films covered with MHPC were used for the analyses of Fourier transform infrared (FT-IR) and water vapour transmission rate (WVTR).
2.4. Accelerated Irradiation
2.5. FT-IR
2.6. Barrier Characteristic
2.7. Statistical Analysis
3. Results
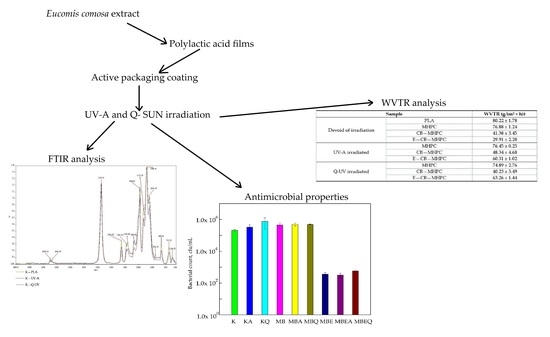
3.1. Antimicrobial Properties
3.2. FT-IR Analysis
3.3. Barrier Characteristic
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Muller, J.; González-Martínez, C.; Chiralt, A. Combination of Poly(lactic) Acid and Starch for Biodegradable Food Packaging. Materials 2017, 10, 952. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, V.; Rosa, M.D.; Iordanskii, A.L. Performance of Poly(lactic acid) Surface Modified Films for Food Packaging Application. Materials 2017, 10, 850. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Aldana, D.; Duarte Villa, E.; De Dios Hernández, M.; Guillermo González Sánchez, G.; Rascón Cruz, Q.; Flores Gallardo, S.; Hilda Piñon Castillo, H.; Ballinas Casarrubias, L. Barrier Properties of Polylactic Acid in Cellulose Based Packages Using Montmorillonite as Filler. Polymers 2014, 6, 2386–2403. [Google Scholar] [CrossRef]
- Musil, J. Flexible antibacterial coatings. Molecules 2017, 22, 813. [Google Scholar] [CrossRef] [PubMed]
- Mizielińska, M.; Lisiecki, S.; Jędra, F.; Kowalska, U.; Tomczak, A. The barrier and the antimicrobial properties of polylactide films covered with exopolysaccharide layers synthesized by Arthrobacter viscosus. Przem. Chem. 2015, 94, 748–751. [Google Scholar] [CrossRef]
- Chu, Z.; Zhao, T.; Li, L.; Fan, J.; Qin, Y. Characterization of Antimicrobial Poly (Lactic Acid)/Nano-Composite Films with Silver and Zinc Oxide Nanoparticles. Materials 2017, 10, 659. [Google Scholar] [CrossRef] [PubMed]
- Mizielińska, M.; Lisiecki, S. Coating of polylactide films to generate their antimicrobial properties. Przem. Chem. 2015, 94, 752–755. [Google Scholar] [CrossRef]
- Han, J.; Salmeri, S.; Le Tien, C.; Lacroix, M. Improvement of Water Barrier Property of Paperboard by Coating Application with Biodegradable Polymers. J. Agric. Food Chem. 2010, 58, 3125–3131. [Google Scholar] [CrossRef] [PubMed]
- Torres-Moreno, M.; Torrescasana, E.; Salas-Salvadó, J.; Blanch, C. Nutritional composition and fatty acids profile in cocoa beans and chocolates with different geographical origin and processing conditions. Food Chem. 2015, 166, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Mizielińska, M.; Kowalska, U.; Pankowski, J.; Bieńkiewicz, G.; Malka, M.; Lisiecki, S. Coating the polyethylene films to generate the antibacterial properties. Przem. Chem. 2017, 96, 1317–1321. [Google Scholar] [CrossRef]
- Mizielińska, M.; Ordon, M.; Pankowski, J.; Bieńkiewicz, G.; Malka, M.; Lisiecki, S.; Bartkowiak, A. Estimation of the antimicrobial properties of the coatings from industrial trials. Przem. Chem. 2017, 96, 1322–1324. [Google Scholar] [CrossRef]
- Mizielińska, M.; Lisiecki, S.; Jotko, M.; Chodzyńska, I.; Bartkowiak, A. The antimicrobial properties of polylactide films covered with ZnO nanoparticles-containing layers. Przem. Chem. 2015, 94, 1000–1003. [Google Scholar] [CrossRef]
- Bartkowiak, A.; Mizielińska, M.; Sumińska, P.; Romanowska-Osuch, A.; Lisiecki, S. Innovations in food packaging materials. In Emerging and Traditional Technologies for Safe, Healthy and Quality Food; Nedović, V., Raspor, P., Lević, J., Šaponjac, V.T., Barbosa-Cánovas, G.V., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 383–412. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Alias, A.K.; Ariffin, F.; Mahmud, S.; Najafi, A.; Ahmad, M. Fabrication and characterization of novel semolina-based antimicrobial films derived from the combination of ZnO nanorods and nanokaolin. J. Food. Sci. Technol. 2017, 54, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, J.; Xu, Q.; Zhang, J. Fabrication of antibacterial casein-based ZnO nanocomposite for flexible coatings. Mater. Des. 2017, 113, 240–245. [Google Scholar] [CrossRef]
- Kwiatkowski, P.; Giedrys–Kalemba, S.; Mizielińska, M.; Artur Bartkowiak, A. Antibacterial activity of rosemary caraway and fennel essential oils. Herba Pol. 2015, 61, 31–39. [Google Scholar] [CrossRef]
- Kwiatkowski, P.; Giedrys–Kalemba, S.; Mizielińska, M.; Artur Bartkowiak, A. Modyfication of PLA foil surface by ethylocellulose and essential oils. J. Microbiol. Biotechnol. Food Sci. 2016, 5, 440–444. [Google Scholar] [CrossRef]
- Taylor, J.L.S.; Van Staden, J. The effect of age, season and growth conditions on anti-inflammatory activity in Eucomis autumnalis (Mill) Chitt. plant extracts. Plant Growth Regul. 2011, 34, 39–47. [Google Scholar] [CrossRef]
- Louw, C.A.M.; Regnier, T.J.C.; Korsten, L. Medicinal bulbous plants of South Africa and their traditional relevance in the control of infectious diseases. J. Ethnopharmacol. 2002, 82, 147–154. [Google Scholar] [CrossRef]
- Salachna, P.; Zawadzińska, A. Effect of daminozide and flurprimidol on growth, flowering and bulb yield of Eucomis autumnalis (Mill.) Chitt. Folia Hortic. 2017, 29, 33–38. [Google Scholar] [CrossRef]
- Masondo, N.A.; Finnie, J.F.; van Staden, J. Pharmacological potential and conservation prospect of the genus Eucomis (Hyacinthaceae) endemic to Southern Africa. J Ethnopharmacol. 2014, 151, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Salachna, P.; Grzeszczuk, M.; Wilas, J. Total phenolic content, phenolic content, photosynthetic pigment concentration and antioxidant activity of leaves and bulbs of selected Eucomis L'Hér. taxa. Fresenius Environ. Bull. 2015, 24, 4220–4225. [Google Scholar]
- Bisi-Johnson, M.A.; Obi, C.L.; Hattori, T.; Oshima, Y.; Li, S.; Kambizi, L.; Eloff, J.N.; Vasaikar, S.D. Evaluation of the antibacterial and anticancer activities of some South African medicinal plants. Complement. Altern. Med. 2011, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Motsei, M.L.; Lindsey, K.L.; van Staden, J.; Jäger, A.K. Screening of traditionally used South African plants for antifungal activity against Candida albicans. J. Ethnopharmacol. 2003, 86, 235–241. [Google Scholar] [CrossRef]
- Salachna, P.; Mizielińska, M.; Soból, M. Exopolysaccharide Gellan Gum and Derived Oligo-Gellan Enhance Growth and Antimicrobial Activity in Eucomis Plants. Polymers 2018, 10, 242. [Google Scholar] [CrossRef]
- Mizielińska, M.; Salachna, P.; Ordon, M.; Łopusiewicz, Ł. Antimicrobial activity of water and acetone extracts of some Eucomis taxa. Asian Pac. J. Trop. Med. 2017, 10, 892–895. [Google Scholar] [CrossRef] [PubMed]
- Mizielińska, M.; Kowalska, U.; Jarosz, M.; Sumińska, P. A comparison of the effects of packaging containing nano ZnO or polylysine on the microbial purity and texture of cod (Gadus morhua) fillets. Nanomaterials 2018, 8, 158. [Google Scholar] [CrossRef] [PubMed]
- Mizielińska, M.; Łopusiewicz, Ł.; Mężyńska, M.; Bartkowiak, A. The influence of accelerated UV-A and Q-SUN irradiation on the antimicrobial properties of coatings containing ZnO nanoparticles. Molecules 2017, 22, 1556. [Google Scholar] [CrossRef] [PubMed]
- Nguyena, T.V.; Dao, P.H.; Khanh Linh Duong, K.L.; Duong, Q.H.; Vu, Q.T.; Nguyena, A.H.; Mac, V.P.; Lea, T.L. Effect of R-TiO2 and ZnO nanoparticles on the UV-shielding efficiency of water-borne acrylic coating. Prog. Org. Coat. 2017, 110, 114–121. [Google Scholar] [CrossRef]
- Marvizadeh, M.M.; Oladzadabbasabadi, N.; Nafchi, A.M.; Jokar, M. Preparation and characterization of bionanocomposite film based on tapioca starch/bovine gelatin/nanorod zinc oxide. Int. J. Biol. Macromol. 2017, 99, 1–7. [Google Scholar] [CrossRef] [PubMed]
- El-Feky, O.M.; Hassan, E.A.; Fadel, S.M.; Hassan, M.L. Use of ZnO nanoparticles for protecting oil paintings on paper support against dirt, fungal attack, and UV aging. J. Cult. Herit. 2014, 15, 165–172. [Google Scholar] [CrossRef]
- Kairyte, K.; Kadys, A.; Luksiene, Z. Antibacterial and antifungal activity of photoactivated ZnO nanoparticles in suspension. J. Photochem. Photobiol. Biol. 2013, 128, 78–84. [Google Scholar] [CrossRef] [PubMed]
- ASTM Standard Test Method for Determining the Activity of Incorporated Antimicrobial Agent(s) in Polymeric or Hydrophobic Materials; E 2180-01; ASTM: West Conshohocken, PA, USA, 2002.
- Nichols, M.; Boisseau, J.; Pattison, L.; Campbell, D.; Quill, J.; Zhang, J.; Smith, D.; Henderson, K.; Seebergh, J.; Berry, D.; et al. An improved accelerated weathering protocol to anticipate Florida exposure behavior of coatings. J. Coat. Technol. Res. 2013, 10, 153–173. [Google Scholar] [CrossRef]
- DIN 53122-1 ISO 2528:1995. Available online: http://sklep.pkn.pl/pn-iso-2528-2000p.html (accessed on 7 June 2016).
- ISO 2528:1995. Available online: http://sklep.pkn.pl/pn-iso-2528-2000p.html (accessed on 7 June 2016).
- Seda Tıglı Aydın, R.; Akyol, E.; Hazer, B. Influence of Soybean Oil Blending with Polylactic Acid (PLA) Films: In Vitro and In Vivo Evaluation. J. Am. Oil Chem. Soc. 2017, 94, 413–424. [Google Scholar] [CrossRef]
- Yingfeng, Z.; Yiqiang, W.; Jiyou, G.; Yanhua, Z. The UV Aging Properties of Maleic Anhydride Esterified Starch/Polylactic Acid Composites. J. Wuhan Univ. Technol. 2017, 32, 917–977. [Google Scholar] [CrossRef]
- Van Cong, D.; Trang, N.T.T.; Giang, N.V.; Lam, T.D.; Hoang, T. Effect of TiO2-Crystal Forms on the Photo-Degradation of EVA/PLA Blend Under Accelerated Weather Testing. J. Electron. Mater. 2016, 45, 2536–2546. [Google Scholar] [CrossRef]
- Punitha, S.; Uvarani, R.; Panneerselvam, A.; Nithiyanantham, S. Physico-chemical studies on some saccharides in aqueous cellulose solutions at different temperatures—Acoustical and FTIR analysis. J. Saudi Chem. Soc. 2014, 18, 657–665. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, X.; Cai, H. Green synthesis of monodisperse silver nanoparticles using hydroxy propyl methyl cellulose. J. Alloys Compd. 2014, 583, 267–271. [Google Scholar] [CrossRef]
- Vesela, A.; Barros, A.S.; Synytsya, A.; Delgadillo, I.; Copikova, J.; Manuel Coimbra, A. Infrared spectroscopy and outer product analysis for quantification of fat, nitrogen, and moisture of cocoa powder. Anal. Chim. Acta 2007, 601, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Suparman; Rahayu, W.S.; Sundhani, E.; Dwi Saputri, S. The use of Fourier Transform Infrared Spectroscopy (FTIR) and Gas Chromatography Mass Spectroscopy (GCMS) for Halal Authentication in Imported Chocolate with Various Variants. J. Food. Pharm. Sci. 2015, 2, 6–11. [Google Scholar]
- Díaz-Tena, E.; Rodríguez-Ezquerro, A.; López de Lacalle Marcaide, L.N.; Gurtubay Bustinduy, L.; Elías Sáenz, A. A sustainable process for material removal on pure copper by use of extremophile bacteria. J. Clean. Prod. 2014, 84, 752–760. [Google Scholar] [CrossRef]
- Díaz-Tena, E.; Barona, A.; Gallastegui, G.; Rodrigez, A.; López de Lacalle, L.N.; Elías, A. Biomachining: Metal etching viamicroorganisms. Crit. Rev. Biotechnol. 2017, 37, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, R.; Natesan Rajeswari, N. ZnO/PBAT nanocomposite films: Investigation on the mechanical and biological activity for food packaging. Polym. Adv. Technol. 2017, 28, 20–27. [Google Scholar] [CrossRef]
- Valdés, A.; Ramos, M.; Beltrán, A.; Jiménez, A.; Garrigós, M.C. State of the Art of Antimicrobial Edible Coatings for Food Packaging Applications. Coatings 2017, 7, 56. [Google Scholar] [CrossRef]
- Djenane, D.; Roncalés, P. Carbon Monoxide in Meat and Fish Packaging: Advantages and Limits. Foods 2018, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Shakila, R.J.; Jeevithan, E.; Arumugam, V.; Jeyasekaran, G. Suitability of antimicrobial grouper bone gelatin films as edible coatings for vacuum-packaged fish steaks. J. Aquat. Food Prod. Technol. 2016, 25, 724–734. [Google Scholar] [CrossRef]
- Jongberg, S.; Tørngren, M.A.; Skibsted, L.H. Protein Oxidation and Sensory Quality of Brine-Injected Pork Loins Added Ascorbate or Extracts of Green Tea or Maté during Chill-Storage in High-Oxygen Modified Atmosphere. Medicines 2018, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Campos, D.; Piccirillo, C.; Pullar, R.C.; Castro, P.M.L.; Pintado, M.M.E. Characterization and antimicrobial properties of food packaging methylcellulose films containing stem extract of Ginja cherry. J. Sci. Food. Agric. 2014, 94, 2097–2103. [Google Scholar] [CrossRef] [PubMed]
- Quesada, J.; Sendra, E.; Navarro, C.; Sayas-Barberá, E. Antimicrobial Active Packaging including Chitosan Films with Thymus vulgaris L. Essential Oil for Ready-to-Eat Meat. Foods 2016, 5, 57. [Google Scholar] [CrossRef] [PubMed]
- Rahman, P.M.; Abdul Mujeeb, V.M.; Muraleedharan, K. Flexible chitosan-nano ZnO antimicrobial pouches as a new materialfor extending the shelf life of raw meat. Int. J. Biol. Macromol. 2017, 97, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, L.N.; López, M.; Montes-Díaz, E.; Espadín, A.; Tecante, A.; Gimeno, M.; Shirai, K. Inhibition of Listeria monocytogenes in Fresh Cheese Using Chitosan-Grafted Lactic Acid Packaging. Molecules 2016, 21, 469. [Google Scholar] [CrossRef] [PubMed]







| Sample | WVTR (g/(m2 × h)) | |
|---|---|---|
| Devoid of irradiation | PLA | 80.22 ± 1.78 |
| MHPC | 76.88 ± 1.24 | |
| CB—MHPC | 41.38 ± 3.45 | |
| E—CB—MHPC | 29.91 ± 2.28 | |
| UV-A irradiated | MHPC | 76.45 ± 0.25 |
| CB—MHPC | 48.34 ± 4.68 | |
| E—CB—MHPC | 60.31 ± 1.02 | |
| Q-UV irradiated | MHPC | 74.89 ± 2.76 |
| CB—MHPC | 40.23 ± 3.49 | |
| E—CB—MHPC | 63.26 ± 1.44 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizielińska, M.; Kowalska, U.; Salachna, P.; Łopusiewicz, Ł.; Jarosz, M. The Influence of Accelerated UV-A and Q-SUN Irradiation on the Antibacterial Properties of Hydrophobic Coatings Containing Eucomis comosa Extract. Polymers 2018, 10, 421. https://doi.org/10.3390/polym10040421
Mizielińska M, Kowalska U, Salachna P, Łopusiewicz Ł, Jarosz M. The Influence of Accelerated UV-A and Q-SUN Irradiation on the Antibacterial Properties of Hydrophobic Coatings Containing Eucomis comosa Extract. Polymers. 2018; 10(4):421. https://doi.org/10.3390/polym10040421
Chicago/Turabian StyleMizielińska, Małgorzata, Urszula Kowalska, Piotr Salachna, Łukasz Łopusiewicz, and Michał Jarosz. 2018. "The Influence of Accelerated UV-A and Q-SUN Irradiation on the Antibacterial Properties of Hydrophobic Coatings Containing Eucomis comosa Extract" Polymers 10, no. 4: 421. https://doi.org/10.3390/polym10040421
APA StyleMizielińska, M., Kowalska, U., Salachna, P., Łopusiewicz, Ł., & Jarosz, M. (2018). The Influence of Accelerated UV-A and Q-SUN Irradiation on the Antibacterial Properties of Hydrophobic Coatings Containing Eucomis comosa Extract. Polymers, 10(4), 421. https://doi.org/10.3390/polym10040421