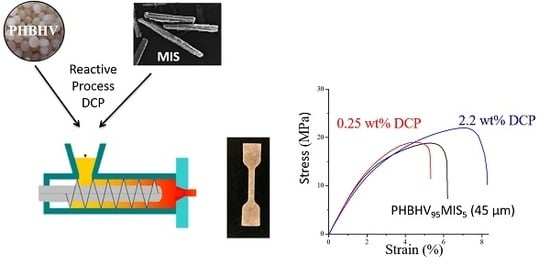
Biocomposites Based on Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate) (PHBHV) and Miscanthus giganteus Fibers with Improved Fiber/Matrix Interface
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemical Treatment of Miscanthus giganteus Fibers
2.3. Composite Manufacturing
2.4. Materials Characterization
2.4.1. Gel Fraction
2.4.2. Mechanical Testing
2.4.3. Scanning Electron Microscopy (SEM)
2.4.4. Fourier Transform Infrared Spectroscopy (FTIR)
2.4.5. Differential Scanning Calorimetry (DSC)
2.4.6. X-ray Diffraction (XRD)
3. Results
3.1. Evaluation of PHBHV Grafting onto MIS Surface during Processing Evaluated by FTIR-ATR Analysis
3.2. Tensile Properties
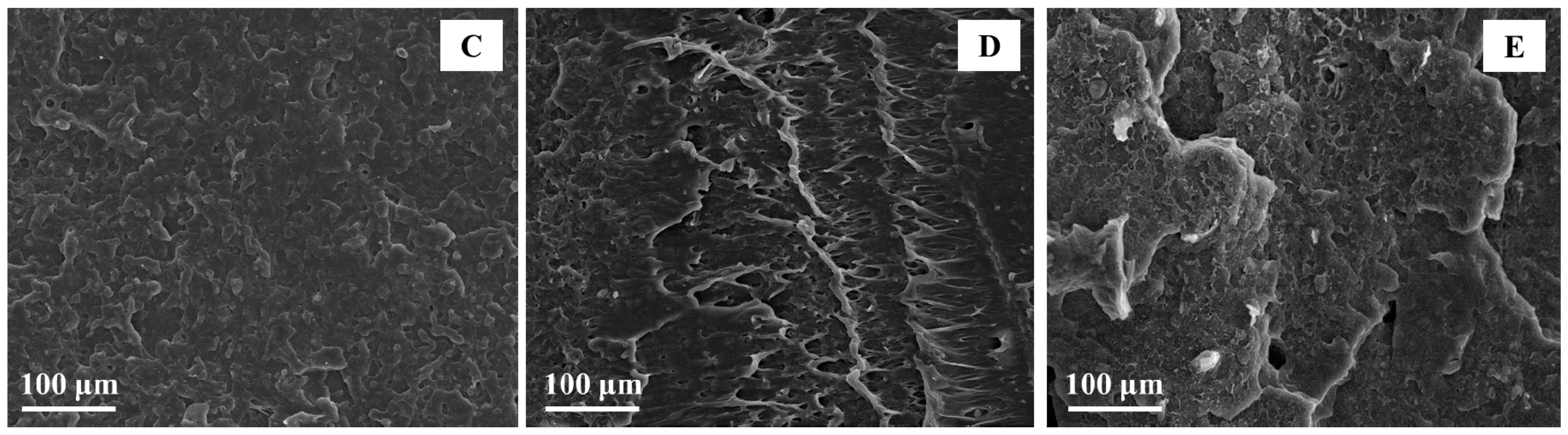
3.3. Fracture Facies Morphology
3.4. Characterization of Biocomposites by DSC and XRD Analyses
3.5. Analytical Models
3.5.1. Use of a Model Involving Three Phases
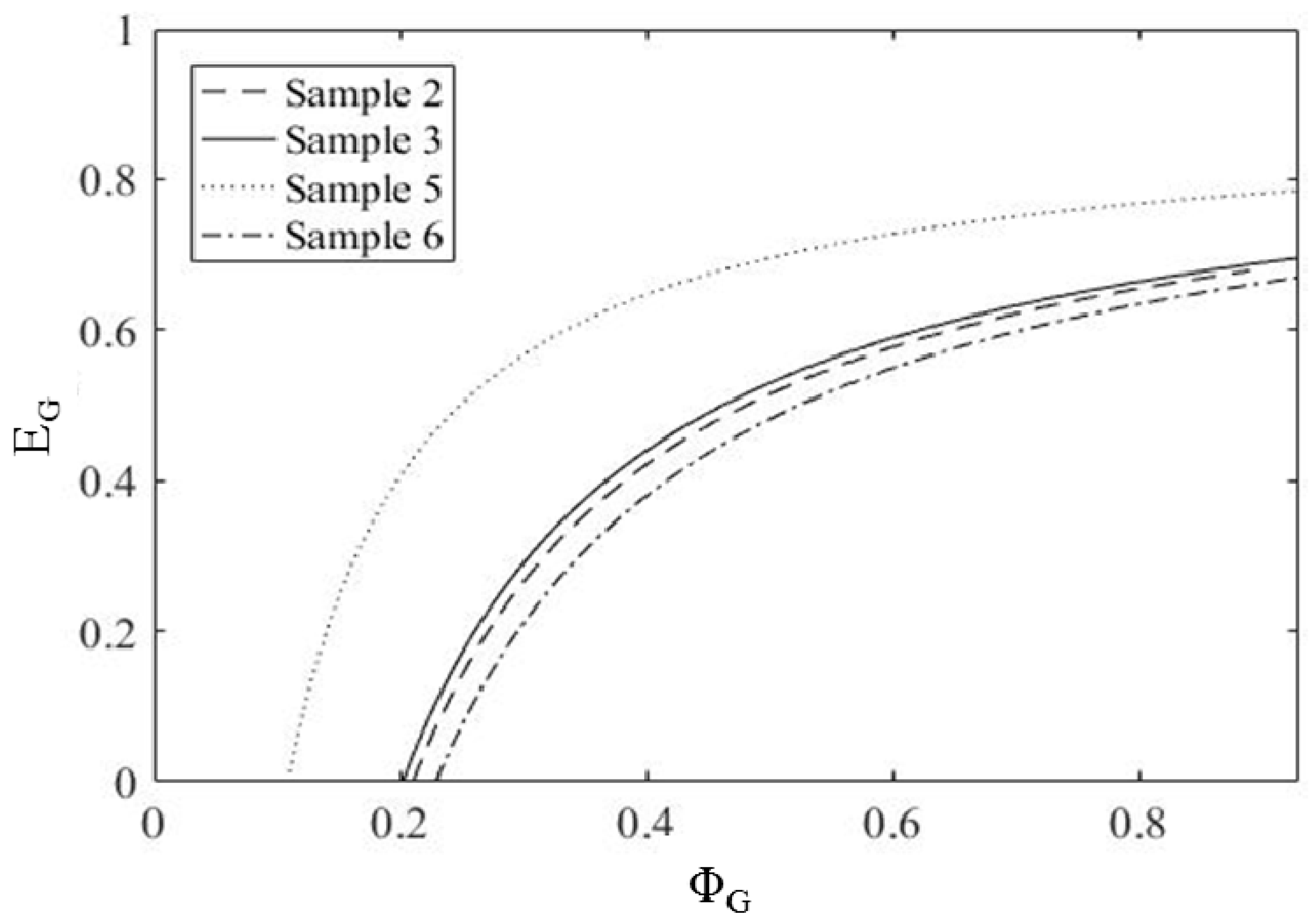
3.5.2. Evaluation of EG and ΦG by a Mathematical Approach
3.5.3. Results of Modeling Approaches
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
Appendix A1. ASTM D638 Standard Test Method for Plastics Tensile Properties
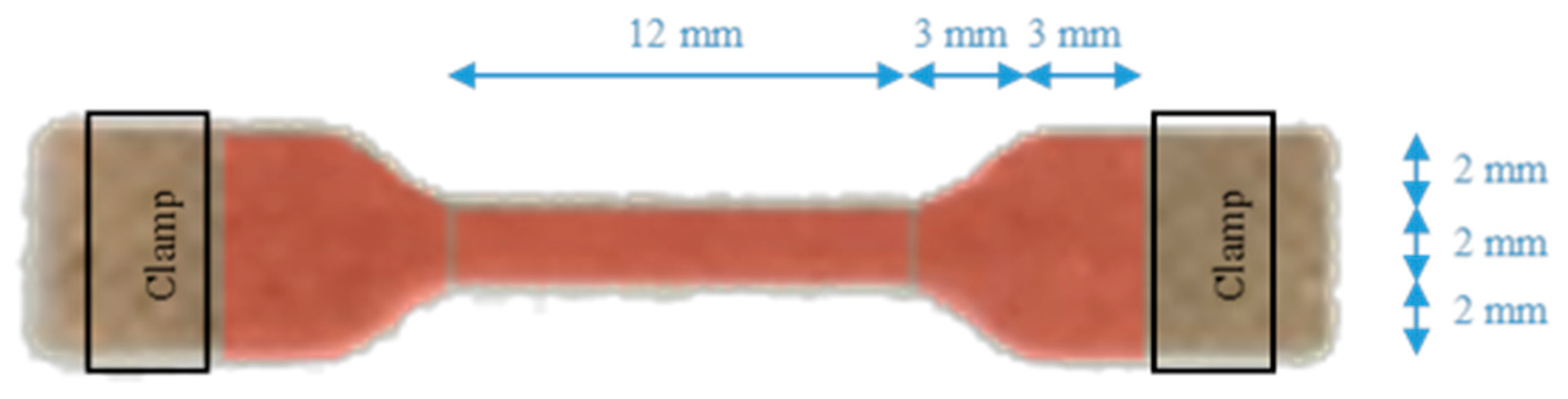
Appendix A2. Realization of Specimens of PHBHV90MIS10 (2.2% DCP)
| Sample | E (Mpa) | σmax (Mpa) | εr (%) |
|---|---|---|---|
| PHBHV90MIS10 (DCP) | 1099 ± 65 | 22 ± 2.0 | 7 ± 1.0 |

References
- Mohanty, A.K.; Misra, M.; Drzal, L.T. Natural Fibers, Biopolymers, and Biocomposites; CRC Press: New York, NY, USA, 2005. [Google Scholar]
- Mohanty, A.; Misra, M.; Hinrichsen, G. Biofibers, biodegradable polymers and biocomposites: An overview. Macromol. Mater. Eng. 2000, 276, 1–24. [Google Scholar] [CrossRef]
- Zhang, K.; Mohanty, A.K.; Misra, M. Fully biodegradable and biorenewable ternary blends from Polylactide, Poly(3-hydroxybutyrate-co-hydroxyvalerate) and Poly(butylene succinate) with balanced properties. ACS Appl. Mater. Interfaces 2012, 4, 3091–3101. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, T.; Mohanty, S.; Nayak, S.K. A review of the recent developments in biocomposites based on natural fibres and their application perspectives. Compos. Part A Appl. Sci. Manuf. 2015, 77, 1–25. [Google Scholar] [CrossRef]
- Somleva, M.N.; Peoples, O.P.; Snell, K.D. PHA bioplastics, biochemicals, and energy from crops. Plant Biotechnol. J. 2013, 11, 233–252. [Google Scholar] [CrossRef] [PubMed]
- Laycock, B.; Halley, P.; Pratt, S.; Werker, A.; Lant, P. The chemomechanical properties of microbial polyhydroxyalkanoates. Progress Polym. Sci. 2014, 39, 397–442. [Google Scholar] [CrossRef]
- Kai, D.; Loh, X.J. Polyhydroxyalkanoates: Chemical modifications toward biomedical applications. ACS Sustain. Chem. Eng. 2013, 2, 106–119. [Google Scholar] [CrossRef]
- Chen, G.-Q.; Wu, Q. The application of polyhydroxyalkanoates as tissue engineering materials. Biomaterials 2005, 26, 6565–6578. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-Q.; Patel, M.K. Plastics derived from biological sources: Present and future: A technical and environmental review. Chem. Rev. 2011, 112, 2082–2099. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y. Plastic bacteria? Progress and prospects for polyhydroxyalkanoate production in bacteria. Trends Biotechnol. 1996, 14, 431–438. [Google Scholar] [CrossRef]
- Sudesh, K. Molecular design and biosynthesis of biodegradable polyesters. Polym. Adv. Technol. 2000, 11, 865–872. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. ZnO-reinforced poly (3-hydroxybutyrate-co-3-hydroxyvalerate) bionanocomposites with antimicrobial function for food packaging. ACS Appl. Mater. Interfaces 2014, 6, 9822–9834. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, T.; Mussone, P.; Khalil, H.; Bressler, D. Progress in bio-based plastics and plasticizing modifications. J. Mater. Chem. A 2013, 1, 13379–13398. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Misra, M.; Drzal, L.T. Sustainable Bio-Composites from Renewable Resources: Opportunities and Challenges in the Green Materials World. J. Polym. Environ. 2002, 10, 19–26. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Mohanty, A.K.; Drzal, L.; Pourboghrat, F.; Misra, M. Renewable resource-based green composites from recycled cellulose fiber and poly (3-hydroxybutyrate-co-3-hydroxyvalerate) bioplastic. Biomacromolecules 2006, 7, 2044–2051. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Mohanty, A.K.; Sugie, T.; Takai, Y.; Hamada, H. Renewable resource based biocomposites from natural fiber and polyhydroxybutyrate-co-valerate (PHBV) bioplastic. Compos. Part A Appl. Sci. Manuf. 2008, 39, 875–886. [Google Scholar] [CrossRef]
- Bledzki, A.; Jaszkiewicz, A. Mechanical performance of biocomposites based on PLA and PHBV reinforced with natural fibres—A comparative study to PP. Compos. Sci. Technol. 2010, 70, 1687–1696. [Google Scholar] [CrossRef]
- Nagarajan, V.; Mohanty, A.K.; Misra, M. Sustainable green composites: Value addition to agricultural residues and perennial grasses. ACS Sustain. Chem. Eng. 2013, 1, 325–333. [Google Scholar] [CrossRef]
- Nagarajan, V.; Misra, M.; Mohanty, A.K. New engineered biocomposites from poly (3-hydroxybutyrate-co-3-hydroxyvalerate)(PHBV)/poly (butylene adipate-co-terephthalate)(PBAT) blends and switchgrass: Fabrication and performance evaluation. Ind. Crops Prod. 2013, 42, 461–468. [Google Scholar] [CrossRef]
- Nyambo, C.; Mohanty, A.K.; Misra, M. Polylactide-based renewable green composites from agricultural residues and their hybrids. Biomacromolecules 2010, 11, 1654–1660. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Chen, F.; Qian, J.; Huang, J.; Wolcott, M.; Liu, L.; Zhang, J. Reinforcing and toughening effects of bamboo pulp fiber on poly (3-hydroxybutyrate-co-3-hydroxyvalerate) fiber composites. Ind. Eng. Chem. Res. 2009, 49, 572–577. [Google Scholar] [CrossRef]
- Ahankari, S.S.; Mohanty, A.K.; Misra, M. Mechanical behaviour of agro-residue reinforced poly(3-hydroxybutyrate-co-3-hydroxyvalerate), (PHBV) green composites: A comparison with traditional polypropylene composites. Compos. Sci. Technol. 2011, 71, 653–657. [Google Scholar] [CrossRef]
- Shibata, M.; Ozawa, K.; Teramoto, N.; Yosomiya, R.; Takeishi, H. Biocomposites made from short abaca fiber and biodegradable polyesters. Macromol. Mater. Eng. 2003, 288, 35–43. [Google Scholar] [CrossRef]
- Wollerdorfer, M.; Bader, H. Influence of natural fibres on the mechanical properties of biodegradable polymers. Ind. Crops Prod. 1998, 8, 105–112. [Google Scholar] [CrossRef]
- Avella, M.; Bogoeva-Gaceva, G.; Bužarovska, A.; Errico, M.E.; Gentile, G.; Grozdanov, A. Poly(lactic acid)-based biocomposites reinforced with kenaf fibers. J. Appl. Polym. Sci. 2008, 108, 3542–3551. [Google Scholar] [CrossRef]
- Bogoeva-Gaceva, G.; Avella, M.; Malinconico, M.; Buzarovska, A.; Grozdanov, A.; Gentile, G.; Errico, M. Natural fiber eco-composites. Polym. Compos. 2007, 28, 98–107. [Google Scholar] [CrossRef]
- Faruk, O.; Bledzki, A.K.; Fink, H.-P.; Sain, M. Biocomposites reinforced with natural fibers: 2000–2010. Progress Polym. Sci. 2012, 37, 1552–1596. [Google Scholar] [CrossRef]
- Murnen, H.K.; Balan, V.; Chundawat, S.P.; Bals, B.; Sousa, L.D.C.; Dale, B.E. Optimization of ammonia fiber expansion (AFEX) pretreatment and enzymatic hydrolysis of Miscanthus x giganteus to fermentable sugars. Biotechnol. Progress 2007, 23, 846–850. [Google Scholar] [CrossRef] [PubMed]
- Fischer, G.; Prieler, S.; van Velthuizen, H. Biomass potentials of miscanthus, willow and poplar: Results and policy implications for Eastern Europe, Northern and Central Asia. Biomass Bioenergy 2005, 28, 119–132. [Google Scholar] [CrossRef]
- Collura, S.; Azambre, B.; Weber, J.-V. Thermal behaviour of Miscanthus grasses, an alternative biological fuel. Environ. Chem. Lett. 2007, 5, 49. [Google Scholar] [CrossRef]
- Dohleman, F.G.; Long, S.P. More Productive Than Maize in the Midwest: How Does Miscanthus Do It? Plant Physiol. 2009, 150, 2104–2115. [Google Scholar] [CrossRef] [PubMed]
- Widholm, J.; Zabotina, O.; McIsaac, G.; David, M.; Voigt, T.; Boersma, N.; Long, S. Miscanthus: A promising biomass crop. Adv. Bot. Res. 2010, 56, 75–137. [Google Scholar]
- Beale, C.V.; Long, S.P. Can perennial C4 grasses attain high efficiencies of radiant energy conversion in cool climates? Plant Cell Environ. 1995, 18, 641–650. [Google Scholar] [CrossRef]
- Kaack, K.; Schwarz, K.-U. Morphological and mechanical properties of Miscanthus in relation to harvesting, lodging, and growth conditions. Ind. Crops Prod. 2001, 14, 145–154. [Google Scholar] [CrossRef]
- Park, H.-J.; Oh, S.-W.; Wen, M.-Y. Manufacture and properties of Miscanthus–wood particle composite boards. J. Wood Sci. 2012, 58, 459–464. [Google Scholar] [CrossRef]
- Heaton, E.A.; Dohleman, F.G.; Long, S.P. Meeting US biofuel goals with less land: The potential of Miscanthus. Glob. Chang. Biol. 2008, 14, 2000–2014. [Google Scholar] [CrossRef]
- Bourmaud, A.; Pimbert, S. Investigations on mechanical properties of poly (propylene) and poly (lactic acid) reinforced by miscanthus fibers. Compos. Part A Appl. Sci. Manuf. 2008, 39, 1444–1454. [Google Scholar] [CrossRef]
- Avella, M.; Martuscelli, E.; Raimo, M. Review Properties of blends and composites based on poly(3-hydroxy)butyrate (PHB) and poly(3-hydroxybutyrate-hydroxyvalerate) (PHBV) copolymers. J. Mater. Sci. 2000, 35, 523–545. [Google Scholar] [CrossRef]
- Raj, R.; Kokta, B.; Maldas, D.; Daneault, C. Use of wood fibers in thermoplastic composites: VI. Isocyanate as a bonding agent for polyethylene–wood fiber composites. Polym. Compos. 1988, 9, 404–411. [Google Scholar] [CrossRef]
- Joseph, K.; Thomas, S.; Pavithran, C. Effect of chemical treatment on the tensile properties of short sisal fibre-reinforced polyethylene composites. Polymer 1996, 37, 5139–5149. [Google Scholar] [CrossRef]
- Wong, S.; Shanks, R.; Hodzic, A. Interfacial improvements in poly(3-hydroxybutyrate)-flax fibre composites with hydrogen bonding additives. Compos. Sci. Technol. 2004, 64, 1321–1330. [Google Scholar] [CrossRef]
- Lee, S.G.; Choi, S.S.; Park, W.H.; Cho, D. Characterization of surface modified flax fibers and their biocomposites with PHB. Macromol. Symp. 2003, 197, 89–100. [Google Scholar] [CrossRef]
- Kusumi, R.; Inoue, Y.; Shirakawa, M.; Miyashita, Y.; Nishio, Y. Cellulose alkyl ester/poly (ε-caprolactone) blends: Characterization of miscibility and crystallization behaviour. Cellulose 2008, 15, 1–16. [Google Scholar] [CrossRef]
- Wong, S.; Shanks, R.; Hodzic, A. Properties of Poly (3-hydroxybutyric acid) Composites with Flax Fibres Modified by Plasticiser Absorption. Macromol. Mater. Eng. 2002, 287, 647–655. [Google Scholar] [CrossRef]
- Carlmark, A.; Malmström, E.E. ATRP grafting from cellulose fibers to create block-copolymer grafts. Biomacromolecules 2003, 4, 1740–1745. [Google Scholar] [CrossRef] [PubMed]
- Lönnberg, H.; Zhou, Q.; Brumer, H.; Teeri, T.T.; Malmström, E.; Hult, A. Grafting of cellulose fibers with poly (ε-caprolactone) and poly (l-lactic acid) via ring-opening polymerization. Biomacromolecules 2006, 7, 2178–2185. [Google Scholar] [CrossRef] [PubMed]
- Samain, X.; Langlois, V.; Renard, E.; Lorang, G. Grafting biodegradable polyesters onto cellulose. J. Appl. Polym. Sci. 2011, 121, 1183–1192. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Drzal, L.T.; Desai, S.M.; Misra, M.; Mulukutla, P. Polyhydroxyalcanoates D’anhydride Fonctionnalises, Preparation et Utilisation Associees. Patent No. WO2005078018A1, 25 August 2005. [Google Scholar]
- Srubar, W.V.; Pilla, S.; Wright, Z.C.; Ryan, C.A.; Greene, J.P.; Frank, C.W.; Billington, S.L. Mechanisms and impact of fiber–matrix compatibilization techniques on the material characterization of PHBV/oak wood flour engineered biobased composites. Compos. Sci. Technol. 2012, 72, 708–715. [Google Scholar] [CrossRef]
- Wei, L.; McDonald, A.G.; Stark, N.M. Grafting of bacterial polyhydroxybutyrate (PHB) onto cellulose via in situ reactive extrusion with dicumyl peroxide. Biomacromolecules 2015, 16, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Cao, J.; McDonald, A.G. Interfacial Improvements in a Green Biopolymer Alloy of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and Lignin via in Situ Reactive Extrusion. ACS Sustain. Chem. Eng. 2016, 4, 3465–3476. [Google Scholar] [CrossRef]
- Rodi, E.G.; Mangeon, C.; Dessauw, E.; Sansalone, V.; Lemaire, T.; Renard, E.; Langlois, V. Functionalization of Miscanthus by Photoactivated Thiol−Ene Addition to Improve Interfacial Adhesion with Polycaprolactone. ACS Sustain. Chem. Eng. 2016, 4, 5475–5482. [Google Scholar] [CrossRef]
- Sapieha, S.; Allard, P.; Zang, Y. Dicumyl peroxide-modified cellulose/LLDPE composites. J. Appl. Polym. Sci. 1990, 41, 2039–2048. [Google Scholar] [CrossRef]
- Suwanda, D.; Balke, S. The reactive modification of polyethylene. II: Mathematical modeling. Polym. Eng. Sci. 1993, 33, 1592–1605. [Google Scholar] [CrossRef]
- Pedernera, M.N.; Sarmoria, C.; Vallés, E.M.; Brandolin, A. An improved kinetic model for the peroxide initiated modification of polyethylene. Polym. Eng. Sci. 1999, 39, 2085–2095. [Google Scholar] [CrossRef]
- Asteasuain, M.; Sarmoria, C.; Brandolin, A. Peroxide modification of polyethylene. Prediction of molecular weight distributions by probability generating functions. Polymer 2002, 43, 2363–2373. [Google Scholar] [CrossRef]
- Brandolin, A.; Sarmoria, C.; Failla, M.D.; Vallés, E.M. Mathematical Modeling of the Reactive Modification of High-Density Polyethylene. Effect of Vinyl Content. Ind. Eng. Chem. Res. 2007, 46, 7561–7570. [Google Scholar] [CrossRef]
- Gloor, P.; Tang, Y.; Kostanska, A.; Hamielec, A. Chemical modification of polyolefins by free radical mechanisms: A modelling and experimental study of simultaneous random scission, branching and crosslinking. Polymer 1994, 35, 1012–1030. [Google Scholar] [CrossRef]
- Tobita, H. Simulation model for the modification of polymers via crosslinking and degradation. Polymer 1995, 36, 2585–2596. [Google Scholar] [CrossRef]
- Zhu, S. Molecular weight distribution in free-radical polymer modification with cross-linking: Effect of chain-length-dependent termination. Macromolecules 1996, 29, 456–461. [Google Scholar] [CrossRef]
- Johnston, R.T. Modelling peroxide cross-linking in polyolefins. In Proceedings of the SPE ANTEC, San Francisco, CA, USA, 5–9 May 2002. [Google Scholar]
- Berthet, M.A.; Gontard, N.; Angellier-Coussy, H. Impact of fibre moisture content on the structure/mechanical properties relationships of PHBV/wheat straw fibres biocomposites. Compos. Sci. Technol. 2015, 117, 386–391. [Google Scholar] [CrossRef]
- Cyras, V.P.; Iannace, S.; Kenny, J.M.; Vázquez, A. Relationship between processing and properties of biodegradable composites based on PCL/starch matrix and sisal fibers. Polym. Compos. 2001, 22, 104–110. [Google Scholar] [CrossRef]
- Keller, A. Compounding and mechanical properties of biodegradable hemp fibre composites. Compos. Sci. Technol. 2003, 63, 1307–1316. [Google Scholar] [CrossRef]
- Nemat-Nasser, S.; Hori, M. Micromechanics: Overall Properties of Heterogeneous Materials; Elsevier Science Publishers: Amsterdam, The Netherlands, 1993; Volume 37. [Google Scholar]
- Sansalone, V.; Naili, S.; Lemaire, T. Nanostructure and effective elastic properties of bone fibril. Bioinspir. Biomim. Nanobiomater. 2012, 1, 154–165. [Google Scholar] [CrossRef]
- Benveniste, Y. A new approach to the application of Mori-Tanaka’s theory in composite materials. Mech. Mater. 1987, 6, 147–157. [Google Scholar] [CrossRef]
- Eshelby, J.D. The determination of the elastic field of an ellipsoidal inclusion, and related problems. Proc. R. Soc. Lond. A Math. Phys. Eng. Sci. 1957, 241, 376–396. [Google Scholar] [CrossRef]
- Kaack, K.; Schwarz, K.U.; Brander, P.E. Variation in morphology, anatomy and chemistry of stems of Miscanthus genotypes differing in mechanical properties. Ind. Crops Prod. 2003, 17, 131–142. [Google Scholar] [CrossRef]
- Rodi, E.G. Influence de la Modification Chimique de L’interface sur la Dispersion des Renforts Lignocellulosiques Dans les Green Wood Plastic Composites (GWPC): Apport de la Modélisation sur L’optimisation des PROPRIETES Mécaniques. Ph.D. Thesis, Université Paris Est, Champs-sur-Marne, France, 2017. [Google Scholar]








| Sample | PHBHVxMIS1−x | MIS Length | DCP (wt %) |
|---|---|---|---|
| PHBHV100 | - | 0 | |
| 1 | PHBHV95MIS5 | 1 mm | 0 |
| 2 | PHBHV95MIS5 | 1 mm | 0.25 |
| 3 | PHBHV95MIS5 | 1 mm | 2.20 |
| 4 | PHBHV95MIS5 | 45 µm | 0 |
| 5 | PHBHV95MIS5 | 45 µm | 0.25 |
| 6 | PHBHV95MIS5 | 45 µm | 2.20 |
| 7 | PHBHV80MIS20 | 1 mm | 0 |
| 8 | PHBHV80MIS20 | 1 mm | 2.20 |
| 9 | PHBHV80MIS20 | 45 µm | 0 |
| 10 | PHBHV80MIS20 | 45 µm | 2.20 |
| Sample | PHBHVxMIS1−x | DCP (wt %) | |
|---|---|---|---|
| MIS100 | -- | 1.3 ± 0.01 | |
| 1 | PHBHV95MIS5 | 0 | 1.3 ± 0.01 |
| 2 | PHBHV95MIS5 | 0.25 | 1.6 ± 0.02 |
| 3 | PHBHV95MIS5 | 2.20 | 3.4 ± 0.04 |
| Sample | PHBHVxMIS1-x | MIS Length | DCP (wt %) | CI |
|---|---|---|---|---|
| PHBHV100 | 0 | 1.07 ± 0.1 | ||
| 1 | PHBHV95MIS5 | 1 mm | 0 | 1.06 ± 0.2 |
| 3 | PHBHV95MIS5 | 1 mm | 2.2 | 0.98 ± 0.1 |
| 4 | PHBHV95MIS5 | 45 μm | 0 | 1.00 ± 0.1 |
| 6 | PHBHV95MIS5 | 45 μm | 2.2 | 0.95 ± 0.3 |
| 7 | PHBHV80MIS20 | 1 mm | 0 | 1.00 ± 0.1 |
| 8 | PHBHV80MIS20 | 1 mm | 2.2 | 0.94 ± 0.2 |
| Sample | PHBHVxMIS1−x | MIS Length | DCP (wt %) | E (MPa) | σmax (MPa) | εr (%) |
|---|---|---|---|---|---|---|
| PHBHV100 | - | - | 889 ± 41 | 23.0 ± 0.5 | 11 ± 1.60 | |
| 1 | PHBHV95MIS5 | 1 mm | - | 1074 ± 44 | 17.0 ± 1.7 | 4.5 ± 0.89 |
| 2 | PHBHV95MIS5 | 1 mm | 0.25 | 1126 ± 37 | 18.0 ± 0.7 | 4.1 ± 0.43 |
| 3 | PHBHV95MIS5 | 1 mm | 2.2 | 962 ± 19 | 20.0 ± 0.9 | 5.9 ± 0.74 |
| 4 | PHBHV95MIS5 | 45 μm | - | 1021 ± 20 | 18.0 ± 0.1 | 6.3 ± 0.27 |
| 5 | PHBHV95MIS5 | 45 μm | 0.25 | 1045 ± 62 | 20.0 ± 1.0 | 5.3 ± 0.60 |
| 6 | PHBHV95MIS5 | 45 μm | 2.2 | 938 ± 55 | 22.1 ± 0.4 | 8.3 ± 1.09 |
| 7 | PHBHV80MIS20 | 1 mm | - | 1267 ± 90 | 15.8 ± 0.7 | 3.9 ± 0.22 |
| 8 | PHBHV80MIS20 | 1 mm | 2.2 | 1358 ± 52 | 22.0 ± 1.0 | 4.8 ± 0.46 |
| 9 | PHBHV80MIS20 | 45 μm | - | 1525 ± 84 | 17.0 ± 0.7 | 3.1 ± 0.30 |
| 10 | PHBHV80MIS20 | 45 μm | 2.2 | - | - | - |
| Sample | PHBHVxMIS1−x | DCP (wt %) | MIS Length | TM1 (°C) | TM2 (°C) | ΔHM (J/g) | Xc (%) |
|---|---|---|---|---|---|---|---|
| PHBHV | - | - | 140 | 156 | 46 | 31 | |
| 1 | PHBHV95MIS5 | - | 1 mm | 140 | 154 | 45 | 32 |
| 3 | PHBHV95MIS5 | 2.2 | 1 mm | 137 | 154 | 44 | 32 |
| 4 | PHBHV95MIS5 | - | 45 μm | 140 | 159 | 48 | 35 |
| 6 | PHBHV95MIS5 | 2.2 | 45 μm | 138 | 156 | 43 | 31 |
| 7 | PHBHV80MIS20 | - | 1 mm | 141 | 155 | 37 | 31 |
| 8 | PHBHV80MIS20 | 2.2 | 1 mm | 133 | 148 | 40 | 31 |
| Constituents | Young‘s Modulus (Gpa) | Volumetric Fraction (%) |
|---|---|---|
| Miscanthus giganteus | 4.5 (a) | 0.22 |
| PHBHV | 0.889 (b) | 0.60 |
| Gel | 0.782 (b) | 0.18 (c) |
| Sample | MT Model 3 Phases | ROM | Experimental | |
|---|---|---|---|---|
| EMT_cyl (Mpa) | EMTMT_sph (Mpa) | EROM (Mpa) | Eexp (Mpa) | |
| 8 | 1655 | 1170 | 1655 | 1358 ± 52 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodi, E.G.; Langlois, V.; Renard, E.; Sansalone, V.; Lemaire, T. Biocomposites Based on Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate) (PHBHV) and Miscanthus giganteus Fibers with Improved Fiber/Matrix Interface. Polymers 2018, 10, 509. https://doi.org/10.3390/polym10050509
Rodi EG, Langlois V, Renard E, Sansalone V, Lemaire T. Biocomposites Based on Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate) (PHBHV) and Miscanthus giganteus Fibers with Improved Fiber/Matrix Interface. Polymers. 2018; 10(5):509. https://doi.org/10.3390/polym10050509
Chicago/Turabian StyleRodi, Erica Gea, Valérie Langlois, Estelle Renard, Vittorio Sansalone, and Thibault Lemaire. 2018. "Biocomposites Based on Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate) (PHBHV) and Miscanthus giganteus Fibers with Improved Fiber/Matrix Interface" Polymers 10, no. 5: 509. https://doi.org/10.3390/polym10050509