In-Situ Incorporation of Alkyl-Grafted Silica into Waterborne Polyurethane with High Solid Content for Enhanced Physical Properties of Coatings
Abstract
:1. Introduction
2. Experiments
2.1. Materials
2.2. Preparation of Waterborne Polyurethane/Silica (WPU/Silica) Hybrid Dispersions with High Solid Content
2.3. Characterization
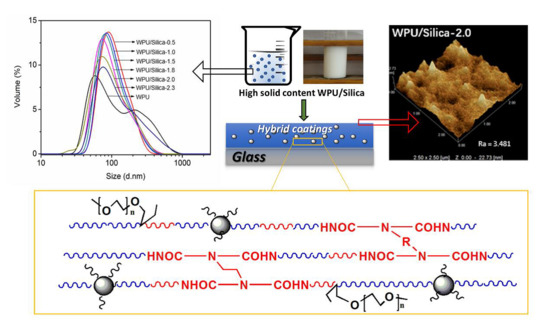
3. Results and Discussion
3.1. Characterization of the Waterborne Polyurethane/Silica Dispersions (WPU/Silica) with High Solid Content
3.2. Structure and Morphology of WPU/Silica Coatings
3.3. Contact Angle and Surface Tension of WPU/Silica Coatings
3.4. Solvent Resistant Properties
3.5. Thermal Properties
3.6. Mechanical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Kang, S.Y.; Ji, Z.; Tseng, L.F.; Turner, S.A.; Villanueva, D.A.; Johnson, R.; Albano, A.; Langer, R. Design and synthesis of waterborne polyurethanes. Adv. Mater. 2018. [Google Scholar] [CrossRef] [PubMed]
- Noreen, A.; Zia, K.M.; Zuber, M.; Tabasum, S.; Saif, M.J. Recent trends in environmentally friendly water-borne polyurethane coatings: A review. Korean J. Chem. Eng. 2016, 33, 388–400. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, R.; Zhang, C.; Gong, S. Preparation and properties of high solid content and low viscosity waterborne polyurethane—Acrylate emulsion with a reactive emulsifier. Polymers 2018, 10, 154. [Google Scholar] [CrossRef]
- Lijie, H.; Yongtao, D.; Zhiliang, Z.; Zhongsheng, S.; Zhihua, S. Synergistic effect of anionic and nonionic monomers on the synthesis of high solid content waterborne polyurethane. Colloids Surf. A 2015, 467, 46–56. [Google Scholar] [CrossRef]
- Peng, S.-J.; Jin, Y.; Cheng, X.-F.; Sun, T.-B.; Qi, R.; Fan, B.-Z. A new method to synthesize high solid content waterborne polyurethanes by strict control of bimodal particle size distribution. Prog. Org. Coat. 2015, 86, 1–10. [Google Scholar] [CrossRef]
- Zhang, F.; Wei, X. Study of ionic/nonionic polyurethane dispersions with high solid content and low viscosity using a complex hydrophilic chain-extending agent. J. Coat. Technol. Res. 2018, 15, 141–148. [Google Scholar] [CrossRef]
- Lee, S.; Kim, B. High solid and high stability waterborne polyurethanes via ionic groups in soft segments and chain termini. J. Colloid Interface Sci. 2009, 336, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Saba, N.; Tahir, P.M.; Jawaid, M. A review on potentiality of nano filler/natural fiber filled polymer hybrid composites. Polymers 2014, 6, 2247–2273. [Google Scholar] [CrossRef]
- Malaki, M.; Hashemzadeh, Y.; Karevan, M. Effect of nano-silica on the mechanical properties of acrylic polyurethane coatings. Prog. Org. Coat. 2016, 101, 477–485. [Google Scholar] [CrossRef]
- Fan, W.; Du, W.; Li, Z.; Dan, N.; Huang, J. Abrasion resistance of waterborne polyurethane films incorporated with pu/silica hybrids. Prog. Org. Coat. 2015, 86, 125–133. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Jia, Z.; Luo, Y.; Jia, D. Use of precipitated silica with silanol groups as an inorganic chain extender in polyurethane. Mater. Des. 2015, 87, 324–330. [Google Scholar] [CrossRef]
- Yu, F.; Huang, H.-X. Simultaneously toughening and reinforcing poly(lactic acid)/thermoplastic polyurethane blend via enhancing interfacial adhesion by hydrophobic silica nanoparticles. Polym. Test. 2015, 45, 107–113. [Google Scholar] [CrossRef]
- Verdolotti, L.; Lavorgna, M.; Lamanna, R.; Di Maio, E.; Iannace, S. Polyurethane-silica hybrid foam by sol-gel approach: Chemical and functional properties. Polymer 2015, 56, 20–28. [Google Scholar] [CrossRef]
- Ding, J.; Ye, L. Structure and property of polyurethane/Sio2 composite elastomer prepared via in situ polymerization. J. Appl. Polym. Sci. 2013, 127, 2732–2738. [Google Scholar] [CrossRef]
- Han, Y.; Chen, Z.; Dong, W.; Xin, Z. Improved water resistance, thermal stability, and mechanical properties of waterborne polyurethane nanohybrids reinforced by fumed silica via in situ polymerization. High Perform. Polym. 2015, 27, 824–832. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, H.; Tian, X.; Xue, M.; Ding, X.; Ye, X.; Cui, Z. Surface and mechanical properties of hydrophobic silica contained hybrid films of waterborne polyurethane and fluorinated polymethacrylate. Polymer 2014, 55, 187–194. [Google Scholar] [CrossRef]
- Khani, M.M.; Woo, D.; Mumpower, E.L.; Benicewicz, B.C. Poly(alkyl methacrylate)-grafted silica nanoparticles in polyethylene nanocomposites. Polymer 2017, 109, 339–348. [Google Scholar] [CrossRef]
- Meier-Westhues, U. Polyurethanes: Coatings, Adhesives and Sealants; Vincentz Network GmbH & Co KG: Hemer, Germany, 2007. [Google Scholar]
- García-Pacios, V.; Jofre-Reche, J.A.; Costa, V.; Colera, M.; Martín-Martínez, J.M. Coatings prepared from waterborne polyurethane dispersions obtained with polycarbonates of 1,6-hexanediol of different molecular weights. Prog. Org. Coat. 2013, 76, 1484–1493. [Google Scholar] [CrossRef]
- Kim, B.; Yang, J.; Yoo, S.; Lee, J. Waterborne polyurethanes containing ionic groups in soft segments. Colloid Polym. Sci. 2003, 281, 461–468. [Google Scholar] [CrossRef]
- Radovsky, B.; Gokhman, L.; Gurary, E.; Dukhovnyy, B. Use of moony formula for the determination of critical concentration of structuring of disperse systems of bitumen type. Colloid J. Acad. Sci. USSR 1979, 4, 729. [Google Scholar]
- Chen, X.; Jiang, J.; Yan, F.; Tian, S.; Li, K. A novel low temperature vapor phase hydrolysis method for the production of nano-structured silica materials using silicon tetrachloride. RSC Adv. 2014, 4, 8703. [Google Scholar] [CrossRef]
- Jia-Hu, G.; Yu-Cun, L.; Tao, C.; Su-Ming, J.; Hui, M.; Ning, Q.; Hua, Z.; Tao, Y.; Wei-Ming, H. Synthesis and properties of a nano-silica modified environmentally friendly polyurethane adhesive. RSC Adv. 2015, 5, 4990–4997. [Google Scholar] [CrossRef]
- Lin, J.; Wu, X.; Zheng, C.; Zhang, P.; Huang, B.; Guo, N.; Jin, L. Synthesis and properties of epoxy-polyurethane/silica. Appl. Surf. Sci. 2014, 303, 67–75. [Google Scholar] [CrossRef]
- Sadeghi, M.; Semsarzadeh, M.A.; Barikani, M.; Chenar, M.P. Gas separation properties of polyether-based polyurethane-silica nanocomposite membranes. J. Membr. Sci. 2011, 376, 188–195. [Google Scholar] [CrossRef]
- Liu, F.; Miao, L.; Wang, Y.; Xue, X.; Yang, H. Green fabrication of ultraviolet curable epoxy acrylate-silica hybrid coatings. Prog. Org. Coat. 2017, 109, 38–44. [Google Scholar] [CrossRef]
- Su, C. Facile fabrication of a lotus-effect composite coating via wrapping silica with polyurethane. Appl. Surf. Sci. 2010, 256, 2122–2127. [Google Scholar] [CrossRef]
- Quéré, D. Wetting and roughness. Annu. Rev. Mater. Res. 2008, 38, 71–99. [Google Scholar] [CrossRef]
- Kaelble, D.H. Physical Chemistry of Adhesion; Wiley-Interscience: Hoboken, NJ, USA, 1971. [Google Scholar]
- Ma, X.-Y.; Zhang, W.-D. Effects of flower-like zno nanowhiskers on the mechanical, thermal and antibacterial properties of waterborne polyurethane. Polym. Degrad. Stab. 2009, 94, 1103–1109. [Google Scholar] [CrossRef]
- Wu, C.L.; Zhang, M.Q.; Rong, M.Z.; Friedrich, K. Silica nanoparticles filled polypropylene: Effects of particle surface treatment, matrix ductility and particle species on mechanical performance of the composites. Compos. Sci. Technol. 2005, 65, 635–645. [Google Scholar] [CrossRef]
- Thirumal, M.; Khastgir, D.; Singha, N.K.; Manjunath, B.; Naik, Y. Mechanical, morphological and thermal properties of rigid polyurethane foam: Effect of the fillers. Cell. Polym. 2007, 26, 245. [Google Scholar]
- Karimi, M.B.; Hassanajili, S. Short fiber/polyurethane composite membrane for gas separation. J. Membr. Sci. 2017, 543, 40–48. [Google Scholar] [CrossRef]
- Swain, S.; Sharma, R.A.; Bhattacharya, S.; Chaudhary, L. Effects of nano-silica/nano-alumina on mechanical and physical properties of polyurethane composites and coatings. Trans. Electr. Electron. Mater. 2013, 14, 1–8. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Song, L.; Yang, H.; Xing, W.; Lu, H. In situ polymerization of graphene nanosheets and polyurethane with enhanced mechanical and thermal properties. J. Mater. Chem. 2011, 21, 4222–4227. [Google Scholar] [CrossRef]
- Hassanajili, S.; Khademi, M.; Keshavarz, P. Influence of various types of silica nanoparticles on permeation properties of polyurethane/silica mixed matrix membranes. J. Membr. Sci. 2014, 453, 369–383. [Google Scholar] [CrossRef]











| Sample | Surface Free Energy (mJ/m2) | ||
|---|---|---|---|
| γd | γp | γ | |
| WPU | 5.23 ± 0.88 | 27.64 ± 4.43 | 32.87 ± 3.55 |
| WPU/Silica-1.0-B | 9.84 ± 0.51 | 16.80 ± 0.76 | 26.64 ± 0.78 |
| WPU/Silica-1.0 | 9.57 ± 2.16 | 15.70 ± 4.68 | 25.27 ± 2.52 |
| WPU/Silica-2.0 | 12.17 ± 0.93 | 9.37 ± 1.01 | 21.54 ± 1.02 |
| Samples | Temperature (°C) | |||
|---|---|---|---|---|
| 30% Weight Loss | 50% Weight Loss | Tmax1 | Tmax2 | |
| WPU | 326.3 | 361.8 | 315.8 | 393.2 |
| WPU/Silica-0.5 | 331.0 | 370.1 | 319.8 | 404.0 |
| WPU/Silica-1.0 | 334.8 | 383.4 | 320.0 | 413.2 |
| WPU/Silica-1.5 | 339.5 | 386.3 | 321.8 | 416.5 |
| WPU/Silica-2.0 | 339.5 | 388.2 | 322.6 | 419.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Hu, J.; Xin, Z. In-Situ Incorporation of Alkyl-Grafted Silica into Waterborne Polyurethane with High Solid Content for Enhanced Physical Properties of Coatings. Polymers 2018, 10, 514. https://doi.org/10.3390/polym10050514
Han Y, Hu J, Xin Z. In-Situ Incorporation of Alkyl-Grafted Silica into Waterborne Polyurethane with High Solid Content for Enhanced Physical Properties of Coatings. Polymers. 2018; 10(5):514. https://doi.org/10.3390/polym10050514
Chicago/Turabian StyleHan, Yanting, Jinlian Hu, and Zhongyin Xin. 2018. "In-Situ Incorporation of Alkyl-Grafted Silica into Waterborne Polyurethane with High Solid Content for Enhanced Physical Properties of Coatings" Polymers 10, no. 5: 514. https://doi.org/10.3390/polym10050514