Synthesis of Zirconium-Containing Polyhedral Oligometallasilsesquioxane as an Efficient Thermal Stabilizer for Silicone Rubber
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Zr-POSS
2.3. Preparation of SR/Zr-POSS Nanocomposites
2.4. Characterization
2.4.1. Fourier Transform Infrared Spectrometry (FTIR)
2.4.2. 29Si Nuclear Magnetic Resonance Spectrometry (29Si-NMR)
2.4.3. Gel Permeation Chromatography (GPC)
2.4.4. Transmission Electron Microscopy (TEM)
2.4.5. Scanning Electron Microscope (SEM)
2.4.6. Thermogravimetry Analysis (TGA)
2.4.7. Thermogravimetry-Fourier Transform Infrared Spectrometry (TG-FTIR)
2.4.8. X-ray Photoelectron Spectroscopy (XPS)
2.4.9. Mechanical Tests
2.4.10. Cross-link Density Test
2.4.11. Thermal-Oxidative Aging of the SR/Zr-POSS Nanocomposites
3. Results and Discussions
3.1. Characterization of Zr-POSS
3.2. Morphology of the SR/Zr-POSS Nanocomposites
3.3. Thermal-Oxidative Aging Properties of the SR/Zr-POSS Nanocomposites
3.4. Thermal-Oxidative Aging Mechanism of the SR/Zr-POSS Nanocomposites
3.4.1. Thermal-Oxidative Stability
3.4.2. Thermal-Oxidative Stability
3.4.3. Attenuated Total Reflection (ATR)
3.4.4. XPS
3.4.5. TG-FTIR
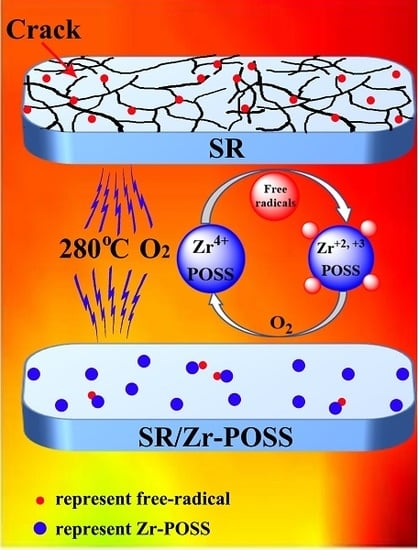
3.4.6. Possible Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boland, C.S.; Khan, U.; Ryan, G.; Barwich, S.; Charifou, R.; Harvey, A.; Backes, C.; Li, Z.L.; Ferreira, M.S.; Möbius, M.E.; et al. Sensitive electromechanical sensors using viscoelastic graphene-polymer nanocomposites. Science 2016, 354, 1257–1260. [Google Scholar] [CrossRef] [PubMed]
- Wåhlander, M.; Nilsson, F.; Andersson, R.L.; Sanchez, C.C.; Taylor, N.; Carlmark, A.; Hillborg, H.; Malmström, E. Tailoring dielectric properties using designed polymer-grafted ZnO nanoparticles in silicone rubber. J. Mater. Chem. A 2017, 5, 14,241–14,258. [Google Scholar] [CrossRef]
- Guo, J.H.; Chen, X.M.; Zhang, Y. Improving the mechanical and electrical properties of ceramizable silicone rubber/halloysite composites and their ceramic residues by incorporation of different borates. Polymers 2018, 10, 388. [Google Scholar] [CrossRef]
- Zhang, Y.; He, J.Y.; Yang, R.J. The effects of phosphorus-based flame retardants and octaphenyl polyhedral oligomeric silsesquioxane on the ablative and flame-retardation properties of room temperature vulcanized silicone rubber insulating composites. Polym. Degrad. Stab. 2016, 125, 140–147. [Google Scholar] [CrossRef]
- Imiela, M.; Anyszka, R.; Bieliński, D.M.; Pędzich, Z.; Zarzecka-Napierała, M.; Szumera, M. Effect of carbon fibers on thermal properties and mechanical strength of ceramizable composites based on silicone rubber. J. Therm. Anal. Calorim. 2016, 124, 197–203. [Google Scholar] [CrossRef]
- Wu, J.; Dong, J.Y.; Wang, Y.S.; Gond, B.K. Thermal oxidation ageing effects on silicone rubber sealing performance. Polym. Degrad. Stab. 2017, 135, 43–53. [Google Scholar] [CrossRef]
- Fei, H.F.; Han, X.J.; Liu, B.Z.; Gao, X.Y.; Wang, Q.; Zhang, Z.J.; Xie, Z.M. Mechanism of the antioxidation effect of α-Fe2O3 on silicone rubbers at high temperature. RSC Adv. 2016, 6, 7717–7722. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Q.; Zheng, J.P. Effect and mechanism of iron oxide modified carbon nanotubes on thermal oxidative stability of silicone rubber. Compos. Sci. Technol. 2014, 99, 1–7. [Google Scholar] [CrossRef]
- Li, H.Y.; Tao, S.; Huang, Y.H.; Su, Z.T.; Zheng, J.P. The improved thermal oxidative stability of silicone rubber by using iron oxide and carbon nanotubes as thermal resistant additives. Compos. Sci. Technol. 2013, 76, 52–60. [Google Scholar] [CrossRef]
- Qiu, X.N.; Cai, H.; Fang, X.; Zheng, J.P. The improved thermal oxidative stability of silicone rubber by incorporating reduced graphene oxide: Impact factors and action mechanism. Polym. Compos. 2018, 39, 1105–1115. [Google Scholar] [CrossRef]
- Bai, L.; Bai, Y.L.; Zheng, J.P. Improving the filler dispersion and performance of silicone rubber/multi-walled carbon nanotube composites by noncovalent functionalization of polymethylphenylsiloxane. J. Mater. Sci. 2017, 52, 7516–7529. [Google Scholar] [CrossRef]
- Chen, D.Z.; Yi, S.P.; Wu, W.B.; Zhong, Y.L.; Liao, J.; Huang, C.; Shi, W.J. Synthesis and characterization of novel room temperature vulcanized RTV) silicone rubbers using Vinyl-POSS derivatives as cross linking agents. Polymer 2010, 51, 3867–3878. [Google Scholar] [CrossRef]
- Chen, D.Z.; Yi, S.P.; Fang, P.F.; Zhong, Y.L.; Huang, C.; Shi, W.J. Synthesis and characterization of novel room temperature vulcanized (RTV) silicone rubbers using octa[(trimethoxysilyl)ethyl]-POSS as cross-linker. React. Funct. Polym. 2011, 74, 502–511. [Google Scholar] [CrossRef]
- Liu, Y.F.; Shi, Y.H.; Zhang, D.; Li, J.L.; Huang, G.S. Preparation and thermal degradation behavior of room temperature vulcanized silicone rubber-g-polyhedral oligomeric silsesquioxanes. Polymer 2013, 54, 6140–6149. [Google Scholar] [CrossRef]
- Hay, M.T.; Seurer, B.; Holmes, D.; Lee, A. A novel linear titanium (IV)-POSS coordination polymer. Macromolecules 2010, 43, 2108–2110. [Google Scholar] [CrossRef]
- Carlos, G.; Manuel, G.; Pilar, G.S.; José, M.H. Monocyclopentadienyl (niobium) compounds with imido and silsesquioxane ligands: synthetic, structural and reactivity studies. Eur. J. Inorg. Chem. 2009, 29, 4401–4415. [Google Scholar]
- Babonneau, F.; Maquet, J. Nuclear magnetic resonance techniques for the structural characterization of siloxane–oxide hybrid materials. Polyhedron 2000, 19, 315–322. [Google Scholar] [CrossRef]
- Kim, Y.H.; Bae, J.Y.; Jin, J.; Bae, B.S. Sol-gel derived transparent zirconium-phenyl siloxane hybrid for robust high refractive index LED encapsulant. ACS Appl. Mater. Interfaces 2014, 6, 3115–3121. [Google Scholar] [CrossRef] [PubMed]
- Tosoni, S.; Chen, H.Y.T.; Pacchioni, G. A DFT study of Ni clusters deposition on titania and zirconia (101) surfaces. Surf. Sci. 2016, 646, 230–238. [Google Scholar] [CrossRef]
- Yin, Z.; Liu, Y.; Liu, Y. Study on preparation of silicon rubber composite and its ablation resistant. Aerosp. Manuf. Technol. 2016, 5, 63–66. [Google Scholar]
- Liu, N.; Wei, K.; Wang, L.; Zheng, S.X. Organic-inorganic polyimides with double decker silsesquioxane in the main chains. Polym. Chem. 2016, 7, 1158–1167. [Google Scholar] [CrossRef]
- Qing, L.Y.; Yan, H.X.; Li, L.; Chen, Z.Y.; Yao, H.H.; Nie, Y.F. Imidazolium ionic liquid modified graphene oxide: as a reinforcing filler and catalyst in epoxy resin. Polymers 2017, 9, 447. [Google Scholar]
- Tang, B.; Dai, W.L.; Sun, X.M.; Wu, G.J.; Guan, N.J.; Hunger, M.; Li, L.D. Mesoporous Zr-Beta zeolites prepared by a post-synthetic strategy as a robust Lewis acid catalyst for the ring-opening aminolysis of epoxides. Green Chem. 2015, 17, 1744–1755. [Google Scholar] [CrossRef]
- Foraita, S.; Liu, Y.; Haller, G.L.; Baráth, E.; Zhao, C.; Lercher, J.A. Controlling hydrodeoxygenation of stearic acid to n-Heptadecane and n-Octadecane by adjusting the chemical properties of Ni/SiO2-ZrO2 catalyst. ChemCatChem 2017, 9, 195–203. [Google Scholar] [CrossRef]
- Min, D.M.; Yan, C.Y.; Huang, Y.; Li, S.T.; Ohki, Y. Dielectric and carrier transport properties of silicone rubber degraded by gamma irradiation. Polymers 2017, 9, 533. [Google Scholar] [CrossRef]
- Hamdani, S.; Longuet, C.; Perrin, D.; Lopez-cuesta, J.; Ganachaud, F. Flame retardancy of silicone-based materials. Polym. Degrad. Stab. 2009, 94, 465–495. [Google Scholar] [CrossRef]
- Jiang, Z.G.; Zhang, J.; Feng, S.Y. Effects of polymethylvinylsilicone oil with side tetraphenylphenyl groups on the radiation resistance of addition-type silicone rubber. J. Appl. Polym. Sci. 2007, 104, 4144–4148. [Google Scholar] [CrossRef]
- Jiang, Z.G.; Feng, S.Y. Progress in studies on radiation effects of polysiloxanes. Prog. Chem. 2005, 17, 1096–1101. [Google Scholar]
- Gu, J.W.; Dong, W.C.; Xu, S.; Tang, Y.S.; Ye, L.; Kong, J. Development of wave-transparent, light-weight composites combined with superior dielectric performance and desirable thermal stabilities. Compos. Sci. Technol. 2017, 144, 185–192. [Google Scholar] [CrossRef]
- Korduban, А.M.; Yashchishyn, I.А.; Konstantinova, Т.Е. X-ray photoelectron spectroscopy of nanopowders of ZrO2-Y2O3-Cr2O3 compounds. Funct. Mater. 2007, 14, 454–455. [Google Scholar]
- Chen, W.J.; Zeng, X.R.; Lai, X.J.; Li, H.Q.; Fang, W.Z.; Hou, F. Suppression effect and mechanism of platinum and nitrogen-containing silane on the tracking and erosion of silicone rubber for high-voltage insulation. ACS Appl. Mater. Interfaces 2016, 8, 21,039–21,045. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.Z.; Zeng, X.R.; Lai, X.J.; Li, H.Q.; Xie, C.X.; Chen, W.J.; Zhang, Y.J. Investigation of the tracking and erosion resistance of cured liquid silicone rubber containing ureido-modified MQ silicone resin. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 3668–3675. [Google Scholar] [CrossRef]
- Wang, Z.; Li, H.Y.; Zheng, J.P. TG-MS study on the effect of multi-walled carbon nanotubes and nano-Fe2O3 on thermo-oxidative stability of silicone rubber. J. Therm. Anal. Calorim. 2016, 126, 733–742. [Google Scholar] [CrossRef]












| Samples | Zr-POSS Content (a phr) | T5 (°C) | T1max (°C) | Tmax (°C) | Rmax (wt %/min) |
|---|---|---|---|---|---|
| SR | 0 | 411.5 | 369.0 | 529.1 | 32.5 |
| SR/Zr-POSS-1 | 1 | 415.3 | 427.1 | 520.1 | 7.00 |
| SR/Zr-POSS-2 | 2 | 423.6 | 398.5 | 512.7 | 6.14 |
| SR/Zr-POSS-3 | 3 | 428.1 | 406.2 | 522.8 | 6.32 |
| SR/Zr-POSS-4 | 4 | 443.2 | 386.7 | 518.5 | 6.34 |
| Samples | 1 Mc | Decreasing Amplitude (%) | |
|---|---|---|---|
| Before Aging | After Aging | ||
| SR | 4338 ± 163 | 1526 ± 134 | 64.8 |
| SR/Zr-POSS-1 | 6985 ± 123 | 5668 ± 147 | 18.8 |
| SR/Zr-POSS-2 | 7597 ± 138 | 6669 ± 129 | 12.2 |
| SR/Zr-POSS-3 | 7616 ± 148 | 7208 ± 152 | 5.4 |
| SR/Zr-POSS-4 | 6845 ± 126 | 6827 ± 135 | 0.3 |
| Samples | Aging | AbsSi–O–Si (1008 cm−1) | AbsSi-CH3 (788 cm−1) | Conservation Rate (%) |
|---|---|---|---|---|
| SR | Before | 0.760 | 0.957 | 69.0 |
| After | 0.763 | 0.663 | ||
| SR/Zr-POSS-1 | Before | 0.738 | 0.930 | 91.2 |
| After | 0.773 | 0.890 | ||
| SR/Zr-POSS-2 | Before | 0.734 | 0.937 | 92.4 |
| After | 0.763 | 0.900 | ||
| SR/Zr-POSS-3 | Before | 0.732 | 0.936 | 93.5 |
| After | 0.638 | 0.763 | ||
| SR/Zr-POSS-4 | Before | 0.714 | 0.910 | 95.0 |
| After | 0.723 | 0.875 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, J.; Lai, X.; Li, H.; Zeng, X.; Zhang, Z. Synthesis of Zirconium-Containing Polyhedral Oligometallasilsesquioxane as an Efficient Thermal Stabilizer for Silicone Rubber. Polymers 2018, 10, 520. https://doi.org/10.3390/polym10050520
Qiu J, Lai X, Li H, Zeng X, Zhang Z. Synthesis of Zirconium-Containing Polyhedral Oligometallasilsesquioxane as an Efficient Thermal Stabilizer for Silicone Rubber. Polymers. 2018; 10(5):520. https://doi.org/10.3390/polym10050520
Chicago/Turabian StyleQiu, Jiedong, Xuejun Lai, Hongqiang Li, Xingrong Zeng, and Zhuopeng Zhang. 2018. "Synthesis of Zirconium-Containing Polyhedral Oligometallasilsesquioxane as an Efficient Thermal Stabilizer for Silicone Rubber" Polymers 10, no. 5: 520. https://doi.org/10.3390/polym10050520
APA StyleQiu, J., Lai, X., Li, H., Zeng, X., & Zhang, Z. (2018). Synthesis of Zirconium-Containing Polyhedral Oligometallasilsesquioxane as an Efficient Thermal Stabilizer for Silicone Rubber. Polymers, 10(5), 520. https://doi.org/10.3390/polym10050520