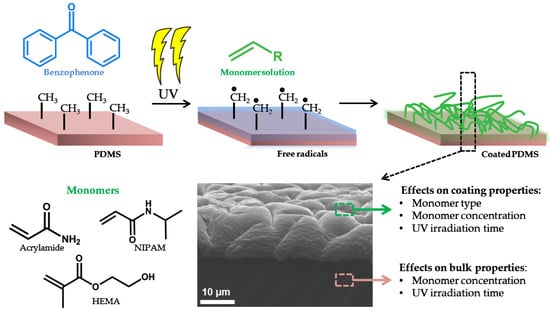
The Relationship between Bulk Silicone and Benzophenone-Initiated Hydrogel Coating Properties
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Modification of PDMS Substrates
2.3. Instrumentation
3. Results and Discussion
3.1. Monomer Type, Concentration, and UV-Irradiation Time Affect Coating Characteristics
3.2. Bulk Material Properties Are Affected by Coating Procedures
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Abbasi, F.; Mirzadeh, H.; Simjoo, M. Hydrophilic interpenetrating polymer networks of poly(dimethyl siloxane) (PDMS) as biomaterial for cochlear implants. J. Biomater. Sci. Polym. Ed. 2006, 17, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chiao, M. Anti-fouling Coatings of Poly(dimethylsiloxane) Devices for Biological and Biomedical Applications. J. Med. Biol. Eng. 2015, 35, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Carman, M.L.; Estes, T.G.; Feinberg, A.W.; Schumacher, J.F.; Wilkerson, W.; Wilson, L.H.; Callow, M.E.; Callow, J.A.; Brennan, A.B. Engineered antifouling microtopographies—Correlating wettability with cell attachment. Biofouling 2006, 22, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Compoint, F.; Fall, D.; Piombini, H.; Belleville, P.; Montouillout, Y.; Duquennoy, M. Sol-gel-processed hybrid silica-PDMS layers for the optics of high-power laser flux systems. J. Mater. Sci. 2016, 51, 5031–5045. [Google Scholar] [CrossRef]
- Srisa-art, M.; Furutani, Y. Simple and Rapid Fabrication of PDMS Microfluidic Devices Compatible with FTIR Microspectroscopy. Bull. Chem. Soc. Jpn. 2016, 89, 195–202. [Google Scholar] [CrossRef]
- Hu, S.; Ren, X.; Bachman, M.; Sims, C.E.; Li, G.P.; Allbritton, N.L. Tailoring the Surface Properties of Poly(dimethylsiloxane) Microfluidic Devices. Langmuir 2004, 20, 5569–5574. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.; Candler, R.N. Non-planar PDMS microfluidic channels and actuators: A review. Lab Chip 2017, 17, 3948–3959. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Gao, H.; Guan, Z.; Wang, L.; Yang, J. Study on hydrophobicity transfer of RTV coatings based on a modification of absorption and cohesion theory. IEEE Trans. Dielectr. Electr. Insul. 2006, 13, 1317–1324. [Google Scholar] [CrossRef]
- Ferreira, P.; Carvalho, A.; Correia, T.R.; Antunes, B.P.; Correia, I.J.; Alves, P. Functionalization of polydimethylsiloxane membranes to be used in the production of voice prostheses. Sci. Technol. Adv. Mater. 2013, 14, 55006–55013. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; Kim, J.K.; Jeong, O.C. Measurement of nonlinear mechanical properties of PDMS elastomer. Microelectron. Eng. 2011, 88, 1982–1985. [Google Scholar] [CrossRef]
- Seo, J.; Lee, L.P. Effects on wettability by surfactant accumulation/depletion in bulk polydimethylsiloxane (PDMS). Sens. Actuators B Chem. 2006, 119, 192–198. [Google Scholar] [CrossRef]
- Ebara, M.; Hoffman, J.M.; Stayton, P.S.; Hoffman, A.S. Surface modification of microfluidic channels by UV-mediated graft polymerization of non-fouling and ‘smart’ polymers. Radiat. Phys. Chem. 2007, 76, 1409–1413. [Google Scholar] [CrossRef]
- Makamba, H.; Kim, J.H.; Lim, K.; Park, N.; Hahn, J.H. Surface modification of poly(dimethylsiloxane) microchannels. Electrophoresis 2003, 24, 3607–3619. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, K.; Miyoshi, H.; Fujii, S.; Hirai, T.; Takahara, A.; Nakao, A.; Iwasaki, Y.; Morigaki, K.; Ishihara, K.; Yusa, S.-I. Poly(dimethylsiloxane) (PDMS) surface patterning by biocompatible photo-crosslinking block copolymers. RSC Adv. 2015, 5, 46686–46693. [Google Scholar] [CrossRef]
- Xiao, D.; Le, T.V.; Wirth, M.J. Surface Modification of the Channels of Poly(dimethylsiloxane) Microfluidic Chips with Polyacrylamide for Fast Electrophoretic Separations of Proteins. Anal. Chem. 2004, 76, 2055–2061. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Ziats, N.P.; Azeez, A.; Brunstedt, M.R.; Stack, S.; Bonfield, T.L. Protein adsorption and macrophage activation on polydimethylsiloxane and silicone rubber. J. Biomater. Sci. Polym. Ed. 1996, 7, 159–169. [Google Scholar] [CrossRef]
- Lee, S.; Vörös, J. An Aqueous-Based Surface Modification of Poly(dimethylsiloxane) with Poly(ethylene glycol) to Prevent Biofouling. Langmuir 2005, 21, 11957–11962. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Brook, M.A.; Sheardown, H. Silicone elastomers for reduced protein adsorption. Biomaterials 2004, 25, 2273–2282. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Brook, M.A.; Chen, Y.; Sheardown, H. Surface properties of PEO–silicone composites: Reducing protein adsorption. J. Biomater. Sci. Polym. Ed. 2005, 16, 531–548. [Google Scholar] [CrossRef] [PubMed]
- Efimenko, K.; Wallace, W.E.; Genzer, J. Surface Modification of Sylgard-184 Poly(dimethyl siloxane) Networks by Ultraviolet and Ultraviolet/Ozone Treatment. J. Colloid Interface Sci. 2002, 254, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Grzybowski, B.A.; Haag, R.; Bowden, N.; Whitesides, G.M. Generation of Micrometer-Sized Patterns for Microanalytical Applications Using a Laser Direct-Write Method and Microcontact Printing. Anal. Chem. 1998, 70, 4645–4652. [Google Scholar] [CrossRef]
- Duffy, D.C.; McDonald, J.C.; Schueller, O.J.A.; Whitesides, G.M. Rapid Prototyping of Microfluidic Systems in Poly(dimethylsiloxane). Anal. Chem. 1998, 70, 4974–4984. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chao, T.; Chen, S.; Jiang, S. Superlow Fouling Sulfobetaine and Carboxybetaine Polymers on Glass Slides. Langmuir 2006, 22, 10072–10077. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Cao, Z. Ultralow-Fouling, Functionalizable, and Hydrolyzable Zwitterionic Materials and Their Derivatives for Biological Applications. Adv. Mater. 2010, 22, 920–932. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Chaudhury, M.K.; Owen, M.J.; Orbeck, T. The Mechanisms of Hydrophobic Recovery of Polydimethylsiloxane Elastomers Exposed to Partial Electrical Discharges. J. Colloid Interface Sci. 2001, 244, 200–207. [Google Scholar] [CrossRef]
- Keefe, A.J.; Brault, N.D.; Jiang, S. Suppressing Surface Reconstruction of Superhydrophobic PDMS Using a Superhydrophilic Zwitterionic Polymer. Biomacromolecules 2012, 13, 1683–1687. [Google Scholar] [CrossRef] [PubMed]
- Tugulu, S.; Klok, H.-A. Surface Modification of Polydimethylsiloxane Substrates with Nonfouling Poly(Poly(ethylene glycol)methacrylate) Brushes. Macromol. Symp. 2009, 279, 103–109. [Google Scholar] [CrossRef]
- Xiao, D.; Zhang, H.; Wirth, M. Chemical Modification of the Surface of Poly(dimethylsiloxane) by Atom-Transfer Radical Polymerization of Acrylamide. Langmuir 2002, 18, 9971–9976. [Google Scholar] [CrossRef]
- Keskin, D.; Clodt, J.I.; Hahn, J.; Abetz, V.; Filiz, V. Postmodification of PS-b-P4VP Diblock Copolymer Membranes by ARGET ATRP. Langmuir 2014, 30, 8907–8914. [Google Scholar] [CrossRef] [PubMed]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2002, 54, 3–12. [Google Scholar] [CrossRef]
- Prucker, O.; Brandstetter, T.; Rühe, J. Surface-attached hydrogel coatings via C,H-insertion crosslinking for biomedical and bioanalytical applications (Review). Biointerphases 2018, 13, 010801. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.P.; Dean, D.; Engel, P.S.; Mikos, A.G. Photoinitiated Polymerization of Biomaterials. Ann. Rev. Mater. Res. 2001, 31, 171–181. [Google Scholar] [CrossRef]
- Schneider, M.H.; Willaime, H.; Tran, Y.; Rezgui, F.; Tabeling, P. Wettability Patterning by UV-Initiated Graft Polymerization of Poly(acrylic acid) in Closed Microfluidic Systems of Complex Geometry. Anal. Chem. 2010, 82, 8848–8855. [Google Scholar] [CrossRef] [PubMed]
- Yagci, Y.; Jockusch, S.; Turro, N.J. Photoinitiated Polymerization: Advances, Challenges, and Opportunities. Macromolecules 2010, 43, 6245–6260. [Google Scholar] [CrossRef]
- Riga, E.; Saar, J.; Erath, R.; Hechenbichler, M.; Lienkamp, K. On the Limits of Benzophenone as Cross-Linker for Surface-Attached Polymer Hydrogels. Polymers 2017, 9, 686. [Google Scholar] [CrossRef]
- Karaca Balta, D.; Karahan, Ö.; Avci, D.; Arsu, N. Synthesis, photophysical and photochemical studies of benzophenone based novel monomeric and polymeric photoinitiators. Prog. Org. Coat. 2015, 78, 200–207. [Google Scholar] [CrossRef]
- Decker, C. Photoinitiated crosslinking polymerisation. Prog. Polym. Sci. 1996, 21, 593–650. [Google Scholar] [CrossRef]
- Kheur, M.; Sethi, T.; Coward, T.; Kakade, D.; Rajkumar, M. Evaluation of the effect of ultraviolet stabilizers on the change in color of pigmented silicone elastomer: An in vitro study. J. Indian Prosthodont. Soc. 2016, 16, 276–281. [Google Scholar] [PubMed]
- Song, L.; Ye, Q.; Ge, X.; Misra, A.; Spencer, P. Tris(trimethylsilyl)silane as a co-initiator for dental adhesive: Photo-polymerization kinetics and dynamic mechanical property. Dent. Mater. 2016, 32, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.H.; Liu, N.; Sun, G. UV-induced graft polymerization of acrylamide on cellulose by using immobilized benzophenone as a photo-initiator. Eur. Polym. J. 2009, 45, 2443–2449. [Google Scholar] [CrossRef]
- Backman, D.E.; LeSavage, B.L.; Shah, S.B.; Wong, J.Y. A Robust Method to Generate Mechanically Anisotropic Vascular Smooth Muscle Cell Sheets for Vascular Tissue Engineering. Macromol. Biosci. 2017, 17, 1600434. [Google Scholar] [CrossRef] [PubMed]
- Hemmilä, S.; Cauich-Rodríguez, J.V.; Kreutzer, J.; Kallio, P. Rapid, simple, and cost-effective treatments to achieve long-term hydrophilic PDMS surfaces. Appl. Surf. Sci. 2012, 258, 9864–9875. [Google Scholar] [CrossRef]
- Huh, C.; Youn, B.; Lee, S. Degradation in silicone rubber used for outdoor insulator by UV radiation. In Proceedings of the 6th International Conference on Properties and Applications of Dielectric Materials, Xi’an, China, 21–26 June 2000; Volume 1, pp. 367–370. [Google Scholar]
- Yuk, H.; Zhang, T.; Parada, G.A.; Liu, X.; Zhao, X. Skin-inspired hydrogel–elastomer hybrids with robust interfaces and functional microstructures. Nat. Commun. 2016, 7, 12028. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, M.E.; Gadepalli, V.S.; Elmak, A.A.; Lee, W.; Rao, R.R.; Yadavalli, V.K. Large area micropatterning of cells on polydimethylsiloxane surfaces. J. Biol. Eng. 2014, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Cui, L.; Chen, R.; Xu, X.; Chen, J.; Yin, L. Facile Fabrication of Hierarchically Thermoresponsive Binary Polymer Pattern for Controlled Cell Adhesion. Macromol. Rapid Commun. 2018, 39, 1700572. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.H.; Tran, Y.; Tabeling, P. Benzophenone Absorption and Diffusion in Poly(dimethylsiloxane) and Its Role in Graft Photo-polymerization for Surface Modification. Langmuir 2011, 27, 1232. [Google Scholar] [CrossRef] [PubMed]
- Goda, T.; Konno, T.; Takai, M.; Moro, T.; Ishihara, K. Biomimetic phosphorylcholine polymer grafting from polydimethylsiloxane surface using photo-induced polymerization. Biomaterials 2006, 27, 5151–5160. [Google Scholar] [CrossRef] [PubMed]








| Sample | Young’s Modulus (MPa) | Elongation at Break (%) |
|---|---|---|
| PDMS | 3.7 ± 0.1 | 76.0 ± 9 |
| 10 wt %—5 min | 4.9 ± 0.5 | 68.2 ± 13 |
| 10 wt %—15 min | 8.2 ± 3 * | 34.0 ± 5 |
| 10 wt %—30 min | 6.2 ± 3 * | 22.2 ± 21 |
| 10 wt %—60 min A | - | - |
| 5 wt %—5 min | 6.1 ± 2 # | 65.2 ± 7 |
| 10 wt %—5 min | 4.9 ± 0.5 # | 68.2 ± 13 |
| 20 wt %—5 min | 7.2 ± 1 | 34.0 ± 6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keskin, D.; Mokabbar, T.; Pei, Y.; Van Rijn, P. The Relationship between Bulk Silicone and Benzophenone-Initiated Hydrogel Coating Properties. Polymers 2018, 10, 534. https://doi.org/10.3390/polym10050534
Keskin D, Mokabbar T, Pei Y, Van Rijn P. The Relationship between Bulk Silicone and Benzophenone-Initiated Hydrogel Coating Properties. Polymers. 2018; 10(5):534. https://doi.org/10.3390/polym10050534
Chicago/Turabian StyleKeskin, Damla, Taraneh Mokabbar, Yutao Pei, and Patrick Van Rijn. 2018. "The Relationship between Bulk Silicone and Benzophenone-Initiated Hydrogel Coating Properties" Polymers 10, no. 5: 534. https://doi.org/10.3390/polym10050534