Polymorphic Behavior and Phase Transition of Poly(1-Butene) and Its Copolymers
Abstract
:1. Introduction
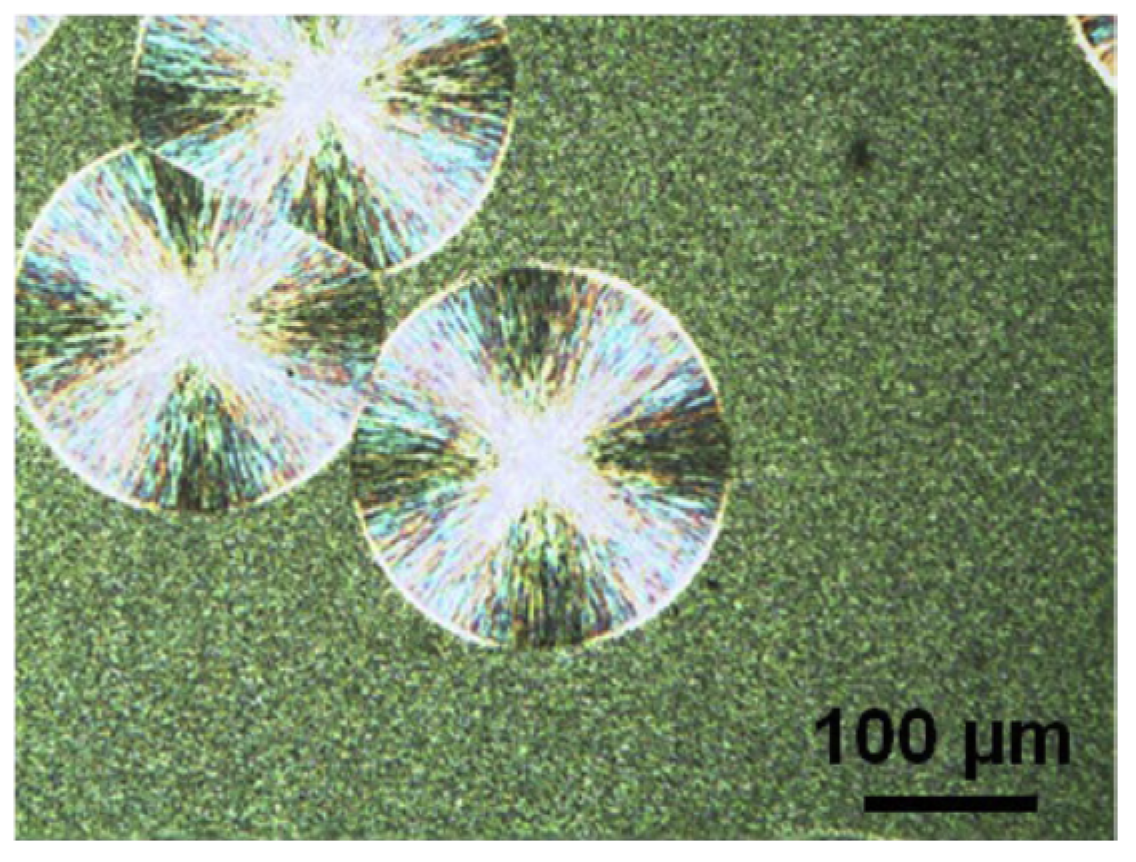
2. Polymorphic Behavior of iPBu
3. Crystal Structural Control of iPBu
3.1. Influence of Temperature on the Polymorphism of iPBu
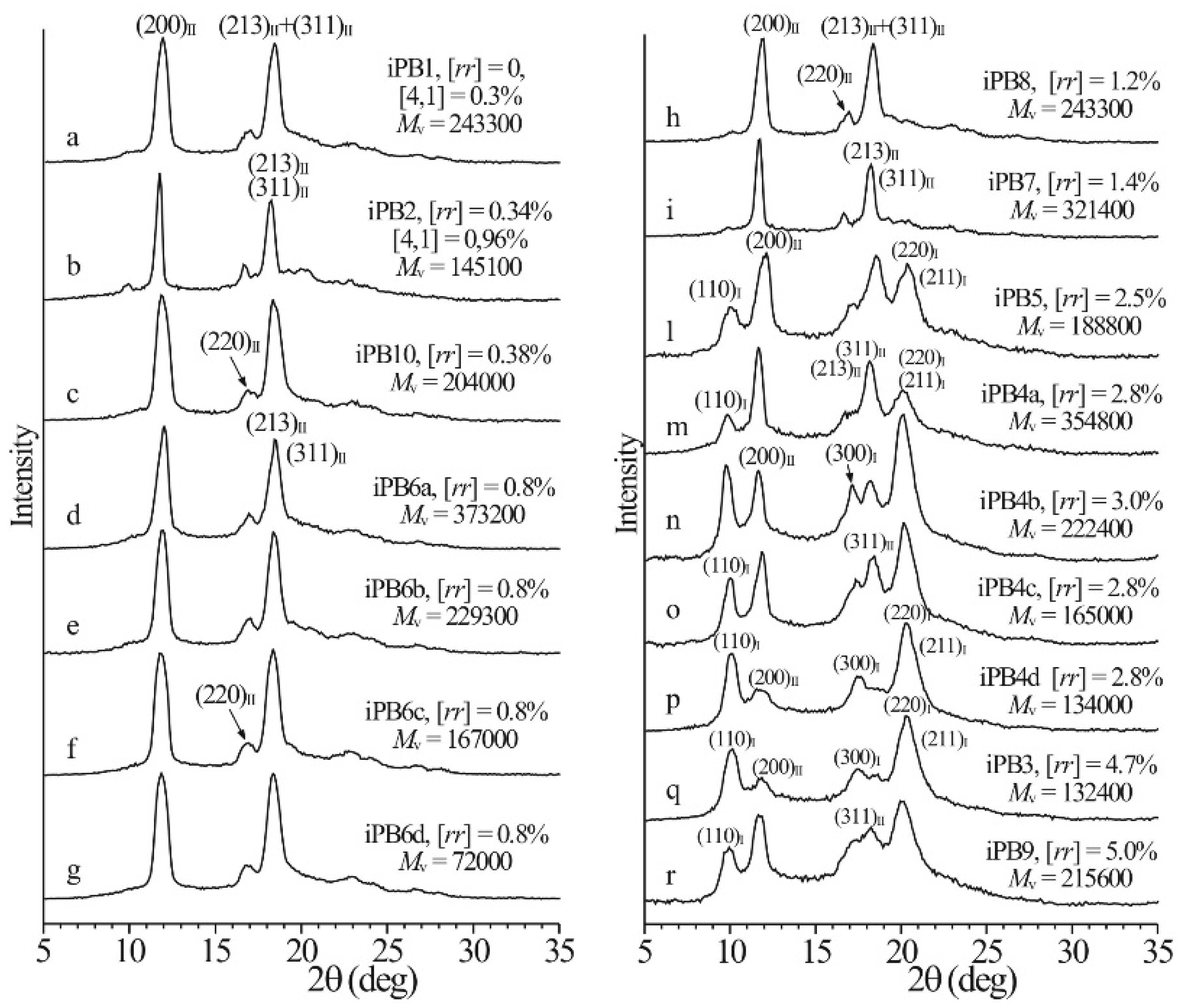
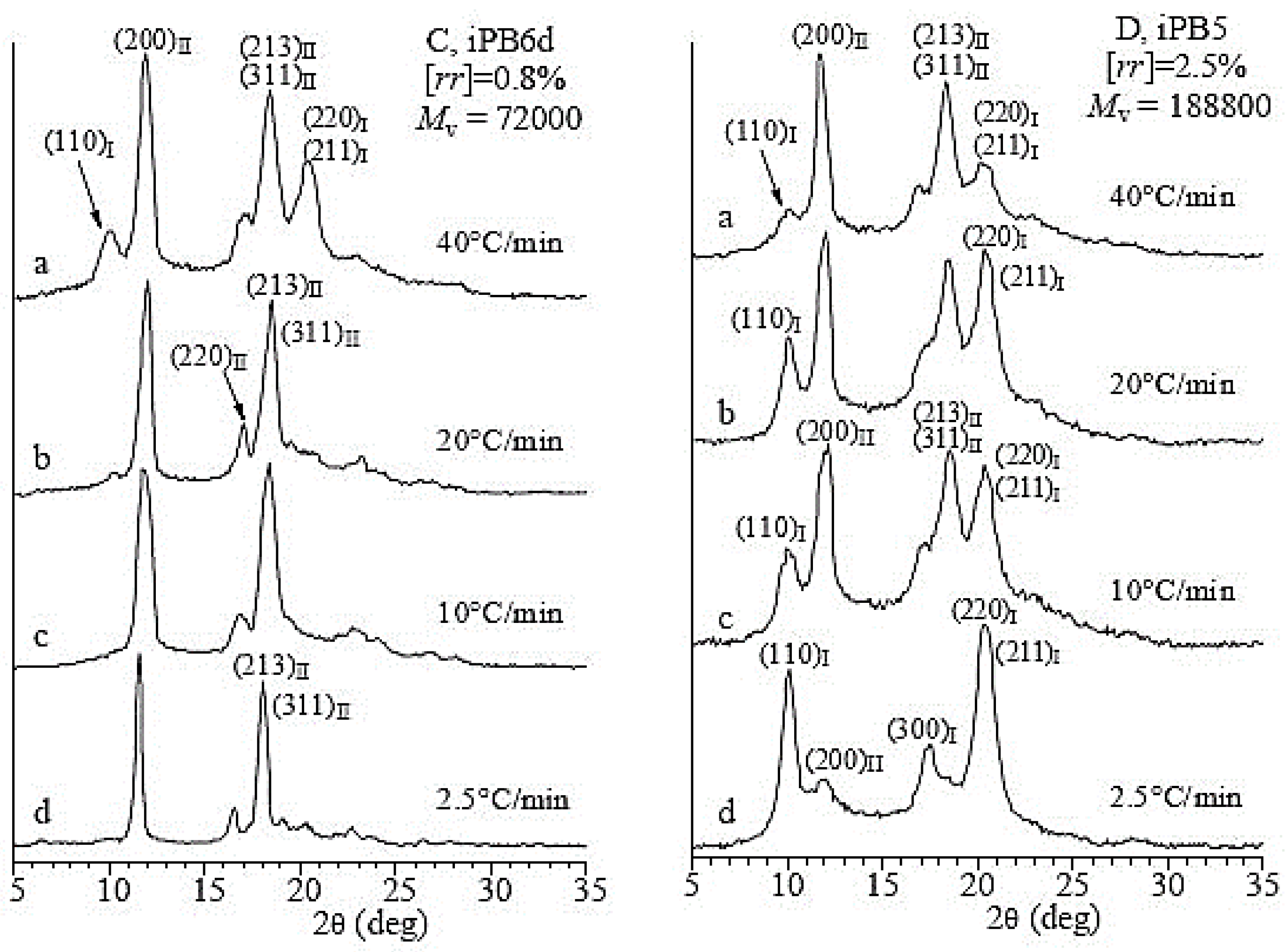
3.2. Influence of Molecular Characteristics on the Polymorphism of iPBu
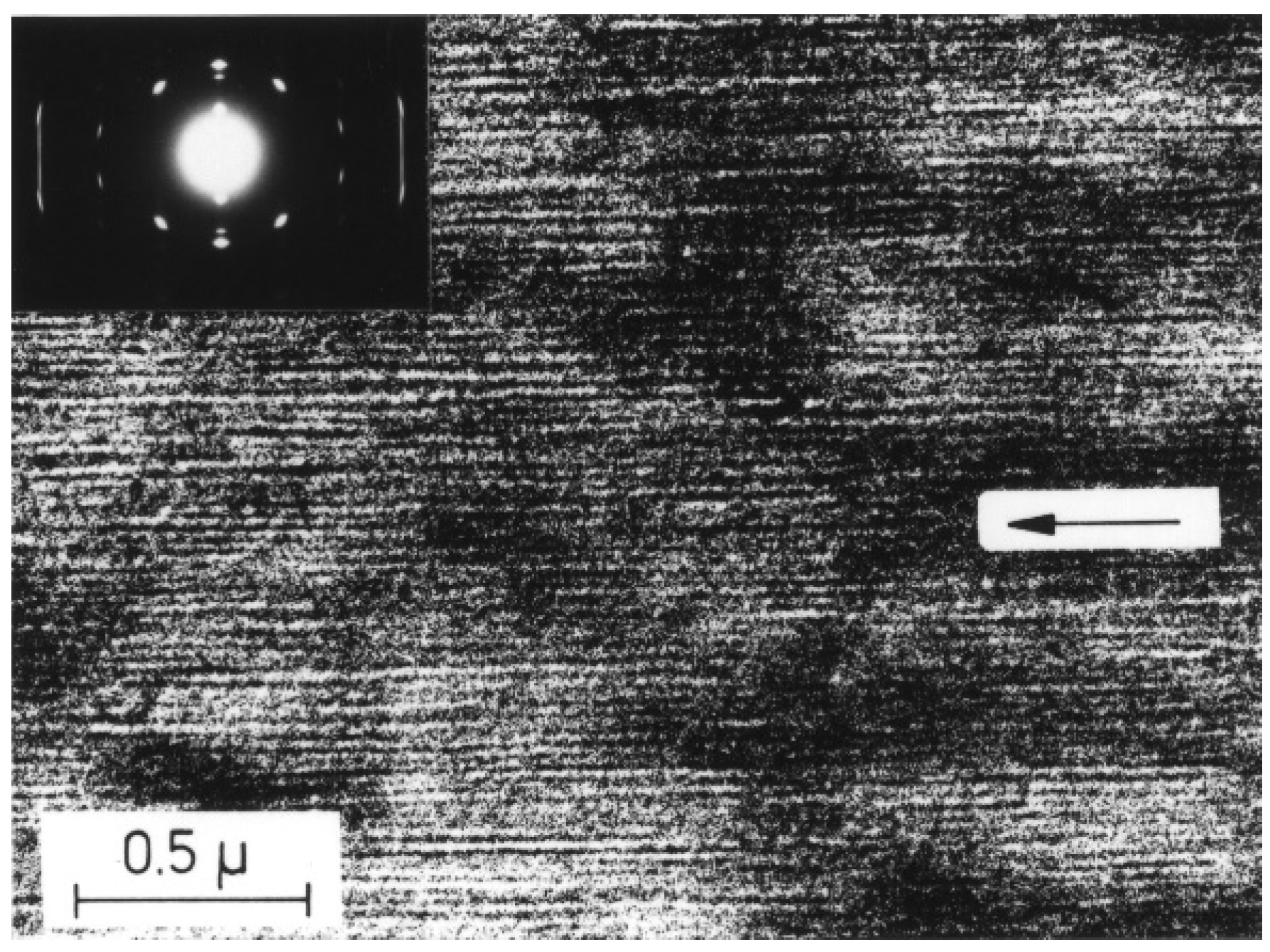
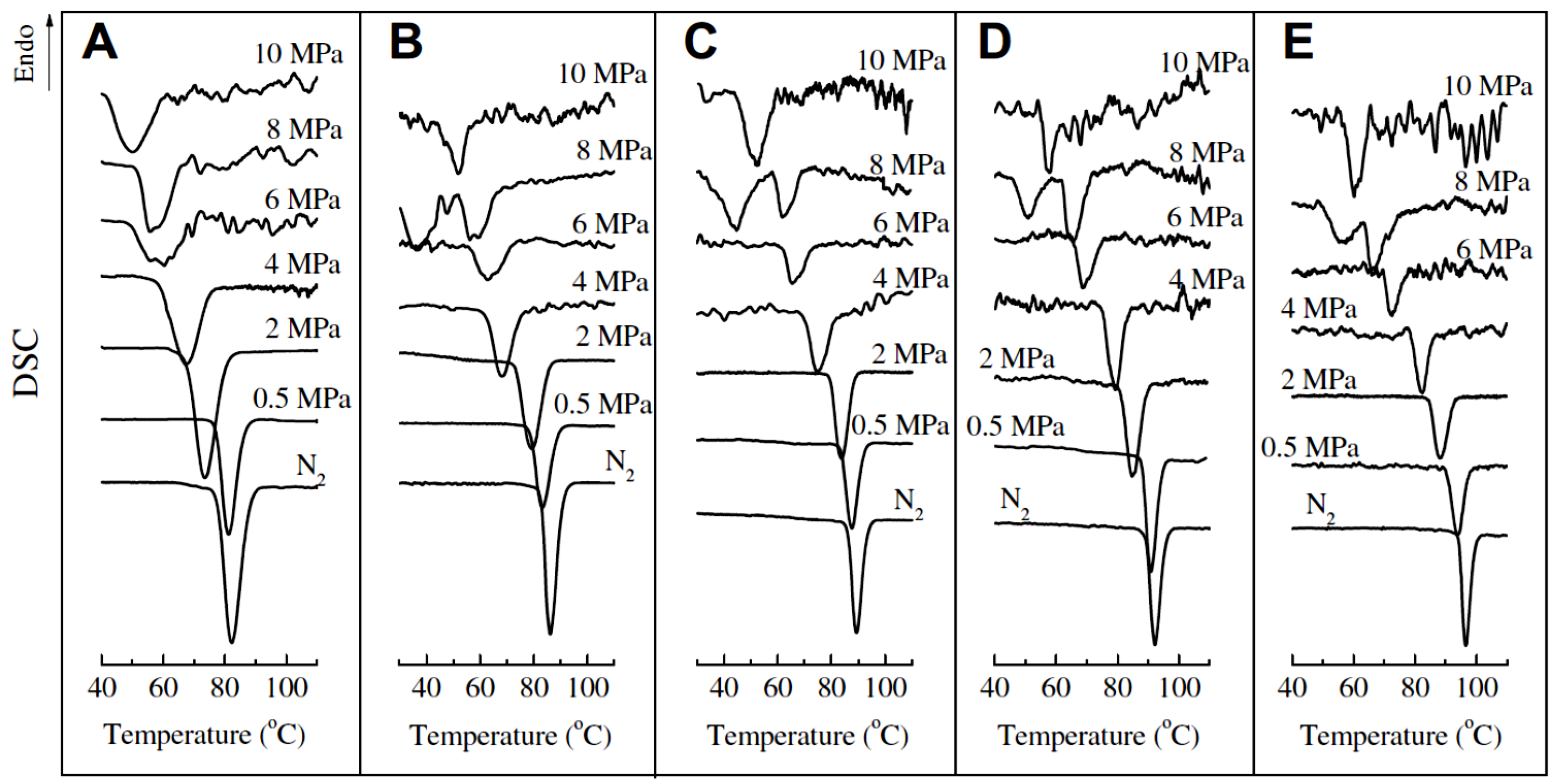
3.3. Influence of External Fields on the Polymorphism of iPBu
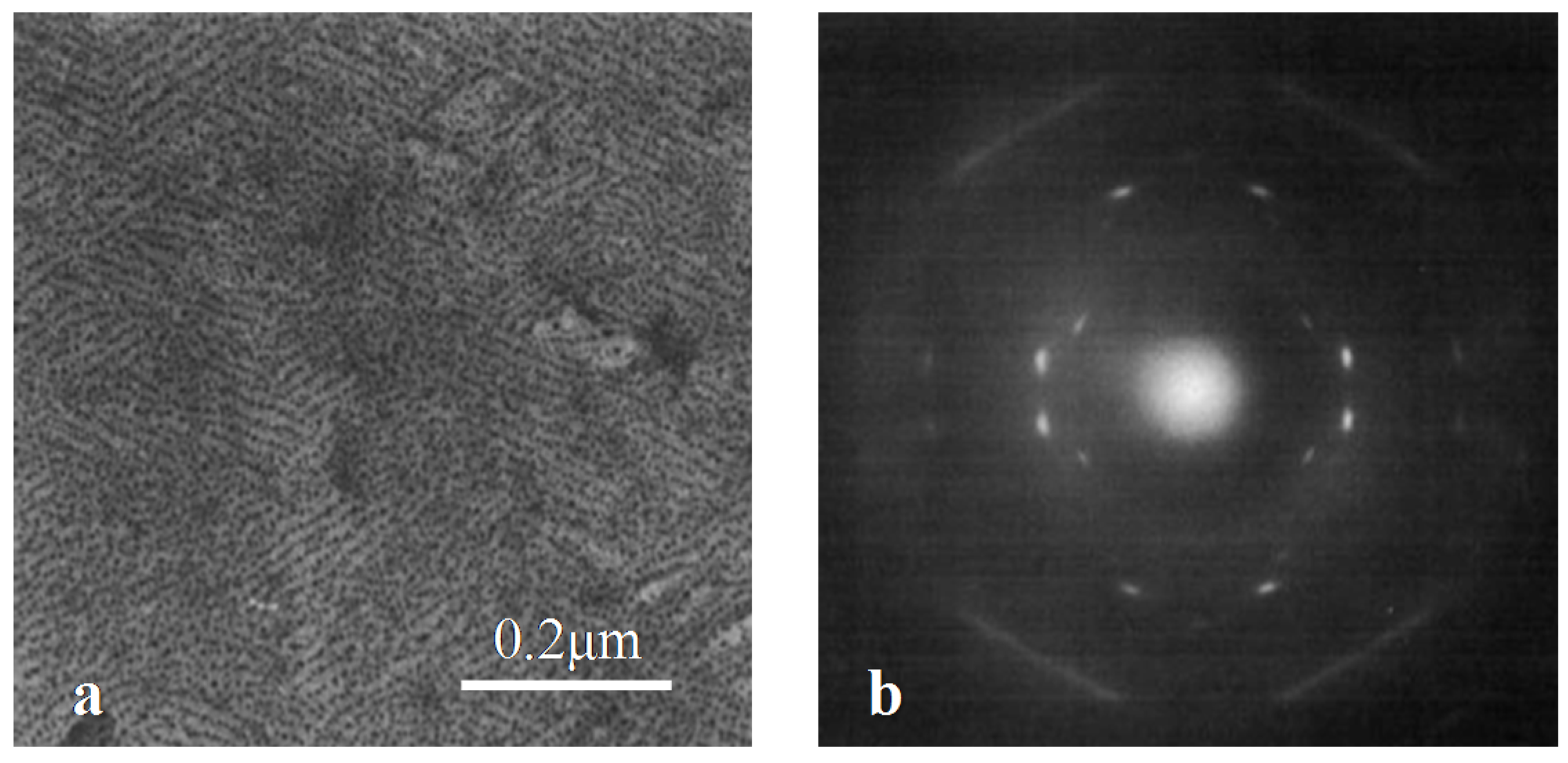
3.4. Influence of Heterogeneous Entities on the Polymorphism of iPBu
4. Phase Transition of Polymorphic Polymers
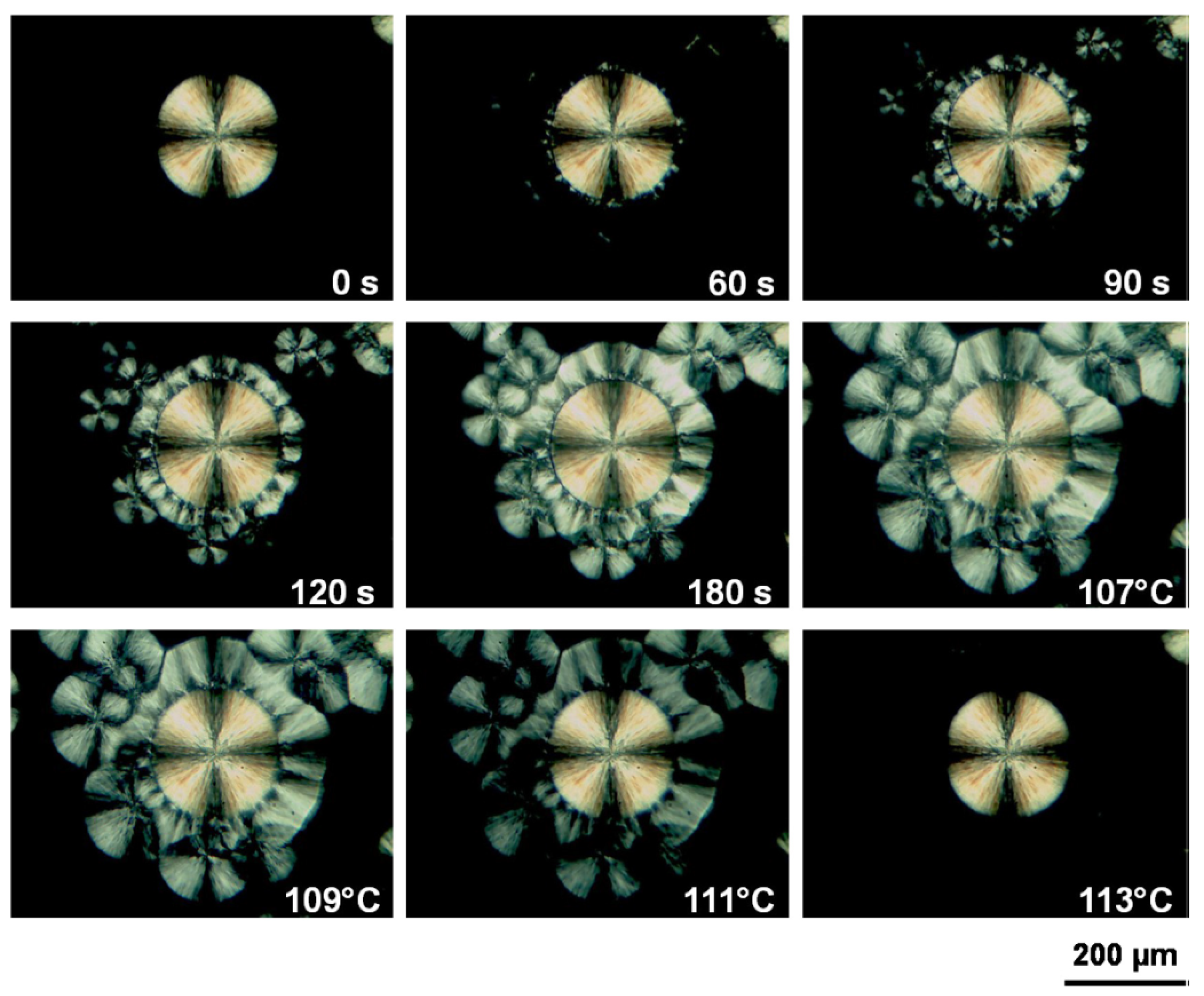
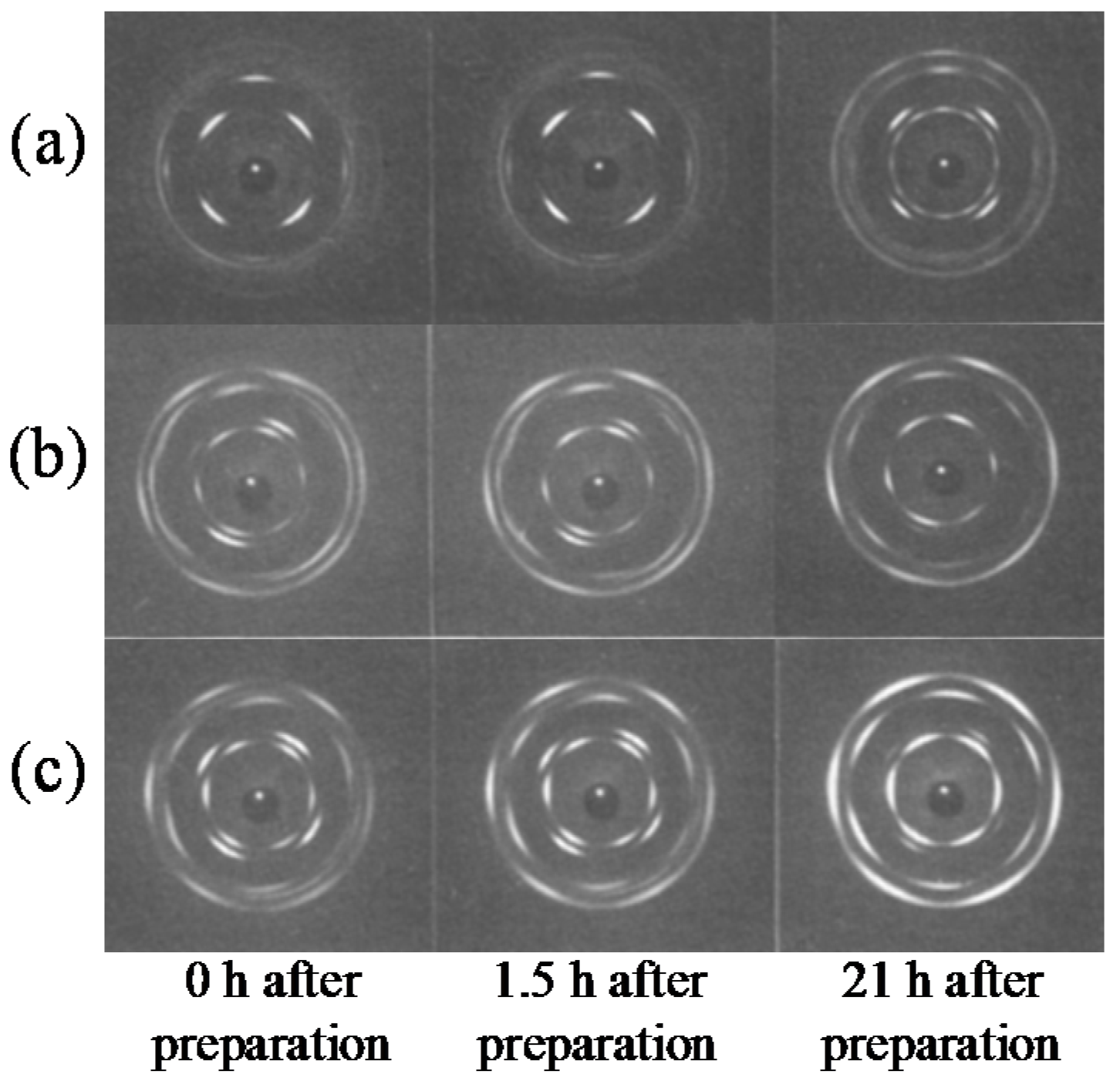
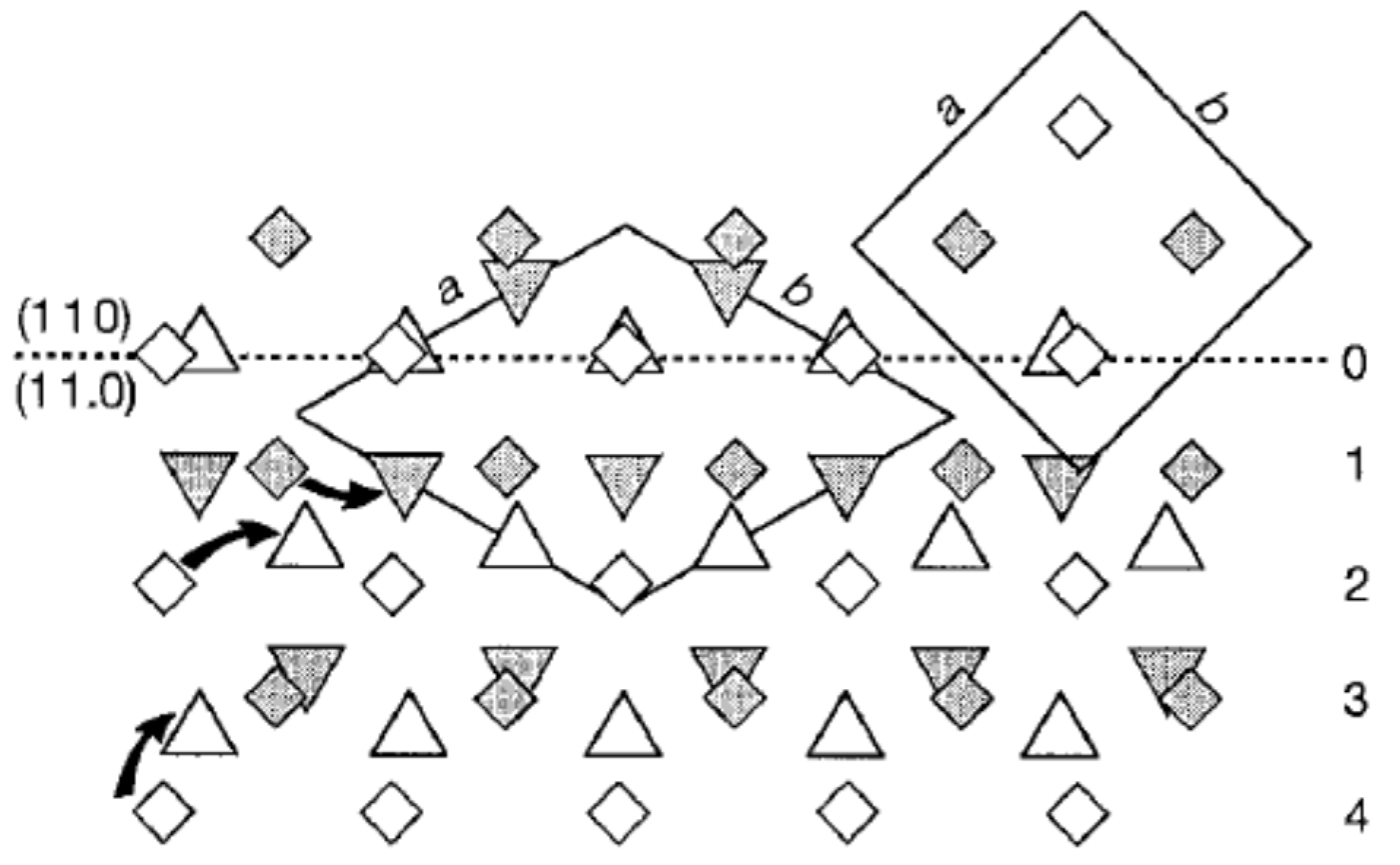
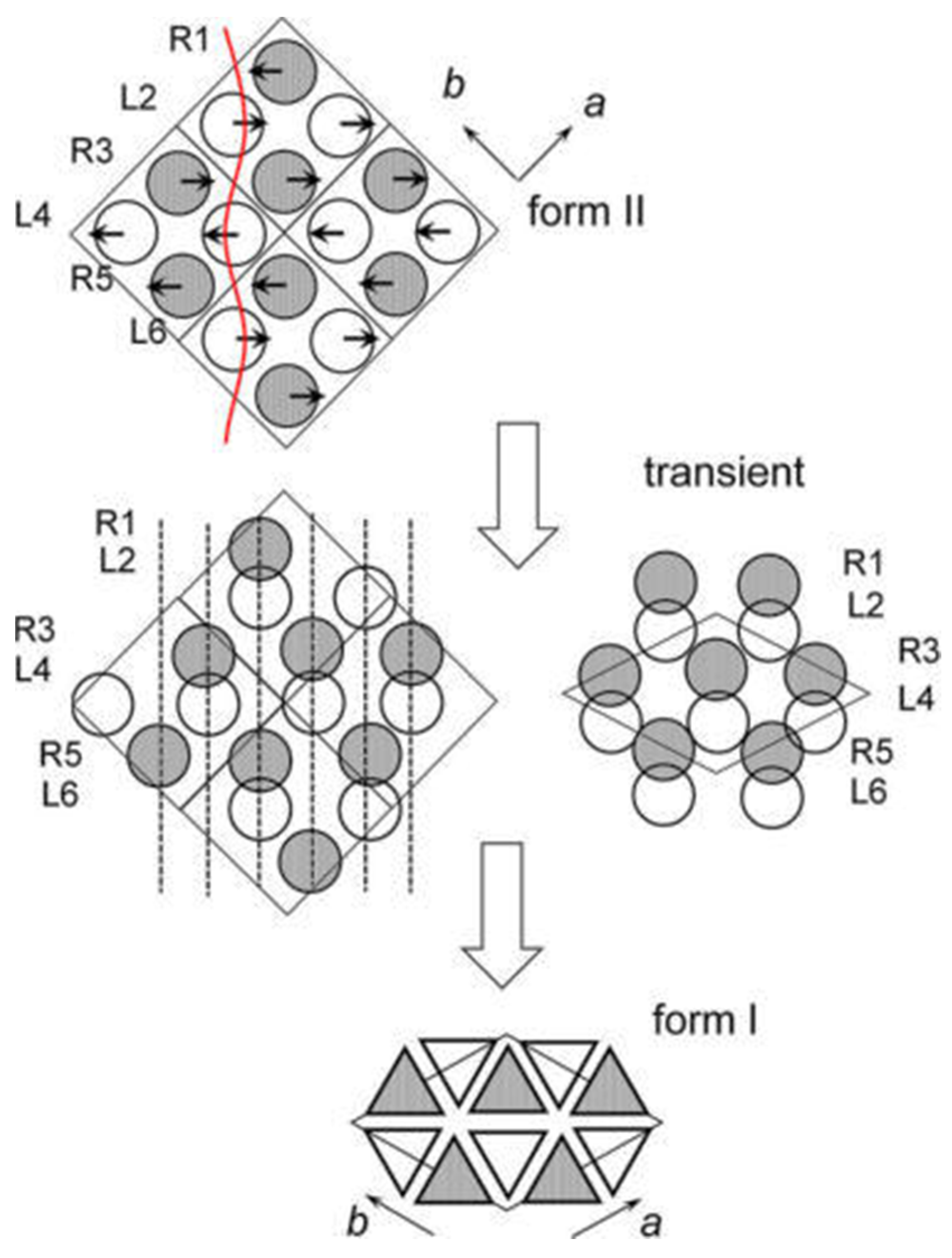
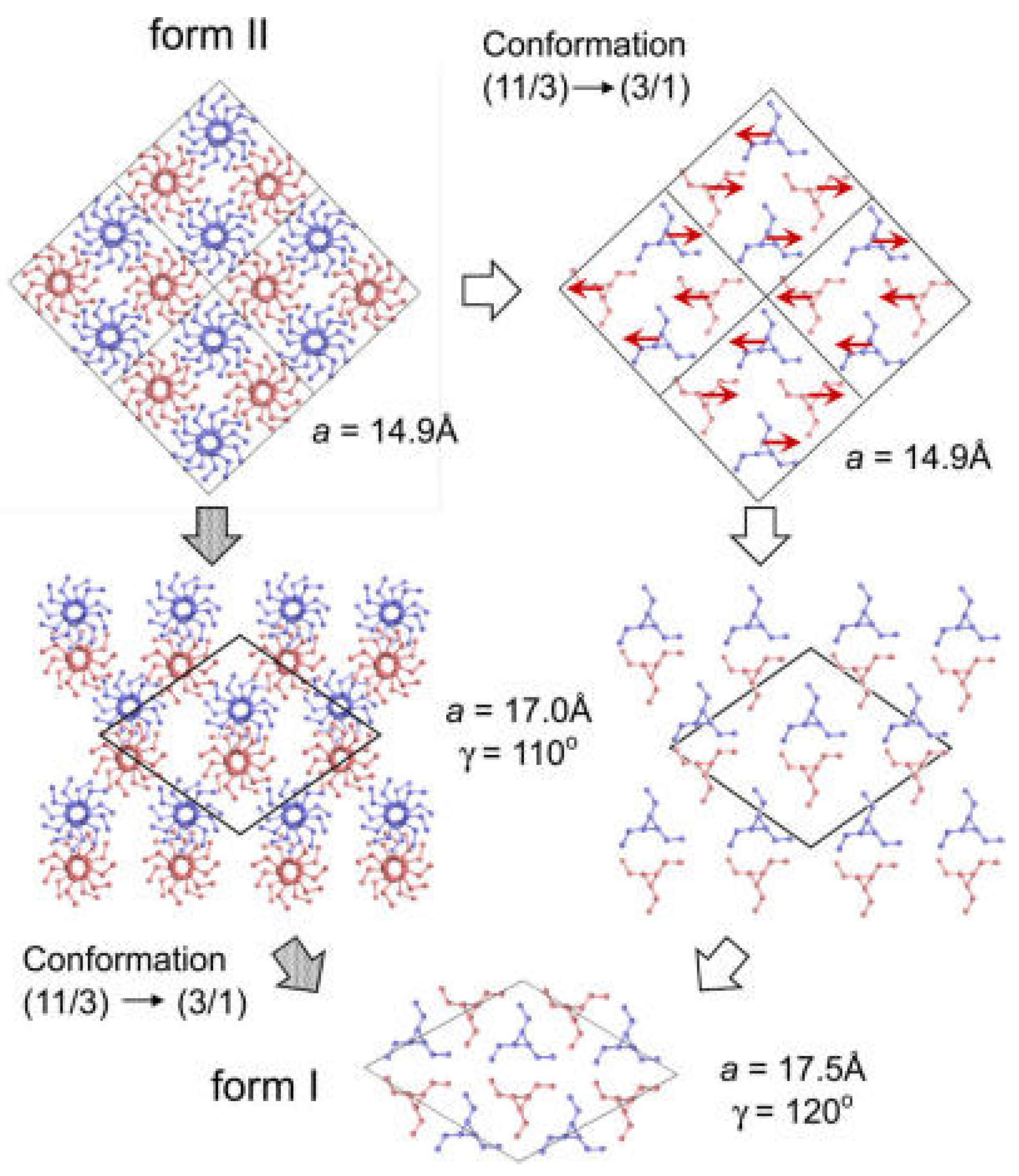
4.1. Phase Transition Mechanism of iPBu
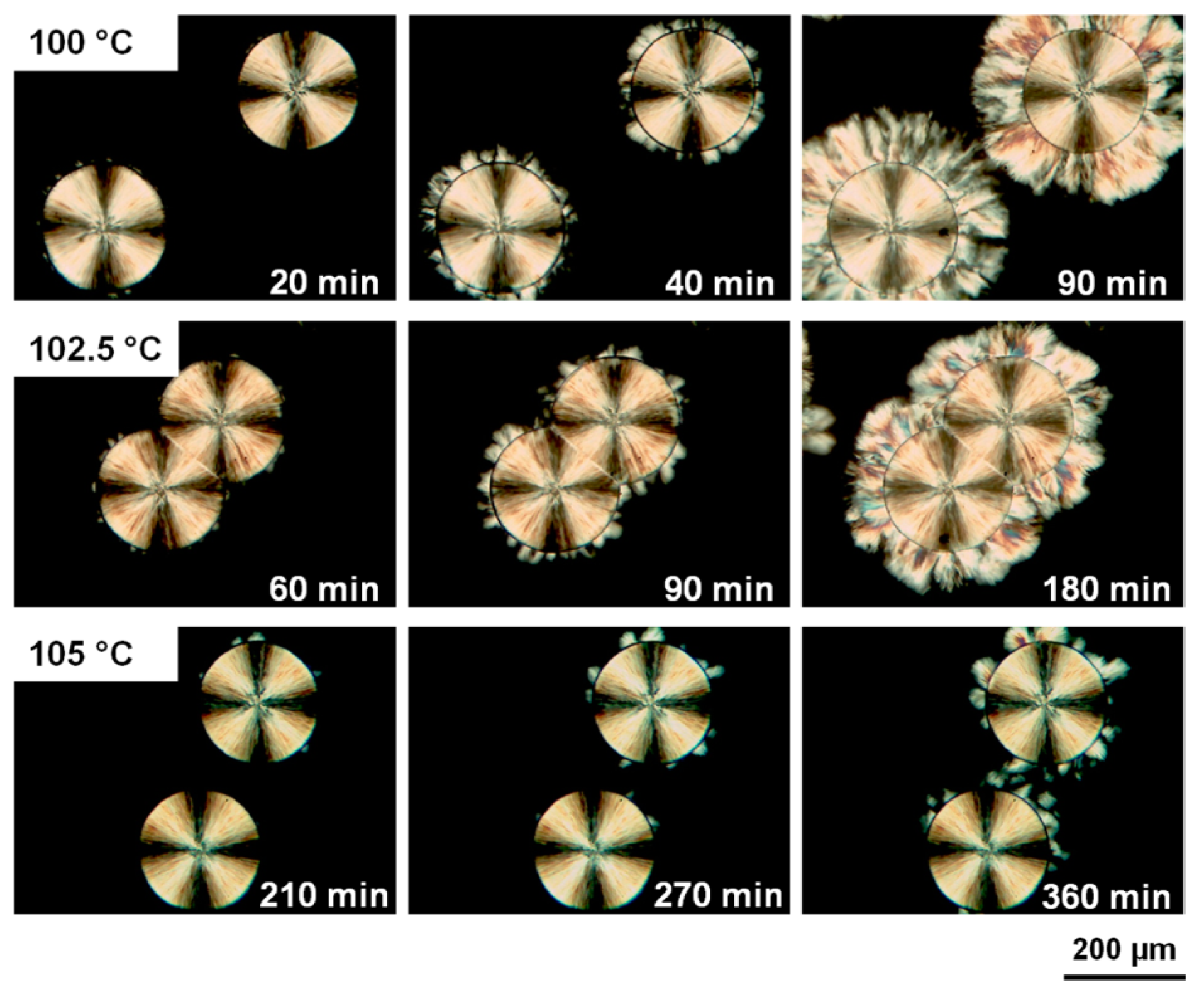
4.2. Phase Transition Kinetics of iPBu
4.3. Factors Influencing the Phase Transition of iPBu
5. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pan, P.; Inoue, Y. Polymorphism and isomorphism in biodegradable polyesters. Prog. Polym. Sci. 2009, 34, 605–640. [Google Scholar] [CrossRef]
- Corradini, P.; Guerra, G. Polymorphism in polymers. Adv. Polym. Sci. 1992, 100, 183–217. [Google Scholar]
- Brückner, S.; Meille, S.V.; Petraccone, V.; Pirozzi, B. Polymorphism in isotactic polypropylene. Prog. Polym. Sci. 1991, 16, 361–404. [Google Scholar] [CrossRef]
- Lotz, B.; Wittmann, J.C.; Lovinger, A.J. Structure and morphology of polypropylenes: A molecular analysis. Polymer 1996, 37, 4979–4992. [Google Scholar] [CrossRef]
- Woo, E.M.; Sun, Y.S.; Yang, C.P. Polymorphism, thermal behavior, and crystal stability in syndiotactic polystyrene vs. its miscible blends. Prog. Polym. Sci. 2001, 26, 945–983. [Google Scholar] [CrossRef]
- Martins, P.; Lopes, A.C.; Lanceros-Mendez, S. Electroactive phases of poly(vinylidene fluoride): Determination, processing and applications. Prog. Polym. Sci. 2014, 39, 683–706. [Google Scholar] [CrossRef]
- Lovinger, A.J. Annealing of poly(viny1idene fluoride) and formation of a fifth phase. Macromolecules 1982, 15, 40–44. [Google Scholar] [CrossRef]
- Nandi, A.K.; Mandelkern, L. The influence of chain structure on the equilibrium melting temperature of poly(vinylidene fluoride). J. Polym. Sci. Part B Polym. Phys. 1991, 29, 1287–1297. [Google Scholar] [CrossRef]
- Gregorio, R., Jr. Determination of the α, β, and γ crystalline phases of poly(vinylidene fluoride) films prepared at different conditions. J. Appl. Polym. Sci. 2006, 100, 3272–3279. [Google Scholar] [CrossRef]
- Lotz, B. A new ε crystal modification found in stereodefective isotactic polypropylene samples. Macromolecules 2014, 47, 7612–7624. [Google Scholar] [CrossRef]
- Lotz, B. Structural polymorphism of crystalline polymers: Electron and atomic force microscopy contributions. Macrornol. Symp. 1995, 94, 97–104. [Google Scholar] [CrossRef]
- Thomann, R.; Wang, C.; Kressler, J.; Mülhaupt, R. On the γ-phase of isotactic polypropylene. Macromolecules 1996, 29, 8425–8434. [Google Scholar] [CrossRef]
- Gou, Q.; Li, H.; Yu, Z.; Chen, E.; Zhang, Y.; Yan, S. Effect of crystallization temperature and propylene sequence length on the crystalline structure of propylene-ethylene random copolymers. Chin. Sci. Bull. 2008, 53, 1804–1812. [Google Scholar] [CrossRef]
- Gou, Q.; Li, H.; Chen, E.; Zhang, Y.; Yan, S. Crystallization behavior of a propylene-1-butene random copolymer in its α and γ modifications. Colloid Polym. Sci. 2007, 285, 1149–1155. [Google Scholar] [CrossRef]
- Luciani, L.; Seppälä, J.; Löfgren, B. Poly-1-butene: Its preparation, properties and challenges. Prog. Polym. Sci. 1988, 13, 37–62. [Google Scholar] [CrossRef]
- Lotz, B.; Thierry, A. Spherulite morphology of form III isotactic poly(1-butene). Macromolecules 2003, 36, 286–290. [Google Scholar] [CrossRef]
- Kaszonyiova, M.; Rybnikar, K.; Geil, P.H. Polymorphism of isotactic poly(butene-1). J. Macromol. Sci. Phys. 2005, 44, 377–396. [Google Scholar] [CrossRef]
- Siracusa, V.; Blanco, I.; Romani, S.; Tylewicz, U.; Rocculi, P.; Rosa, M.D. Poly(lactic acid)-Modified Films for Food Packaging Application: Physical, Mechanical, and Barrier Behavior. J. Appl. Polym. Sci. 2012, 125, E390–E401. [Google Scholar] [CrossRef]
- Blanco, I.; Siracusa, V. Kinetic study of the thermal and thermo-oxidative degradations of polylactide-modified films for food packaging. J. Therm. Anal. Calorim. 2013, 112, 1171–1177. [Google Scholar] [CrossRef]
- Tashiro, K.; Asanaga, H.; Ishino, K.; Tazaki, R.; Kobayashi, M. Development of a new software for the X-ray structural analysis of polymer crystals by utilizing the X-ray imaging plate system. J. Polym. Sci. Part B Polym. Phys. 1997, 35, 1677–1700. [Google Scholar] [CrossRef]
- Miller, R.L.; Nielsen, L.E. Crystallographic data for various polymers. II. J. Polym. Sci. 1961, 55, 643–656. [Google Scholar] [CrossRef]
- Danusso, F.; Gianotti, G. The three polymorphs of isotactic polybutene-1: Dilatometric and thermodynamic fusion properties. Makromol. Chem. 1963, 61, 139–156. [Google Scholar] [CrossRef]
- Tashiro, K.; Hu, J.; Wang, H.; Hanesaka, M.; Saiani, A. Refinement of the crystal structures of forms I and II of isotactic polybutene-1 and a proposal of phase transition mechanism between them. Macromolecules 2016, 49, 1392–1404. [Google Scholar] [CrossRef]
- Turner-Jones, A. Polybutene-1-type II crystalline form. J. Polym. Sci. Part B Polym. Lett. 1963, 1, 455–456. [Google Scholar] [CrossRef]
- Petraccone, V.; Pirozzi, B.; Frasci, A.; Corradini, P. Polymorphism of isotactic poly-α-butene: Conformational analysis of the chain and crystalline structure of form II. Eur. Polym. J. 1976, 12, 323–327. [Google Scholar] [CrossRef]
- Turner-Jones, A. On transformations in isotactic polybutene-1. J. Polym. Sci. Part B Polym. Lett. 1964, 2, 519–521. [Google Scholar]
- Dorset, D.L.; McCourt, M.P.; Kopp, S.; Wittmann, J.C.; Lotz, B. Direct determination of polymer crystal structures by electron crystallography—Isotactic poly(1-butene), Form(III). Acta Crystallogr. Sect. B 1994, 50, 201–208. [Google Scholar] [CrossRef]
- Cojazzi, G.; Malta, V.; Celotti, G.; Zannetti, R. Crystal structure of form III of isotactic poly(1-butene). Makromol. Chem. 1976, 177, 915–926. [Google Scholar] [CrossRef]
- Holland, V.F.; Miller, R.L. Isotactic Polybutene-1 Single Crystals: Morphology. J. Appl. Phys. 1964, 35, 3241–3248. [Google Scholar] [CrossRef]
- Nakafuku, C.; Miyaki, T. Effect of pressure on the melting and crystallization behaviour of isotactic polybutene-1. Polymer 1983, 24, 141–148. [Google Scholar] [CrossRef]
- Danusso, F.; Gianotti, G. Isotactic polybutener-1: Formation and transformation of modification 2. Makromol. Chem. 1965, 88, 149–158. [Google Scholar] [CrossRef]
- Schaffhauser, R.J. On the nature of the form II to form I transformation in isotactic polybutene-1. J. Polym. Sci. Part B Polym. Lett. 1967, 5, 839–841. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, D.; Yan, S. Direct formation of form I isotactic poly(1-butene) single crystals. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 2641–2645. [Google Scholar] [CrossRef]
- Xu, J.; Ma, Y.; Hu, W.; Rehahn, M.; Reiter, G. Cloning polymer single crystals through self-seeding. Nat. Mater. 2009, 8, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M. Direct crystal growth of isotactic polybutene-1 trigonal phase in the melt: In-situ observation. J. Cryst. Growth 2008, 310, 1739–1743. [Google Scholar] [CrossRef]
- Yamashita, M. Crystal growth kinetics and morphology of isotactic polybutene-1 trigonal phase in the melt. J. Cryst. Growth 2009, 311, 560–563. [Google Scholar] [CrossRef]
- Zhang, M.C.; Guo, B.H.; Xu, J. A review on polymer crystallization theories. Crystals 2017, 7, 4. [Google Scholar] [CrossRef]
- Schneider, S.; Drujon, X.; Lotz, B.; Wittmann, J.C. Self-nucleation and enhanced nucleation of polyvinylidene fluoride (α-phase). Polymer 2001, 42, 8787–8798. [Google Scholar] [CrossRef]
- De Rosa, C.; Ruiz de Ballesteros, O.; Di Gennaro, M.; Auriemma, F. Crystallization from the melt of α and β forms of syndiotactic polystyrene. Polymer 2003, 44, 1861–1870. [Google Scholar] [CrossRef]
- Sorrentino, A.; Pantani, R.; Titomanlio, G. Kinetics of melting and characterization of the thermodynamic and kinetic properties of syndiotactic polystyrene. J. Polym. Sci. Part B Polym. Phys. 2007, 45, 196–207. [Google Scholar] [CrossRef]
- Mamun, A.; Umemoto, S.; Okui, N.; Ishihara, N. Self-seeding effect on primary nucleation of isotactic polystyrene. Macromolecules 2007, 40, 6296–6303. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, J.; Sun, X.; Li, H.; Ren, Z.; Yan, S. Crystal structure regulation of ferroelectric poly(vinylidene fluoride) via controlled melt-recrystallization. Ind. Eng. Chem. Res. 2017, 56, 4580–4587. [Google Scholar] [CrossRef]
- Zheng, Y.R.; Zhang, J.; Sun, X.L.; Li, H.H.; Ren, Z.J.; Yan, S.K. Enhanced αγ′ Transition of Poly(vinylidene fluoride) by Step Crystallization and Subsequent Annealing. Chin. J. Polym. Sci. 2018, 36, 598–603. [Google Scholar] [CrossRef]
- Jiang, J.; Zhuravlev, E.; Hu, W.; Schick, C.; Zhou, D. The effect of self-nucleation on isothermal crystallization kinetics of poly(butylene succinate) (PBS) investigated by differential fast scanning calorimetry. Chin. J. Polym. Sci. 2017, 35, 1009–1019. [Google Scholar] [CrossRef]
- Armeniades, C.D.; Baer, E. Effect of pressure on the polymorphism of melt crystallized polybutene-1. J. Macromol. Sci. Phys. 1967, 1, 309–334. [Google Scholar] [CrossRef]
- Yamashita, M.; Hoshino, A.; Kato, M. Isotactic poly(butene-1) trigonal crystal growth in the melt. J. Polym. Sci. Part B Polym. Phys. 2007, 45, 684–697. [Google Scholar] [CrossRef]
- Su, F.; Li, X.; Zhou, W.; Zhu, S.; Ji, Y.; Wang, Z.; Qi, Z.; Li, L. Direct formation of isotactic poly(1-butene) form I crystal from memorized ordered melt. Macromolecules 2013, 46, 7399–7405. [Google Scholar] [CrossRef]
- Cavallo, D.; Zhang, L.; Sics, I.; Alfonso, G.C.; Dumas, P.; Marcod, C.; Ellis, G. The morphology and polymorphism of self-nucleated trigonal isotactic poly(1-butene) studied by synchrotron IR microspectroscopy. CrystEngComm 2016, 18, 816–828. [Google Scholar] [CrossRef]
- Cavallo, D.; Gardella, L.; Portale, G.; Müller, A.J.; Alfonso, G.C. On cross- and self-nucleation in seeded crystallization of isotactic poly(1-butene). Polymer 2013, 54, 4637–4644. [Google Scholar] [CrossRef]
- Cavallo, D.; Gardella, L.; Portale, G.; Müller, A.J.; Alfonso, G.C. Self-nucleation of isotactic poly(1-butene) in the trigonal modification. Polymer 2014, 55, 137–142. [Google Scholar] [CrossRef]
- Yu, L. Nucleation of one polymorph by another. J. Am. Chem. Soc. 2003, 125, 6380–6381. [Google Scholar] [CrossRef] [PubMed]
- Lovinger, A.J.; Chua, J.O.; Gryte, C.C. Studies on the α and β forms of isotactic polypropylene by crystallization in a temperature gradient. J. Polym. Sci. Part B Polym. Phys. 1977, 15, 641–656. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhou, Q.; Ren, Z.; Sun, X.; Li, H.; Li, H.; Yan, S. The αβ-iPP growth transformation of commercial grade iPP during non-isothermal crystallization. CrystEngComm 2015, 17, 9221–9227. [Google Scholar] [CrossRef]
- Lovinger, A.J. Crystallization and morphology of melt-solidified poly(vinylidene fluoride). J. Polym. Sci. Part B Polym. Phys. 1980, 18, 793–809. [Google Scholar] [CrossRef]
- Wang, J.; Ren, Z.; Sun, X.; Li, H.; Yan, S. The βα growth transition of isotactic polypropylene during stepwise crystallization at elevated temperature. Colloid Polym. Sci. 2015, 293, 2823–2830. [Google Scholar] [CrossRef]
- Li, H.; Sun, X.; Yan, S.; Schultz, J.M. Initial stage of iPP β to α growth transition induced by stepwise crystallization. Macromolecules 2008, 41, 5062–5064. [Google Scholar] [CrossRef]
- Chen, S.; Xi, H.; Yu, L. Cross-nucleation between ROY polymorphs. J. Am. Chem. Soc. 2005, 127, 17439–17444. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Yu, L. Kinetics of cross-nucleation between polymorphs. J. Phys. Chem. B 2006, 110, 7098–7101. [Google Scholar] [CrossRef] [PubMed]
- Desgranges, C.; Delhommelle, J. Molecular mechanism for the cross-nucleation between polymorphs. J. Am. Chem. Soc. 2006, 128, 10368–10369. [Google Scholar] [CrossRef] [PubMed]
- Fraschini, C.; Jiménez, L.; Kalala, B.; Prud’homme, R.E. Polymorphism and cross-nucleation in poly(1,3-dioxolan). Polymer 2012, 53, 188–195. [Google Scholar] [CrossRef]
- Nozue, Y.; Seno, S.; Nagamatsu, T.; Hosoda, S.; Shinohara, Y.; Amemiya, Y.; Berda, E.B.; Rojas, G.; Wagener, K.B. Cross nucleation in polyethylene with precisely spaced ethyl branches. ACS Macro Lett. 2012, 1, 772–775. [Google Scholar] [CrossRef]
- Cavallo, D.; Gardella, L.; Portale, G.; Muüller, A.J.; Alfonso, G.C. Kinetics of cross-nucleation in isotactic poly(1-butene). Macromolecules 2014, 47, 870–873. [Google Scholar] [CrossRef]
- Cavallo, D.; Alfonso, G.C. Concomitant crystallization and cross-nucleation in polymorphic polymers. Adv. Polym. Sci. 2017, 277, 1–54. [Google Scholar]
- Lorenzo, A.T.; Arnal, M.L.; Sanchez, J.J.; Müller, A.J. Effect of annealing time on the self-nucleation behavior of semicrystalline polymers. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 1738–1750. [Google Scholar] [CrossRef]
- Fillon, B.; Wittmann, J.C.; Lotz, B.; Thierry, A. Self-nucleation and recrystallization of isotactic polypropylene (α phase) investigated by differential scanning calorimetry. J. Polym. Sci. Part B Polym. Phys. 1993, 31, 1383–1393. [Google Scholar] [CrossRef]
- Fillon, B.; Lotz, B.; Thierry, A.; Wittmann, J.C. Self-nucleation and enhanced nucleation of polymers. Definition of a convenient calorimetric “efficiency scale” and evaluation of nucleating additives in isotactic polypropylene (α phase). J. Polym. Sci. Part B Polym. Phys. 1993, 31, 1395–1405. [Google Scholar]
- Massa, M.V.; Lee, M.S.M.; Dalnoki-Veress, K. Crystal nucleation of polymers confined to droplets: Memory effects. J. Polym. Sci. Part B Polym. Phys. 2005, 43, 3438–3443. [Google Scholar] [CrossRef]
- Li, H.; Jiang, S.; Wang, J.; Wang, D.; Yan, S. Optical microscopic study on the morphologies of isotactic polypropylene induced by its homogeneity fibers. Macromolecules 2003, 36, 2802–2807. [Google Scholar] [CrossRef]
- Li, H.; Zhang, X.; Duan, Y.; Wang, D.; Yan, S. Influence of crystallization temperature on the morphologies of isotactic polypropylene single-polymer composite. Polymer 2004, 45, 8059–8065. [Google Scholar] [CrossRef]
- Li, H.; Zhang, X.; Kuang, X.; Wang, J.; Wang, D.; Li, L.; Yan, S. A scanning electron microscopy study on the morphologies of isotactic polypropylene induced by its own fibers. Macromolecules 2004, 37, 2847–2853. [Google Scholar] [CrossRef]
- Sun, X.; Li, H.; Zhang, X.; Wang, J.; Wang, D.; Yan, S. The effect of fiber molecular weight on the interfacial morphology of iPP fiber/matrix single polymer composites. Macromolecules 2006, 39, 1087–1092. [Google Scholar] [CrossRef]
- Li, H.; Yan, S. Surface-induced polymer crystallization and the resultant structures and morphologies. Macromolecules 2011, 44, 417–428. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, Y.; Zhao, J.; Jiang, Z.; Men, Y. Direct formation of different crystalline forms in butene-1/ethylene copolymer via manipulating melt temperature. Macromolecules 2014, 47, 8653–8662. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, P.; Lu, Y.; Men, Y. Mechanism of polymorph selection during crystallization of random butene-1/ethylene copolymer. Chin. J. Polym. Sci. 2016, 34, 1014–1020. [Google Scholar] [CrossRef]
- Su, Z.; Wang, H.; Dong, J.; Zhang, X.; Dong, X.; Zhao, Y.; Yu, J.; Han, C.C.; Xu, D.; Wang, D. Conformation transition and crystalline phase variation of long chain branched isotactic polypropylenes (LCB-iPP). Polymer 2007, 48, 870–876. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Q.; Men, Y. Molecular weight dependency of crystallization and melting behavior of β-nucleated isotactic polypropylene. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 1301–1308. [Google Scholar] [CrossRef]
- Zhao, J.; Sun, Y.; Men, Y. Melt temperature and initial polymorphs dependencies of polymorphs selection during subsequent crystallization in propylene-ethylene random copolymer. Ind. Eng. Chem. Res. 2017, 56, 198–205. [Google Scholar] [CrossRef]
- Xiang, S.; Jun, S.; Li, G.; Bian, X.; Feng, L.; Chen, X.; Liu, F.; Huang, S. Effects of molecular weight on the crystallization and melting behaviors of poly(l-lactide). Chin. J. Polym. Sci. 2016, 34, 69–76. [Google Scholar] [CrossRef]
- Cao, J.; Wen, N.; Zheng, Y. Effect of long chain branching on the rheological behavior, crystallization and mechanical properties of polypropylene random copolymer. Chin. J. Polym. Sci. 2016, 34, 1158–1171. [Google Scholar] [CrossRef]
- De Rosa, C.; Auriemma, F.; Ruiz de Ballesteros, O.; Esposito, F.; Laguzza, D.; Di Girolamo, R.; Resconi, L. Crystallization properties and polymorphic behavior of isotactic poly(1-butene) from metallocene catalysts: The crystallization of form I from the melt. Macromolecules 2009, 42, 8286–8297. [Google Scholar] [CrossRef]
- De Rosa, C.; Auriemma, F.; Villani, M.; Ruiz de Ballesteros, O.; Di Girolamo, R.; Di Girolamo, R.; Tarallo, O.; Malafronte, A. Mechanical properties and stress-induced phase transformations of metallocene isotactic poly(1-butene): The influence of stereodefects. Macromolecules 2014, 47, 1053–1064. [Google Scholar] [CrossRef]
- De Rosa, C.; Auriemma, F.; Resconi, L. Metalloorganic polymerization catalysis as a tool to probe crystallization properties of polymers: The case of isotactic poly(1-butene). Angew. Chem. Int. Ed. 2009, 48, 9871–9874. [Google Scholar] [CrossRef] [PubMed]
- Turner Jones, A. Crystalline phases in copolymers of butene and 3-methylbutene. J. Polym. Sci. Part B Polym. Lett. 1965, 3, 591–600. [Google Scholar] [CrossRef]
- Stolte, I.; Cavallo, D.; Alfonso, G.C.; Portale, G.; van Drongelen, M.; Androsch, R. Form I’ crystal formation in random butene-1/propylene copolymers as revealed by real-time X-ray scattering using synchrotron radiation and fast scanning chip calorimetry. Eur. Polym. J. 2014, 60, 22–32. [Google Scholar] [CrossRef]
- Ji, Y.; Su, F.; Cui, K.; Huang, N.; Qi, Z.; Li, L. Mixing assisted direct formation of isotactic poly(1-butene) form I’ crystals from blend melt of isotactic poly(1-butene)/polypropylene. Macromolecules 2016, 49, 1761–1769. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, Z.; Li, L. Flow-induced crystallization of polymers: Molecular and thermodynamic considerations. Macromolecules 2016, 49, 1505–1517. [Google Scholar] [CrossRef]
- Ruan, C.; Liang, K.; Liu, E. Macro-micro simulation for polymer crystallization in couette flow. Polymers 2017, 9, 699. [Google Scholar] [CrossRef]
- Petermann, J.; Gohil, R.M. A new method for the preparation of high modulus thermoplastic films. J. Mater. Sci. 1979, 14, 2260–2264. [Google Scholar] [CrossRef]
- Gohil, R.M.; Miles, M.J.; Petermann, J. On the molecular mechanism of the crystal transformation (tetragonal-hexagonal) in polybutene-1. J. Macromol. Sci. Part B Phys. 1982, 21, 189–201. [Google Scholar] [CrossRef]
- Yan, S. Origin of oriented recrystallization of carbon coated pre-oriented ultra-thin polymer films. Macromolecules 2003, 36, 339–345. [Google Scholar] [CrossRef]
- Li, L.; Liu, T.; Zhao, L. Direct melt-crystallization of isotactic poly-1-butene with form I’ using high-pressure CO2. Polymer 2011, 52, 5659–5668. [Google Scholar] [CrossRef]
- Li, L.; Liu, T.; Zhao, L. Direct fabrication of porous isotactic poly-1-butene with form I from the melt using CO2. Macromol. Rapid Commun. 2011, 32, 1834–1838. [Google Scholar] [CrossRef] [PubMed]
- Handa, Y.P.; Zhang, Z.; Wong, B. Effect of compressed CO2 on phase transitions and polymorphism in syndiotactic polystyrene. Macromolecules 1997, 30, 8499–8504. [Google Scholar] [CrossRef]
- Zhang, Z.; Nawaby, A.V.; Day, M. CO2-delayed crystallization of isotactic polypropylene: A kinetic study. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 1518–1525. [Google Scholar] [CrossRef]
- Varga, J. β-Modification of isotactic polypropylene: Preparation, Structure, Processing, Properties, and Application. J. Macromol. Sci. Part B Phys. 2002, 41, 1121–1171. [Google Scholar] [CrossRef]
- Stocker, W.; Schumacher, M.; Graff, S.; Thierry, A.; Wittmann, J.C.; Lotz, B. Epitaxial crystallization and AFM investigation of a frustrated polymer structure: Isotactic poly(propylene), β phase. Macromolecules 1998, 31, 807–814. [Google Scholar] [CrossRef]
- Bai, H.; Huang, C.; Xiu, H.; Zhang, Q.; Fu, Q. Enhancing mechanical performance of polylactide by tailoring crystal morphology and lamellae orientation with the aid of nucleating agent. Polymer 2014, 55, 6924–6934. [Google Scholar] [CrossRef]
- Schneider, S.; Drujon, X.; Wittmann, J.C.; Lotz, B. Impact of nucleating agents of PVDF on the crystallization of PVDF/PMMA blends. Polymer 2001, 42, 8799–8806. [Google Scholar] [CrossRef]
- Shi, S.; Zhang, X.; Liu, Y.; Nie, M.; Wang, Q. Crystalline modification and morphology of polypropylene developed under the combined effects of montmorillonite and self-assembly β nucleating agent. Compos. Sci. Technol. 2016, 135, 76–82. [Google Scholar] [CrossRef]
- Kai, W.; Zhu, B.; He, Y.; Inoue, Y. Crystallization of poly(butylene adipate) in the presence of nucleating agents. J. Polym. Sci. Part B Polym. Phys. 2005, 43, 2340–2351. [Google Scholar] [CrossRef]
- Zhang, H.X.; Park, M.J.; Moon, Y.K.; Ko, E.B.; Zhang, X.Q.; Yoon, K.B. An efficient organic additive to control the crystallization rate of aliphatic polyketone: A non-isothermal crystallization kinetics study. Chin. J. Polym. Sci. 2017, 35, 547–557. [Google Scholar] [CrossRef]
- Liang, R.; Chen, Y.C.; Zhang, C.Q.; Yin, J.; Liu, X.L.; Wang, L.K.; Kong, R.; Feng, X.; Yang, J.J. Crystallization behavior of biodegradable poly(ethylene adipate) modulated by a benign nucleating agent: Zinc phenylphosphonate. Chin. J. Polym. Sci. 2017, 35, 558–568. [Google Scholar] [CrossRef]
- Luo, B.J.; Li, H.F.; Zhang, W.Y.; Zhou, C.B.; Li, J.Q.; Lu, C.H.; He, X.H.; Jiang, S.C. Mechanistic insights into the shear effects on isotactic polypropylene crystallization containing β-nucleating agent. Chin. J. Polym. Sci. 2017, 35, 672–680. [Google Scholar] [CrossRef]
- Zhang, K.J.; Qiu, Z.B. Enhanced crystallization rate of biodegradable poly(ε-caprolactone) by cyanuric acid as an efficient nucleating agent. Chin. J. Polym. Sci. 2017, 35, 1517–1523. [Google Scholar] [CrossRef]
- Thomason, J.L.; Van Rooyen, A.A. Transcrystallized interphase in thermoplastic composites. Part II Influence of interfacial stress, cooling rate, fibre properties and polymer molecular weight. J. Mater. Sci. 1992, 27, 889–907. [Google Scholar] [CrossRef]
- Wang, C.; Liu, C.R. Transcrystallization of polypropylene composites: Nucleating ability of fibres. Polymer 1999, 40, 289–298. [Google Scholar] [CrossRef]
- Wittmann, J.C.; Lotz, B. Epitaxial crystallization of polymers on organic and polymeric substrates. Prog. Polym. Sci. 1990, 15, 909–948. [Google Scholar] [CrossRef]
- Xin, R.; Zhang, J.; Sun, X.; Li, H.; Qiu, Z.; Yan, S. Epitaxial effects on polymer crystallization. Adv. Polym. Sci. 2017, 277, 55–94. [Google Scholar]
- Liu, L.; Ren, Z.; Xiao, C.; He, B.; Dong, H.; Yan, S.; Hu, W.; Wang, Z. Epitaxially-crystallized oriented naphthalene bis(dicarboximide) morphology for significant performance improvement of electron-transporting thin-film transistors. Chem. Commun. 2016, 52, 4902–4905. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Li, Y.; Ren, Z.; Bryce, M.R.; Li, H.; Yan, S. Anisotropic highly-conductive films of poly(3-methylthiophene) from epitaxial electropolymerization on oriented poly(vinylidenefluoride). Chem. Sci. 2014, 5, 3240–3245. [Google Scholar] [CrossRef]
- Zhou, H.; Jiang, S.; Yan, S. Epitaxial crystallization of poly(3-hexylthiophene) on a highly oriented polyethylene thin film from solution. J. Phys. Chem. B 2011, 115, 13449–13454. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Zhang, J.; Li, L.; Wang, Z.; Yang, C.; Takahashi, I.; Ozaki, Y.; Yan, S. A study on the epitaxial ordering process of the polycarprolactone on the highly oriented polyethylene substrate. Macromolecules 2010, 43, 362–366. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Li, H.; Shen, D.; Zhang, J.; Ozaki, Y.; Yan, S. Epitaxial crystallization of isotactic poly(methyl methacrylate) on highly oriented polyethylene. J. Phys. Chem. B 2006, 110, 738–742. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ruan, J.; Yan, S. Epitaxy of PLLA/PCL blends on highly oriented polyethylene substrate. Acta Polym. Sin. 2017, 9, 1524–1529. [Google Scholar]
- Sun, Y.; Li, H.; Huang, Y.; Chen, E.; Gan, Z.; Yan, S. Epitaxial crystallization of poly(butylene adipate) on highly oriented isotactic polypropylene thin film. Polymer 2006, 47, 2455–2459. [Google Scholar] [CrossRef]
- Sun, Y.; Li, H.; Huang, Y.; Chen, E.; Zhao, L.; Gan, Z.; Yan, S. Epitaxial crystallization of poly(butylene adipate) on highly oriented polyethylene thin film. Macromolecules 2005, 38, 2739–2743. [Google Scholar] [CrossRef]
- Kopp, S.; Wittmann, J.C.; Lotz, B. Epitaxial crystallization and crystalline polymorphism of poly(1-butene): Forms III and II. Polymer 1994, 35, 908–915. [Google Scholar] [CrossRef]
- Kopp, S.; Wittmann, J.C.; Lotz, B. Epitaxial crystallization and crystalline polymorphism of poly(1-butene): Form I. Polymer 1994, 35, 916–924. [Google Scholar] [CrossRef]
- Mathieu, C.; Stocker, W.; Thierry, A.; Wittmann, J.C.; Lotz, B. Epitaxy of isotactic poly(1-butene): New substrates, impact and attempt at recognition of helix orientation in form I’ by AFM. Polymer 2001, 42, 7033–7047. [Google Scholar] [CrossRef]
- Kopp, S.; Wittmann, J.C.; Lotz, B. Epitaxy of helical polyolefins. Macrornol. Symp. 1995, 98, 917–923. [Google Scholar] [CrossRef]
- Lotz, B.; Wittmann, J.C. Interfacial interactions and structure of polyolefins. Macrornol. Symp. 1996, 101, 91–94. [Google Scholar] [CrossRef]
- Lotz, B.; Wittmann, J.C. Epitaxy of helical polyolefins: Polymer blends and polymer nucleating agent systems. Makromol. Chem. 1984, 185, 2043–2052. [Google Scholar] [CrossRef]
- Nakamura, K.; Aoike, T.; Usaka, K.; Kanamoto, T. Phase transformation in poly(1-butene) upon drawing. Macromolecules 1999, 32, 4975–4982. [Google Scholar] [CrossRef]
- Natta, G.; Corradisti, P.; Bassi, I.W. Crystal structure of isotactic poly-alpha-butene. NuovoCimento 1960, 1, 52–67. [Google Scholar] [CrossRef]
- Rakus, J.P.; Mason, C.D. The direct formation of modification I’ polybutene-1. Polym. Lett. 1966, 4, 467–468. [Google Scholar] [CrossRef]
- Boor, J.; Youngman, E.A., Jr. Polymorphism in poly-1-butene: Apparent direct formation of modification I. Polym. Lett. 1964, 2, 903–907. [Google Scholar] [CrossRef]
- Clampitt, B.H.; Hughes, R.H. Differential thermal analysis of polybutene-1. J. Polym. Sci. Part C 1964, 6, 43–51. [Google Scholar] [CrossRef]
- Luongo, J.P.; Salovey, R. Infrared characterization of polymorphism in polybutene-1. J. Polym. Sci. Part B 1966, 4, 997–1008. [Google Scholar] [CrossRef]
- Asada, T.; Sasada, J.; Onagi, S. Rheo-optical studies of high polymers. XXI the deformation process and crystal transformation in polybutene-1. Polym. J. 1972, 3, 350–356. [Google Scholar] [CrossRef]
- Fujiwara, Y. Orientation: II-I phase transformation of melt-crystallized oriented lamellae of polybutene-1 by shear deformation. Polym. Bull. 1985, 13, 253–258. [Google Scholar] [CrossRef]
- Yang, Y.C.; Geil, P.H. Deformation mechanisms of isotactic poly(1-butene). Makromol. Chem. 1985, 186, 1961–1978. [Google Scholar] [CrossRef]
- Chau, K.W.; Yang, Y.C.; Geil, P.H. Tetragonal-twinned hexagonal crystal phase transformation in polybutene-1. J. Mater. Sci. 1986, 21, 3002–3014. [Google Scholar] [CrossRef]
- Hsu, C.C.; Geil, P.H. Structure and properties of polybutylene crystallized from the glassy state. 1. X-ray scattering, DSC, and torsion pendulum. J. Macromol. Sci.-Phys. 1986, 25, 433–466. [Google Scholar] [CrossRef]
- Kopp, S.; Wittmann, J.C.; Lotz, B. Phase II to phase I crystal transformation in polybutene-1 single crystals: A reinvestigation. J. Mater. Sci. 1994, 29, 6159–6166. [Google Scholar] [CrossRef]
- Lotz, B.; Mathieu, C.; Thierry, A.; Lovinger, A.J.; De Rosa, C.; Ruiz de Ballesteros, O.; Auriemma, F. Chirality constraints in crystal-crystal transformations: Isotactic poly(1-butene) versus syndiotactic polypropylene. Macromolecules 1998, 31, 9253–9257. [Google Scholar] [CrossRef]
- Tosaka, M.; Kamijo, T.; Tsuji, M.; Kohjiya, S.; Ogawa, T.; Isoda, S.; Kobayashi, T. High-resolution transmission electron microscopy of crystal transformation in solution-grown lamellae of isotactic polybutene-1. Macromolecules 2000, 33, 9666–9672. [Google Scholar] [CrossRef]
- Yan, S.; Petermann, J. Oriented recrystallization of pre-oriented thin polymer films. Polymer 2000, 41, 6679–6681. [Google Scholar] [CrossRef]
- Kaszonyiová, M.; Rybnikář, F. The Three Processes of Phase II-I Transformation of Isotactic Polybutene-1. J. Macromol. Sci. Part B Phys. 2018, 57, 278–286. [Google Scholar] [CrossRef]
- Maruyama, M.; Sakamoto, Y.; Nozaki, K.; Yamamoto, T.; Kajioka, H.; Toda, A.; Yamada, K. Kinetic study of the II-I phase transition of isotactic polybutene-1. Polymer 2010, 51, 5532–5538. [Google Scholar] [CrossRef]
- Li, L.; Liu, T.; Zhao, L.; Yuan, W. CO2-induced phase transition of isotactic poly-1-butene with form III upon heating. Macromolecules 2011, 44, 4836–4844. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, T.; Li, L.; Li, D.; Yuan, W.; Zhao, L. Controlling crystal phase transition from form II to I in isotactic poly-1-butene using CO2. Polymer 2012, 53, 6102–6111. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, K.; Tian, N.; Zhou, W.; Meng, L.; Li, L.; Ma, Z.; Wang, X. Stretch-induced crystal-crystal transition of polybutene-1: An in situ synchrotron radiation wide-angle X-ray scattering study. Macromolecules 2012, 45, 2764–2772. [Google Scholar] [CrossRef]
- Qiao, Y.; Wang, Q.; Men, Y. Kinetics of nucleation and growth of form II to I polymorphic transition in polybutene-1 as revealed by stepwise annealing. Macromolecules 2016, 49, 5126–5136. [Google Scholar] [CrossRef]
- Qiao, Y.; Wang, H.; Men, Y. Retardance of form II to form I transition in polybutene-1 at late stage: A proposal of a new mechanism. Macromolecules 2018, 51, 2232–2239. [Google Scholar] [CrossRef]
- Hsu, T.C.; Geil, P.H. Deformation and stress-induced transformation of polybutene-1. J. Macromol. Sci.-Phys. 1989, 28, 69–95. [Google Scholar] [CrossRef]
- Di Lorenzo, M.L.; Androsch, R.; Righetti, M.C. The irreversible form II to form I transformation in random butene-1/ethylene copolymers. Eur. Polym. J. 2015, 67, 264–273. [Google Scholar] [CrossRef]
- Goldbach, G. Zurumwandlung der polymorphenstruktur von polybuten-1 unter der wirkung mechanischer spannungen. Macromol. Mater. Eng. 1973, 29, 213–227. [Google Scholar]
- Goldbach, G. Spannungsinduzierte modifikations umwandlung II nach I von polybuten-1. Angew. Makromol. Chem. 1974, 39, 175–188. [Google Scholar] [CrossRef]
- Su, F.; Li, X.; Zhou, W.; Chen, W.; Li, H.; Cong, Y.; Hong, Z.; Qi, Z.; Li, L. Accelerating crystalecrystal transition in poly(1-butene) with two-step crystallization: An in-situ microscopic infrared imaging and microbeam X-ray diffraction study. Polymer 2013, 54, 3408–3416. [Google Scholar] [CrossRef]
- Jiang, S.; Duan, Y.; Li, L.; Yan, D.; Yan, S. An afm study on the structure and melting behavior of melt-crystallized isotactic poly(1-butene). Polymer 2004, 45, 6365–6374. [Google Scholar] [CrossRef]
- Shi, J.; Wu, P.; Li, L.; Liu, T.; Zhao, L. Crystalline transformation of isotactic polybutene-1 in supercritical CO2 studied by in-situ fourier transform infrared spectroscopy. Polymer 2009, 50, 5598–5604. [Google Scholar] [CrossRef]
- Jones, A.T. Cocrystallization in copolymers of α-olefins II—Butene-1 copolymers and polybutene type II/I crystal phase transition. Polymer 1966, 7, 23–59. [Google Scholar] [CrossRef]
- Tanaka, A.; Sugimoto, N.; Asada, T.; Onogi, S. Orientation and crystal transformation in polybutene-1 under stress relaxation. Polym. J. 1975, 7, 529–537. [Google Scholar] [CrossRef]
- Chen, W.; Li, X.; Li, H.; Su, F.; Zhou, W.; Li, L. Deformation-induced crystal−crystal transition of polybutene-1: An in situ ftir imaging study. J. Mater. Sci. 2013, 48, 4925–4933. [Google Scholar] [CrossRef]
- Cavallo, D.; Kanters, M.J.W.; Caelers, H.J.M.; Portale, G.; Govaert, L.E. Kinetics of the polymorphic transition in isotactic poly(1-butene) under uniaxial extension. New insights from designed mechanical histories. Macromolecules 2014, 47, 3033–3040. [Google Scholar] [CrossRef]
- Li, L.; Liu, T.; Zhao, L.; Yuan, W.K. CO2-induced crystal phase transition from form II to I in isotactic poly-1-butene. Macromolecules 2009, 42, 2286–2290. [Google Scholar] [CrossRef]
- Stolte, I.; Androsch, R. Kinetics of the melt-Form II phase transition in isotactic random butene-1/ethylene copolymers. Polymer 2013, 54, 7033–7040. [Google Scholar] [CrossRef]
- Azzurri, F.; Alfonso, G.C.; Gomez, M.A.; Marti, M.C.; Ellis, G.; Marco, C. Polymorphic transformation in isotactic 1-butene/ethylene copolymers. Macromolecules 2004, 37, 3755–3762. [Google Scholar] [CrossRef]
- Gianotti, G.; Capizzi, A. Butene-1/propylene copolymers, influence of the comonomeric units on polymorphism. Macromol. Chem. Phys. 1969, 124, 152–159. [Google Scholar] [CrossRef]
- Qiao, Y.; Men, Y. Intercrystalline links determined kinetics of form II to I polymorphic transition in polybutene-1. Macromolecules 2017, 50, 5490–5497. [Google Scholar] [CrossRef]
- Boor, J., Jr.; Mitchell, J.C. Kinetics of crystallization and a crystal-crystal transition in poly-1-butene. J. Polym. Sci. Part A 1963, 1, 59–84. [Google Scholar] [CrossRef]
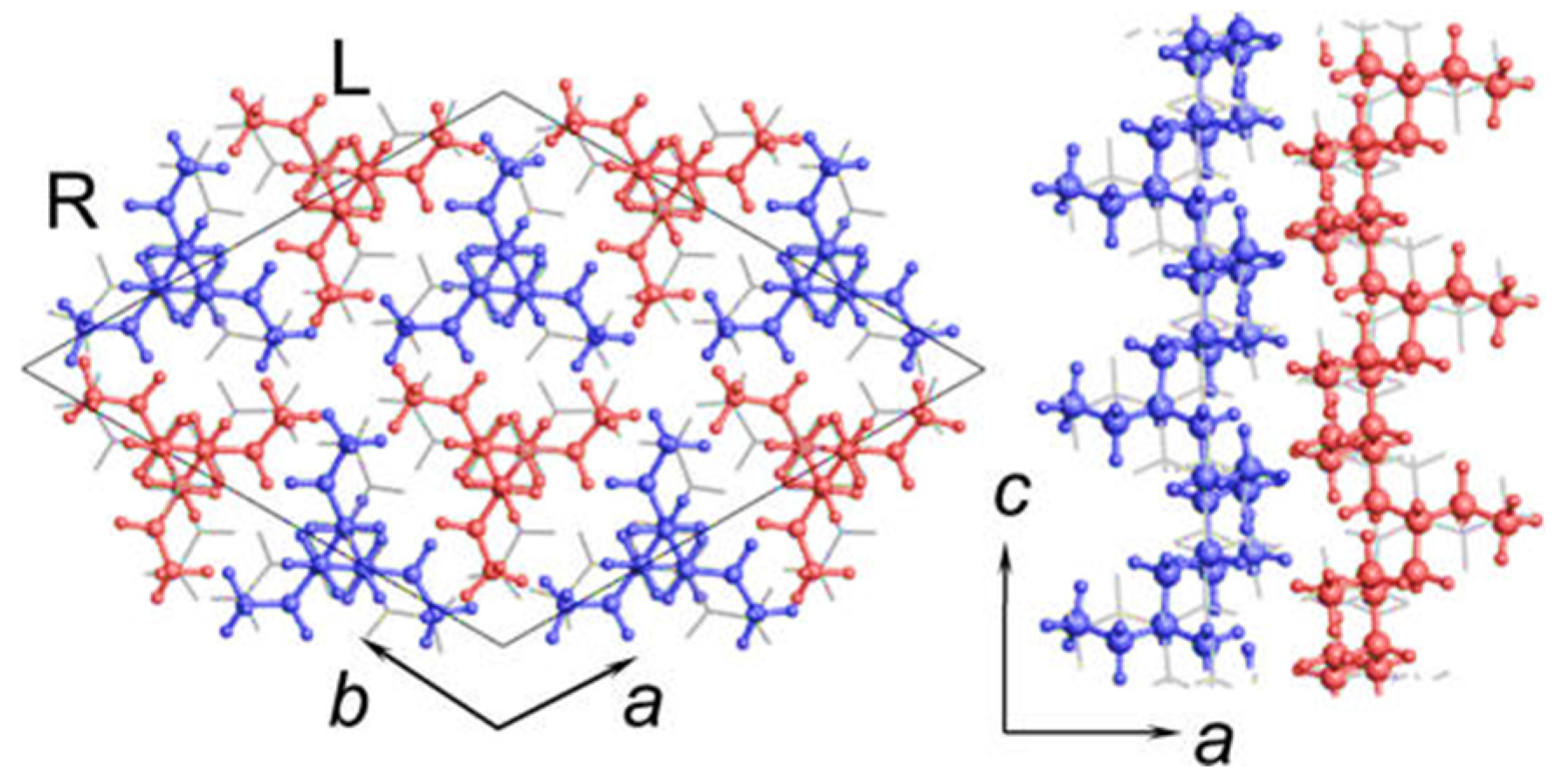
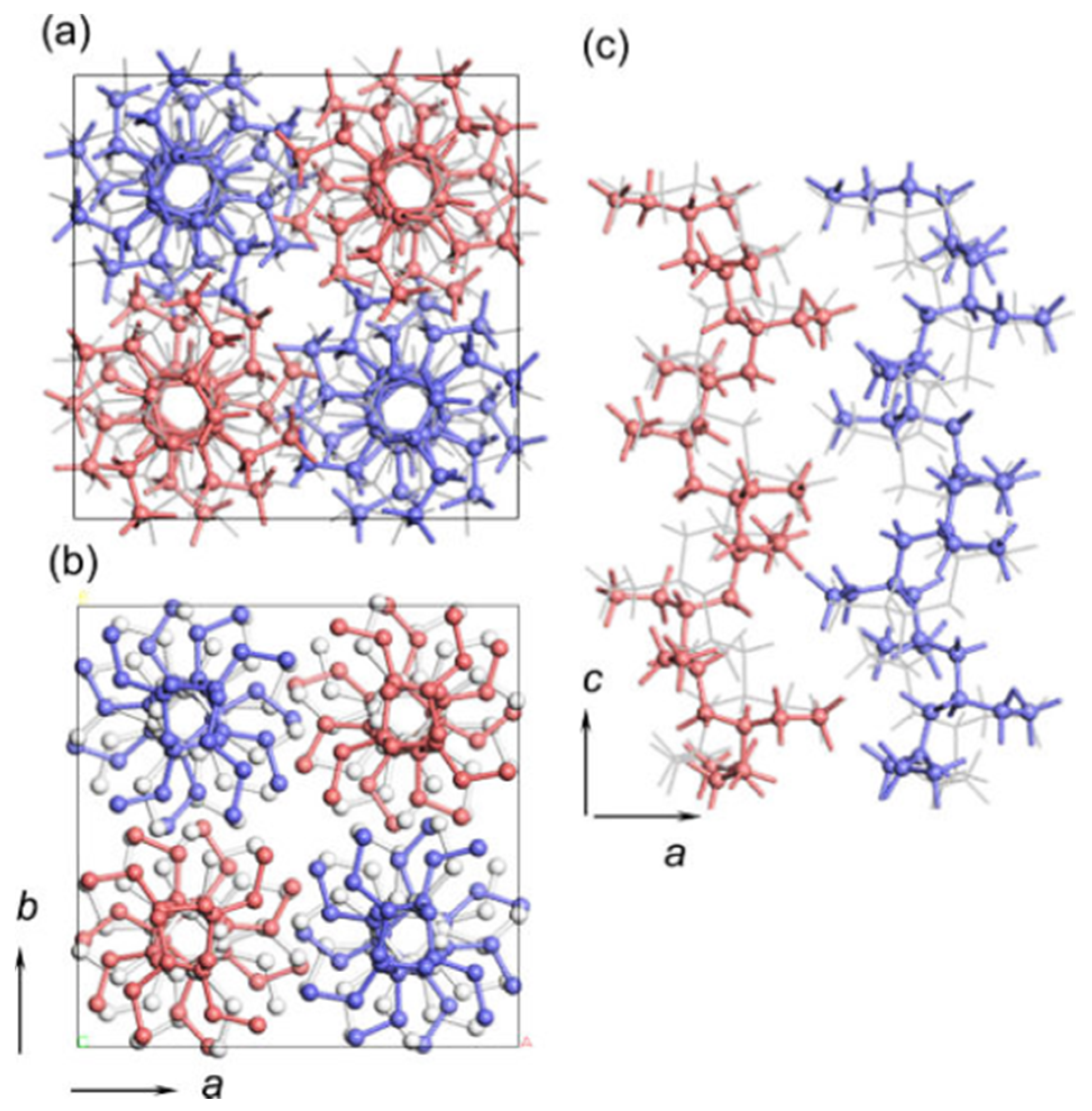
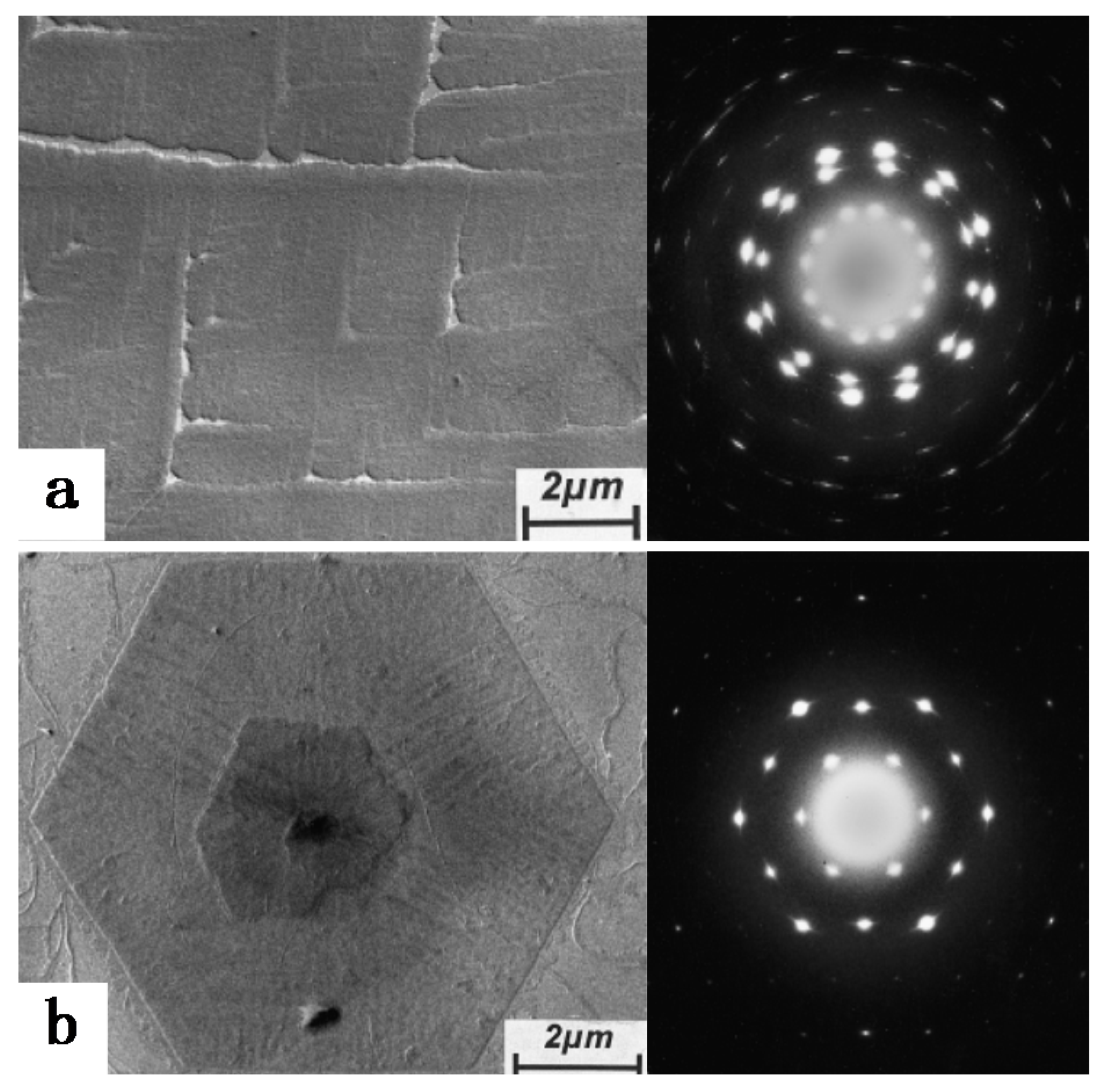

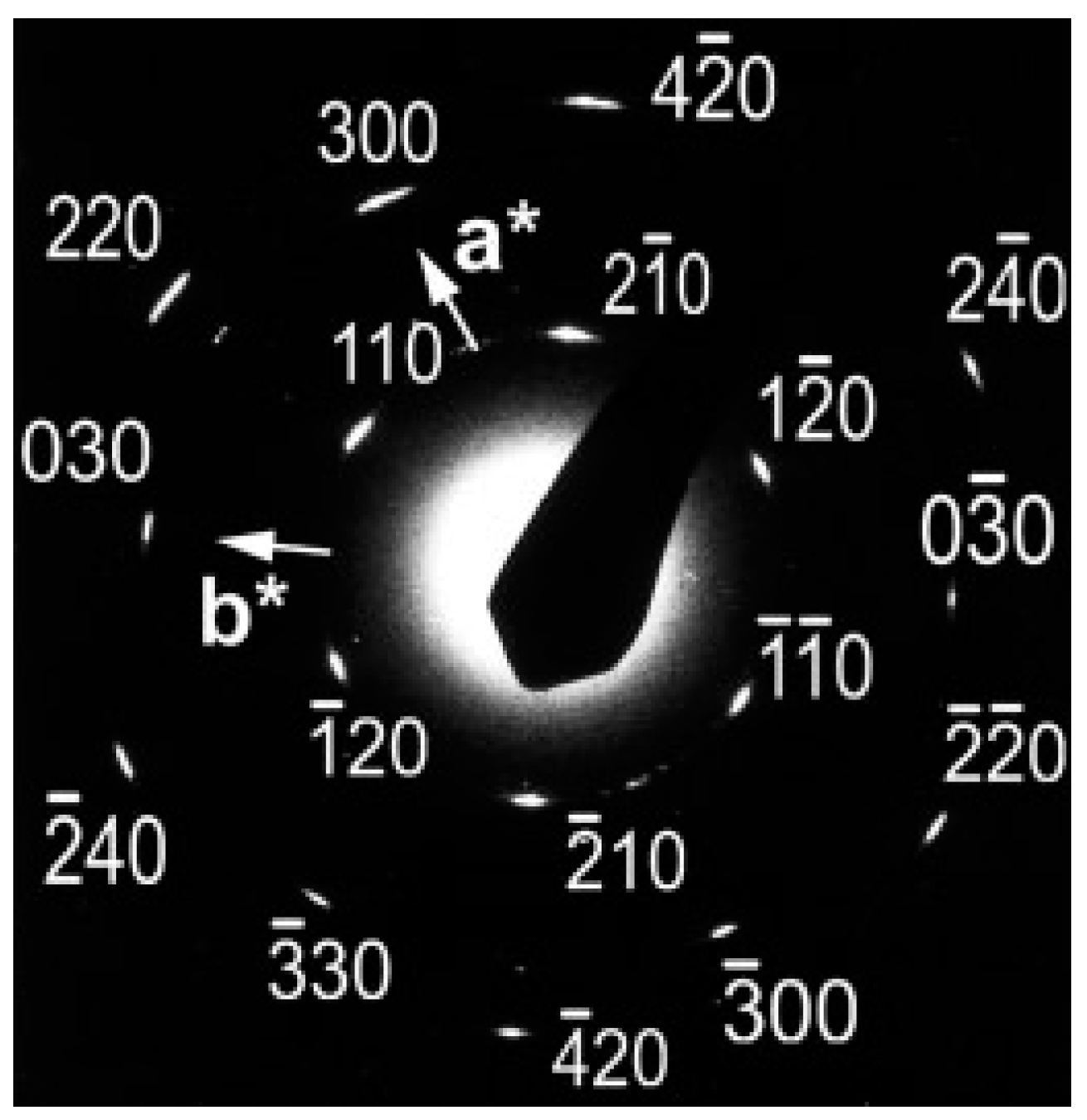



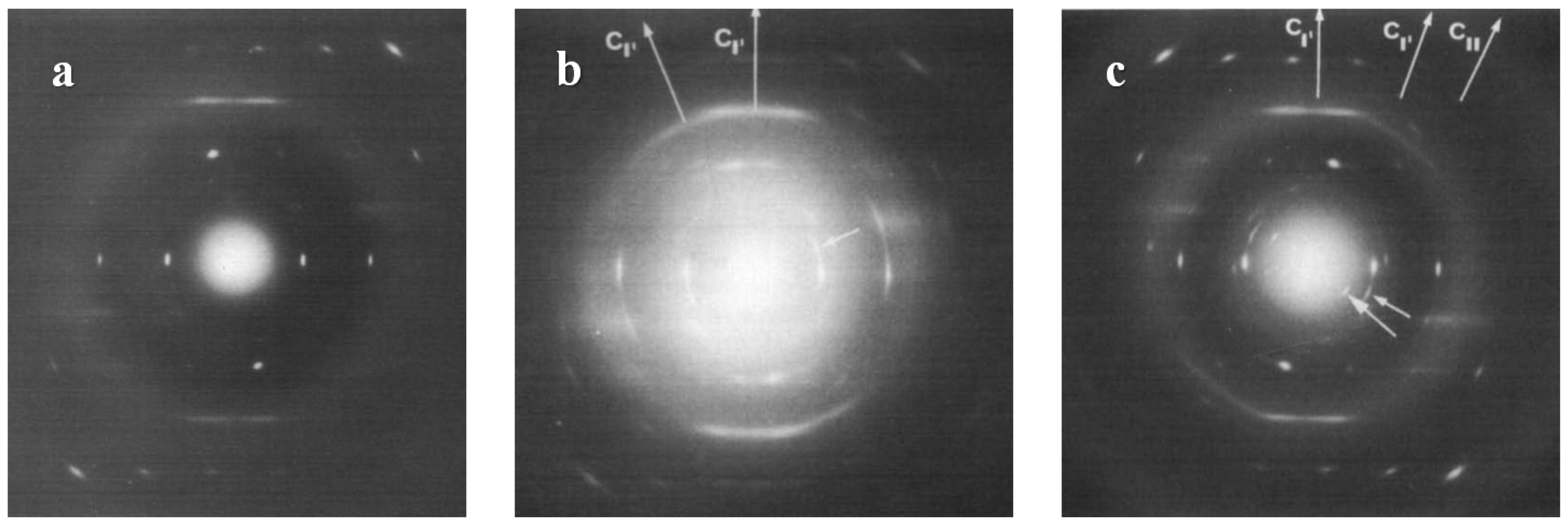

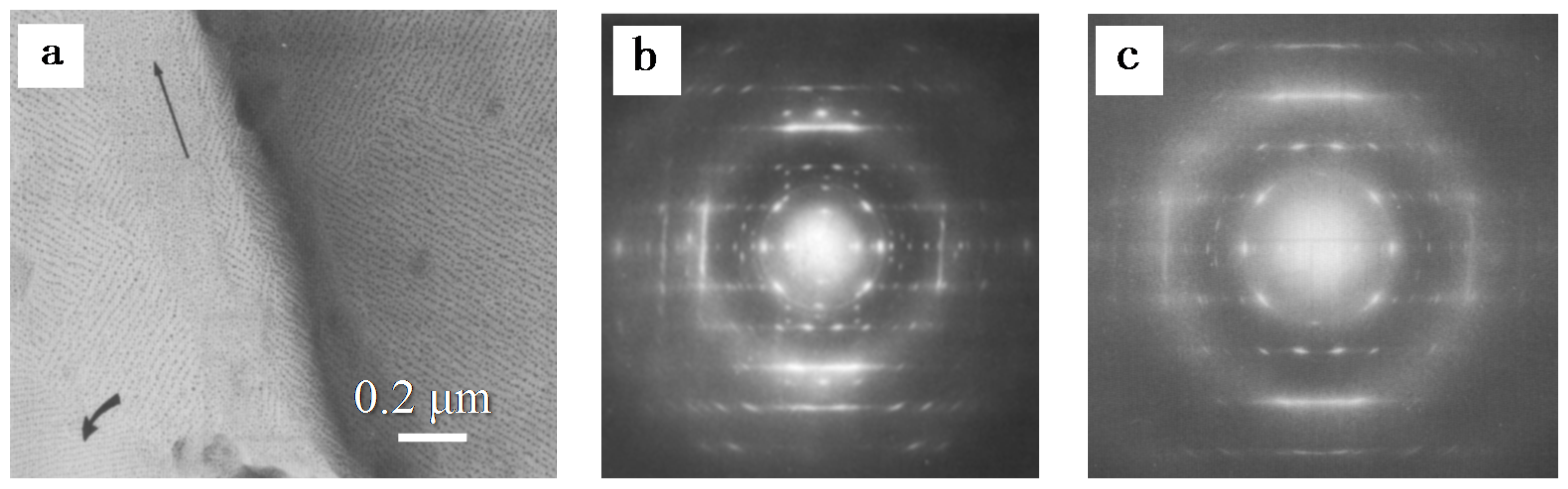

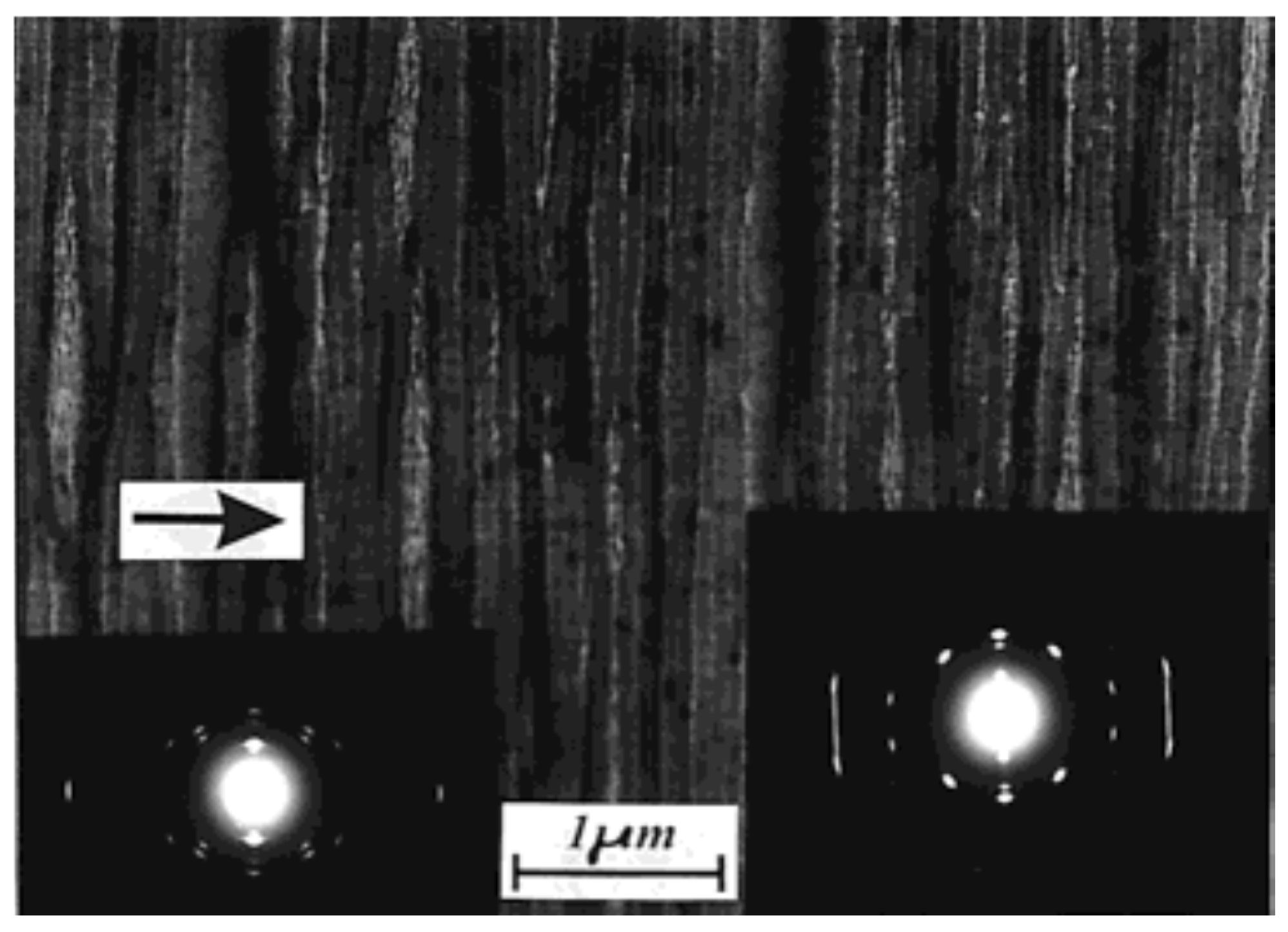
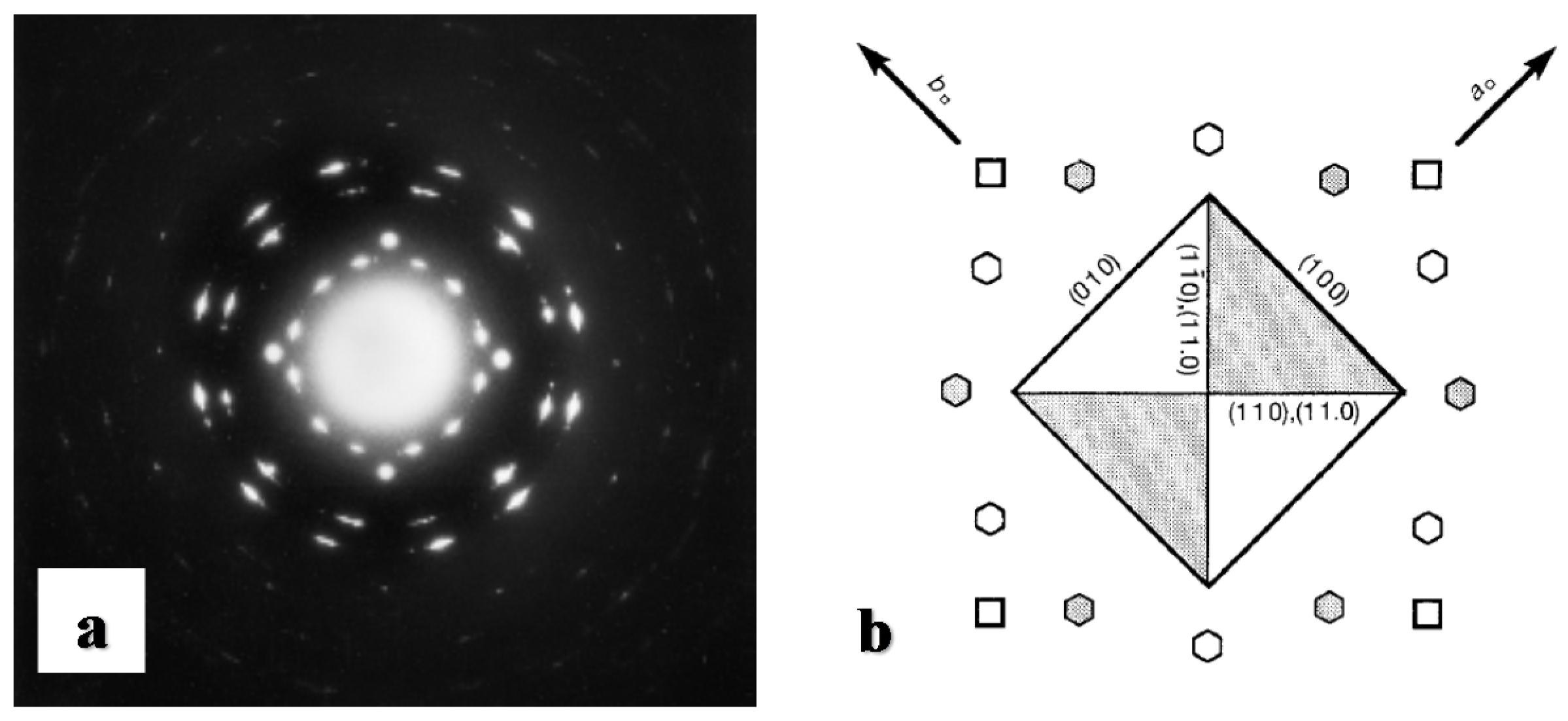


| Substrate | Crystalline phase | Number of orientation | Epitaxial orientation relationships |
|---|---|---|---|
| 2-Quinoxalinol | III | 1 | (110)iPBu // (001)2-Qol CiPBu // [010]2-Qol |
| Benzoic acid | II | 2 (∠94°) | (100) or (010)iPBu // (011)BA CiPBu // [110] or [BA |
| 4-Bromobenzoic acid | II (+) | 2 (∠50°) | (100) or (010)iPBu // (100)4BrBA CiPBu // [032] or [03]4BrBA |
| I’ (−) | 2 (∠22°) | (110)iPBu // (100)4BrBA CiPBu˄ [001]4BrBA = ±11° | |
| 4-Chlorobenzoic acid | I’ (+) | 1 or 2 (∠22°) | (110)iPBu // (100)4ClBA CiPBu˄ [001]4ClBA = ±11° |
| II (−) | 1 | (100)iPBu // (100)4ClBA CiPBu // [032]4ClBA | |
| Potassium hydrogen 4-chlorobenzoate | I’ | 2 (∠60°) | (110)iPBu // (100)PH4ClB CiPBu˄ [001]PH4ClB = ±30° |
| Potassium 4-chlorobenzoate | I’ | 2 (∠60°) | (110)iPBu // (100)P4ClB CiPBu˄ [001]P4ClB = ±30° |
| Potassium 4-bromobenzoate | I’ | 2 (∠60°) | (110)iPBu // (100)P4BrB CiPBu˄ [001]P4BrB = ±30° |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xin, R.; Zhang, J.; Sun, X.; Li, H.; Ren, Z.; Yan, S. Polymorphic Behavior and Phase Transition of Poly(1-Butene) and Its Copolymers. Polymers 2018, 10, 556. https://doi.org/10.3390/polym10050556
Xin R, Zhang J, Sun X, Li H, Ren Z, Yan S. Polymorphic Behavior and Phase Transition of Poly(1-Butene) and Its Copolymers. Polymers. 2018; 10(5):556. https://doi.org/10.3390/polym10050556
Chicago/Turabian StyleXin, Rui, Jie Zhang, Xiaoli Sun, Huihui Li, Zhongjie Ren, and Shouke Yan. 2018. "Polymorphic Behavior and Phase Transition of Poly(1-Butene) and Its Copolymers" Polymers 10, no. 5: 556. https://doi.org/10.3390/polym10050556






