1. Introduction
Polymers are widely used materials in different fields owing to their good mechanical and physicochemical properties as well as their economic accessibility [
1,
2,
3]. The use of polymers for membrane production for applying in different membrane processes enables highly effective separation of liquid and gas mixtures. There are a large number of commercial polymer membranes that have undergone the necessary series of studies and found their application in membrane processes in industry due to their low cost, good film-formability, and mechanical strength. However, it should be noted that polymeric membranes are characterized by a number of drawbacks, such as low resistance to mechanical impurities, relatively poor chemical stability, low thermal resistance, and low permeability for some polymers. Thus, modern production conditions demand improved quality of the final product and productivity for existing processes. In this regard, the development of new membranes is a highly important task.
Among the membrane processes, pervaporation is one of the perspective membrane technologies that can be a good alternative to classical separation processes, applicable for liquid mixtures containing low-molecular weight components. It is widely used for the dehydration of water–organic mixtures, in particular containing an azeotropic and close-boiling components. One of the key factors for the effective performance of the pervaporation (high water content in the permeate and permeability) is the proper choice of a material type for membrane preparation [
4,
5,
6].
Recently, a number of studies have been conducted using different types of hydrophilic polymers, especially poly(acrylic acid), cellulose, hydroxyl ethyl cellulose, chitosan (CS), sodium alginate, and poly(vinyl alcohol) (PVA) as membrane matrices for the pervaporation dehydration purposes [
7,
8]. Among these, PVA is considered to be one of the most widely used polymers in pervaporation, applied for the separation of water–organic mixtures, due to its unique properties such as good film-forming properties, high hydrophilicity, and chemical stability [
9,
10]. However, the permeability of PVA-based membranes is too low due to its glassy and semi-crystalline structure.
The transport characteristics of PVA membranes can be significantly improved using various methods of polymer modification and/or functionalization [
9,
11,
12,
13]. Among them, bulk (volume) and surface modifications are considered the most promising methods to get tailored membrane properties. In this regard, two modification methods were applied to PVA-based membranes in the present study.
Bulk modification was performed by the polymer blending (the addition of CS into PVA matrix) and the creation of a mixed-matrix membrane (by introducing fullerenol (polyhydroxylated fullerene) to PVA).
The blending of polymers is a quite simple bulk modification method capable to acquire characteristic advantages by combining two or more polymers in different concentration ratio for the preparation of the blended membrane with the optimal transport properties [
14].
In this study, to increase the permeability of PVA membranes, the modification of PVA matrix by a well-known biopolymer chitosan (CS) was carried out. Chitosan was chosen owing to its high chemical resistance, biodegradability, nontoxicity, and high number of amino and hydroxyl groups, which actively react with PVA groups and significantly affect the changes in the membrane properties, depending on the polymer matrix content. The PVA-CS composite has been broadly reported and investigated for the application in various fields such as packaging in food industry [
15], as hydrogels in medical and pharmaceutical industries [
16], as films with a high antibacterial activity [
17], and especially as a membrane material for gas separation [
18], filtration [
19], polymer electrolyte membrane electrochemical reactor (PEMER) [
20] and pervaporation [
21]. The literature review on the preparation of blended PVA-CS pervaporation membranes demonstrated that the studies focus on the dependence of the transport properties of the membrane on the weight ratio of both polymers in the membrane matrix. This, in turn, determines the prospects of using the membrane with a blended matrix for pervaporation of various mixtures. The developed blended PVA-CS membranes have been already tested for dehydration of 1,4-dioxane [
22], tetrahydrofuran (THF) [
23], ethylene glycol [
24], ethanol and isopropanol [
21,
25], for separation of benzene/cyclohexane mixtures [
26,
27], binary and ternary mixtures of n-butyl acetate, n-butanol and water [
28]. In the study [
21], the main goals were to determine the optimal composition of the PVA-CS membranes, cross-linked by glutaraldehyde (GA) or by heat-treating at different temperatures, for the dehydration of isopropanol (IPA) by pervaporation (PV) and to investigate interactions among these variables. It was found that the CS content was the most important factor influencing the permeation flux and separation factor. PVA-CS blend membranes were also prepared by cross-linking of the PVA with urea formaldehyde/sulfuric acid (UFS) mixture for PV dehydration of isopropanol and tetrahydrofuran (THF) at 30 °C close to their azeotropic compositions in the study [
23]. A new hollow fiber composite membrane of CS-PVA/polyvinylidene fluoride (PVDF) cross-linked with GA and sulfuric acid was prepared by casting the polymer solutions on PVDF hollow fiber support for PV dehydration of IPA [
29]. The increase of thermostability and a decrease of hydrophilicity were shown after the blending CS and PVA by TGA and contact angles. The PVA and CS membranes, modified by the amino functionalized multi-walled carbon nanotube (NH
2-MWCNT), were used for the dehydration of IPA, whilst the membrane with 10 wt % of NH
2-MWCNT showed the optimal transport performance [
30].
The creation of a mixed-matrix membrane is another promising method of modification, which allows changing the characteristics of the membrane material in a directed and flexible way, depending on the task solved. The main idea for the creating of this membrane type is the combination of the best properties and advantages of both components. These composite materials usually provide an effective integrated approach to solve practical problems, such as separation and recycling. Among the inorganic particles, carbon nanoparticles take a special place due to their physicochemical characteristics (for example, fullerenes [
31], carbon nanotubes [
32], graphene [
33], graphene oxide [
34], and fullerene derivatives [
35], etc.). In this study, fullerenol as water-soluble fullerene derivative was chosen as an inorganic filler for the introduction into PVA-CS matrix, creating a mixed-matrix membrane (bulk modification), which already showed its promising applicability as a modifier and a cross-linking agent for the pervaporation PVA-based membranes in the previous works [
36,
37,
38,
39].
Among the various surface modification methods, the development of supported membranes with a thin selective layer and the deposition of nano-sized layers by layer-by-layer assembly (LbL) can be considered as effective means of adjusting of the membrane performance to obtain desired permeation flux and separation factor [
40]. Membranes, that are covered by PEL layers on the surface, may have surface charge, and also have high hydrophilicity and, as a consequence, a strong affinity for water molecules, which makes polyelectrolytes attractive for their use in the manufacturing of the pervaporation membranes [
40]. Depending on parameters—such as the amount of deposited layer, types of applied polyelectrolytes, their ionic strength, and pH—the structure and morphology of membranes can be varied in order to improve the efficiency of the pervaporation membranes [
41,
42,
43,
44]. In the article [
44], a novel pervaporation LbL polyelectrolyte membrane with a single bilayer of polyethyleneimine (PEI) polycation and Nexar™ on a hydrolyzed polyacrylonitrile (PAN) hollow fiber substrate has been developed for the pervaporation dehydration of ethanol (85 wt %) with a good separation performance and long-term stability. The polyelectrolyte multilayer membrane, prepared by LbL adsorption of polyvinylamine (PVA) as cationic and polyvinylsulfonate (PVSu) as anionic component, was developed. It provided the improved transport properties for the separation of ethanol/water mixtures with low water content (<20 wt %), while the membrane modified by polyvinylsulfate (PVS) and polyacrylate (PAA) was suitable for the dehydration of feed with higher water content [
45]. It was shown that this multilayer composite membrane exhibited improved transport parameters for the ethanol/water separation.
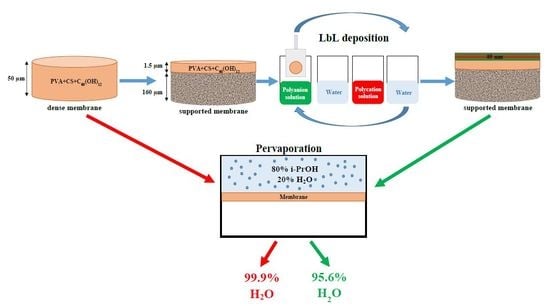
The increase of membrane permeability also can be reached by the creation of a supported membrane, consisting of a thin selective polymeric layer on the surface of a porous support allows to achieve a high permeability for commercial application. At present, supported membranes are used in various fields such as gas separation, water purification, pervaporation, etc. Dense polymeric membranes, which are very thin, usually do not possess sufficient mechanical properties and resistance, therefore, these should be deposited onto a stable support in order to avoid destruction and to create a thin selective layer to improve the performance. Various micro- and ultraporous commercial or prepared supports—consisting of mechanically rigid materials—are used for the development of a supported membrane with a thin selective PVA layer, depending on the separation task. Most of these materials are based on polysulfone and applied for copper ions removal and dehydration of ethanol mixtures [
46,
47]. Additionally, the folowing materials were used: α-Al
2O
3 hollow fiber or ceramic supports [
48,
49], poly(ether sulfone) (PES) [
50,
51], polypropylene [
52], and polyacrylonitrile [
53,
54] for the pervaporation dehydration of various aqueous mixtures, etc. We used supported membranes based on composites PVA-CS and PVA-fullerenol-CS. These were developed by the deposition of a thin selective layer (~1 μm) onto the surface of the commercial ultrafiltration support (UPM-20) based on aromatic polysulfone amide. The choice of the UPM-20 support was based on an earlier study [
55] showing good mechanical and chemical resistance of UPM-20, while other supports (including polyacrylonitrile (PAN)) could be hydrolyzed at elevated temperatures.
The obtained membranes modified in the bulk and on the surface with various methods were examined in separation of a model isopropanol (IPA)-water mixture to evaluate the changes of internal membrane structure and surface characteristics. Isopropanol is one the major industrial solvent and chemical intermediates for synthesis that is used in different fields such as chemical, pharmaceutical, medical, electronics, and cosmetic industries. Most frequently, absolutely dehydrated isopropanol is required. It is well-known that the dehydration of alcohols by traditional separation methods (distillation, rectification) is quite a difficult task due to the formation of azeotropic mixture with water (for example, the composition 12 wt % water–88 wt % IPA [
56]). Energy demanding processes with special definite conditions and the use of harmful organic solvents that form stronger azeotropic mixtures with water, which prevents the recovery of a high-purity target product, are usually needed. Pervaporation with a properly matched membrane can be a promising way to dehydrate isopropanol owing to adequate separation efficiency, low energy consumption, and performance advantages without any additional reagents in comparison to traditional separation methods.
The aim of this study was to develop new PVA-based membranes having enhanced properties for the application to industrial dehydration of alcohol feed. To improve transport properties of PVA membranes, the different techniques for bulk and surface modifications were studied and implemented for the development of supported PVA composite membranes. Bulk modification included introduction of CS and fullerenol to PVA matrix. The choice of these modifiers was due to their nontoxicity, water-solubility, and biodegradability, which allows development of novel green membranes. Moreover, big amounts of highly reactive polar groups of such modifiers contribute to the increase of surface hydrophilicity and permeation flux of PVA-based membranes. The surface modification of obtained membranes based on composites PVA-CS and PVA-fullerenol-CS included the preparation of supported membranes on ultrafiltration commercial UPM-20 support and the deposition of PEL layers (by LbL) for the improved pervaporation performance of composite supported membranes. Moreover, the nature of water soluble PELs and deposited number of PELs were studied to find the optimal composition and conditions of surface modification. The transport properties of developed membranes were evaluated in separation of a model mixture of IPA (80 wt %) and water (20 wt %) by pervaporation. The stability and indelibility of PEL layers (PSS, CS) were studied by scanning electron microscopy (SEM) and measuring the contact angles by water.