Electrospun Polyethylene Terephthalate Nonwoven Reinforced Polypropylene Separator: Scalable Synthesis and Its Lithium Ion Battery Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Composite Separators
2.2. Characterization of Composite Separator
2.3. Electrochenmical Performance of Composite Separator
3. Results and Discussion
3.1. Membrane Characteristics of the Composite Separators
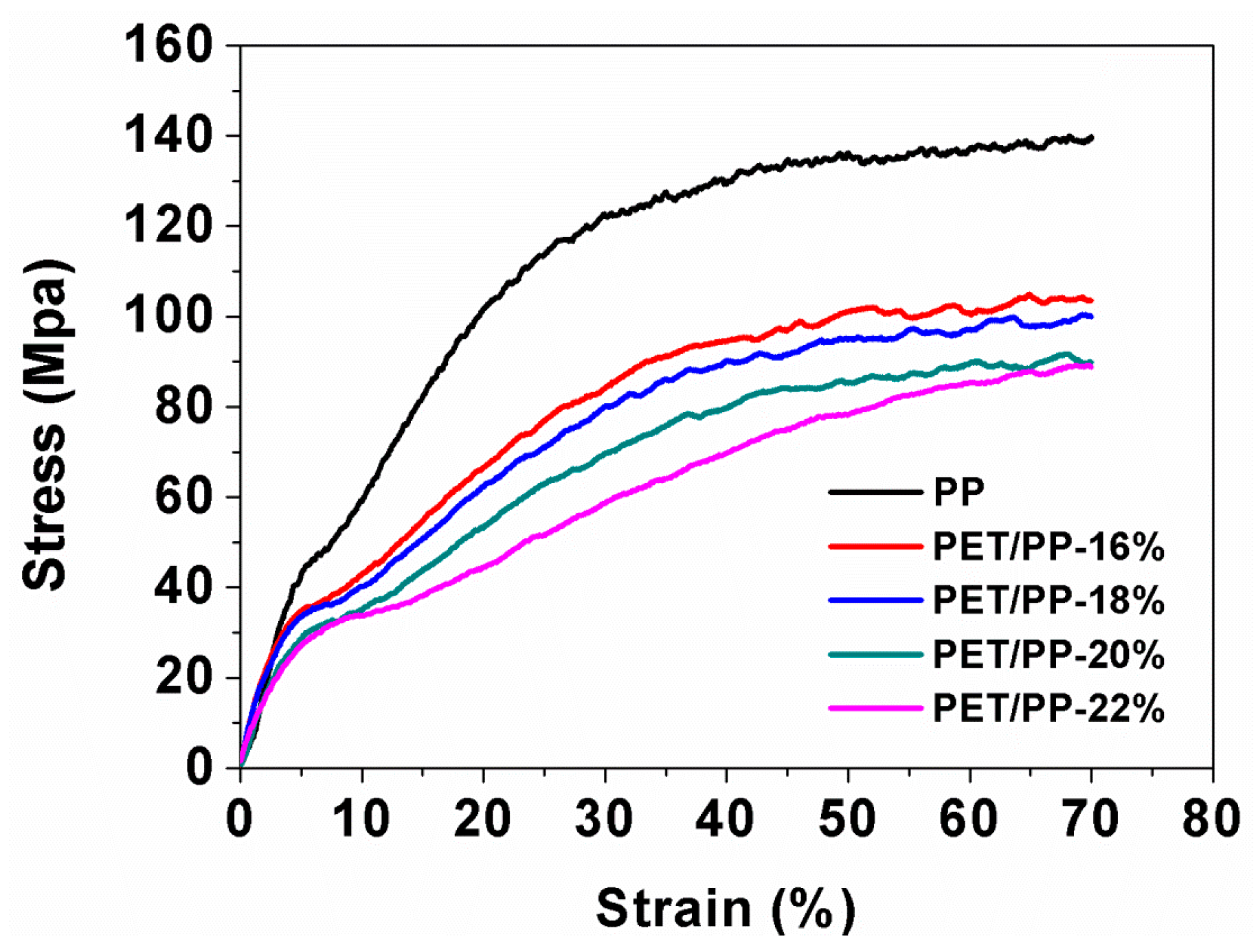
3.2. Mechanical and Thermal Properties of the Composite Separators
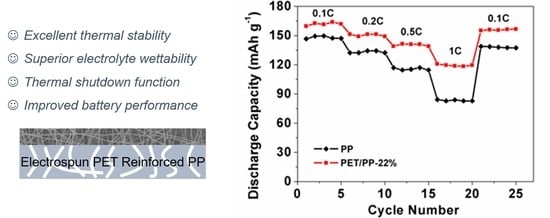
3.3. Electrochemical Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wei, Z.; Meng, S.; Xiong, B.; Ji, D.; Tseng, K.J. Enhanced online model identification and state of charge estimation for lithium-ion battery with a FBCRLS based observer. Appl. Energy 2016, 181, 332–341. [Google Scholar] [CrossRef]
- Wei, Z.; Zhao, J.; Ji, D.; Tseng, K.J. A multi-timescale estimator for battery state of charge and capacity dual estimation based on an online identified model. Appl. Energy 2017, 204, 1264–1274. [Google Scholar] [CrossRef]
- Tang, H.; Zeng, Y.; Liu, D.; Qu, D.; Luo, J.; Binnemans, K.; Vos, D.E.D.; Fransaer, J.; Qu, D.; Sun, S.G. Dual-doped mesoporous carbon synthesized by a novel nanocasting method with superior catalytic activity for oxygen reduction. Nano Energy 2016, 26, 131–138. [Google Scholar] [CrossRef]
- Cai, S.; Meng, Z.; Tang, H.; Wang, Y.; Tsiakaras, P. 3d Co-N-doped hollow carbon spheres as excellent bifunctional electrocatalysts for oxygen reduction reaction and oxygen evolution reaction. Appl. Catal. B Environ. 2017, 217, 477–484. [Google Scholar] [CrossRef]
- Thackeray, M.M.; Wolverton, C.; Isaacs, E.D. Electrical energy storage for transportation—approaching the limits of, and going beyond, lithium-ion batteries. Energy Environ. Sci. 2012, 5, 7854. [Google Scholar] [CrossRef]
- Lee, H.; Yanilmaz, M.; Toprakci, O.; Fu, K.; Zhang, X. A review of recent developments in membrane separators for rechargeable lithium-ion batteries. Energy Environ. Sci. 2014, 7, 3857–3886. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K.S. The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Li, J.; Lei, J.; Liu, D.; Xie, Z.; Qu, D.; Li, K.; Deng, T.; Tang, H. Advanced separators for lithium-ion and lithium-sulfur batteries: A review of recent progress. ChemSusChem 2016, 9, 3023–3039. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.S. A review on the separators of liquid electrolyte li-ion batteries. J. Power Sources 2007, 164, 351–364. [Google Scholar] [CrossRef]
- Zhai, Y.; Xiao, K.; Yu, J.; Ding, B. Fabrication of hierarchical structured sio2/polyetherimide-polyurethane nanofibrous separators with high performance for lithium ion batteries. Electrochim. Acta 2015, 154, 219–226. [Google Scholar] [CrossRef]
- Fang, L.-F.; Shi, J.-L.; Zhu, B.-K.; Zhu, L.-P. Facile introduction of polyether chains onto polypropylene separators and its application in lithium ion batteries. J. Membr. Sci. 2013, 448, 143–150. [Google Scholar] [CrossRef]
- Zhang, J.; Yue, L.; Kong, Q.; Liu, Z.; Zhou, X.; Zhang, C.; Xu, Q.; Zhang, B.; Ding, G.; Qin, B.; et al. Sustainable, heat-resistant and flame-retardant cellulose-based composite separator for high-performance lithium ion battery. Sci. Rep. 2014, 4, 3935. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee, W.-Y.; Kim, K.J.; Yu, J.-S.; Kim, Y.-J. Shutdown-functionalized nonwoven separator with improved thermal and electrochemical properties for lithium-ion batteries. J. Power Sources 2016, 305, 225–232. [Google Scholar] [CrossRef]
- Wang, H.; Pan, L.; Wu, C.; Gao, D.; Chen, S.; Li, L. Pyrogallic acid coated polypropylene membranes as separators for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 20535–20540. [Google Scholar] [CrossRef]
- Xiao, W.; Gao, Z.; Wang, S.; Liu, J.; Yan, C. A novel naa-type zeolite-embedded composite separator for lithium-ion battery. Mater. Lett. 2015, 145, 177–179. [Google Scholar] [CrossRef]
- Byrd, I.; Wu, J. Asymmetric membranes containing micron-size silicon for high performance lithium ion battery anode. Electrochim. Acta 2016, 213, 46–54. [Google Scholar] [CrossRef]
- Juang, R.-S.; Hsieh, C.-T.; Chen, P.-A.; Chen, Y.-F. Microwave-assisted synthesis of titania coating onto polymeric separators for improved lithium-ion battery performance. J. Power Sources 2015, 286, 526–533. [Google Scholar] [CrossRef]
- Raja, M.; Sanjeev, G.; Prem Kumar, T.; Manuel Stephan, A. Lithium aluminate-based ceramic membranes as separators for lithium-ion batteries. Ceram. Int. 2015, 41, 3045–3050. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, P.; Shi, C.; Chen, L.; Dai, J.; Zhao, J. The functional separator coated with core–shell structured silica–poly(methyl methacrylate) sub-microspheres for lithium-ion batteries. J. Membr. Sci. 2015, 474, 148–155. [Google Scholar] [CrossRef]
- Liang, X.; Yang, Y.; Jin, X.; Huang, Z.; Kang, F. The high performances of SiO2/Al2O3-coated electrospun polyimide fibrous separator for lithium-ion battery. J. Membr. Sci. 2015, 493, 1–7. [Google Scholar] [CrossRef]
- Xiang, Y.; Zhu, W.; Qiu, W.; Guo, W.; Lei, J.; Liu, D.; Qu, D.; Xie, Z.; Tang, H.; Li, J. SnO2 functionalized polyethylene separator with enhanced thermal stability for high performance lithium ion battery. ChemistrySelect 2018, 3, 911–916. [Google Scholar] [CrossRef]
- Lee, M.J.; Hwang, J.-K.; Kim, J.H.; Lim, H.-S.; Sun, Y.-K.; Suh, K.-D.; Lee, Y.M. Electrochemical performance of a thermally rearranged polybenzoxazole nanocomposite membrane as a separator for lithium-ion batteries at elevated temperature. J. Power Sources 2016, 305, 259–266. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, P.; Huang, S.; He, X.; Yang, P.; Wu, D.; Sun, D.; Zhao, J. Functional separator consisted of polyimide nonwoven fabrics and polyethylene coating layer for lithium-ion batteries. J. Power Sources 2015, 298, 158–165. [Google Scholar] [CrossRef]
- He, H.; Wang, X.; Liu, W. Effects of pegdma on a pet non-woven fabric embedded pan lithium-ion power battery separator. Solid State Ion. 2016, 294, 31–36. [Google Scholar] [CrossRef]
- Zhang, L.C.; Sun, X.; Hu, Z.; Yuan, C.C.; Chen, C.H. Rice paper as a separator membrane in lithium-ion batteries. J. Power Sources 2012, 204, 149–154. [Google Scholar] [CrossRef]
- Weng, B.; Xu, F.; Alcoutlabi, M.; Mao, Y.; Lozano, K. Fibrous cellulose membrane mass produced via forcespinning® for lithium-ion battery separators. Cellulose 2015, 22, 1311–1320. [Google Scholar] [CrossRef]
- Yanilmaz, M.; Lu, Y.; Li, Y.; Zhang, X. SiO2/polyacrylonitrile membranes via centrifugal spinning as a separator for li-ion batteries. J. Power Sources 2015, 273, 1114–1119. [Google Scholar] [CrossRef]
- Jiang, F.; Nie, Y.; Yin, L.; Feng, Y.; Yu, Q.; Zhong, C. Core–shell-structured nanofibrous membrane as advanced separator for lithium-ion batteries. J. Membr. Sci. 2016, 510, 1–9. [Google Scholar] [CrossRef]
- Liang, X.; Yang, Y.; Jin, X.; Cheng, J. Polyethylene oxide-coated electrospun polyimide fibrous separator for high-performance lithium-ion battery. J. Mater. Sci. Technol. 2016, 32, 200–206. [Google Scholar] [CrossRef]
- Kang, W.; Ma, X.; Zhao, H.; Ju, J.; Zhao, Y.; Yan, J.; Cheng, B. Electrospun cellulose acetate/poly(vinylidene fluoride) nanofibrous membrane for polymer lithium-ion batteries. J. Solid State Electrochem. 2016, 20, 2791–2803. [Google Scholar] [CrossRef]
- Croce, F.; Focarete, M.L.; Hassoun, J.; Meschini, I.; Scrosati, B. A safe, high-rate and high-energy polymer lithium-ion battery based on gelled membranes prepared by electrospinning. Energy Environ. Sci. 2011, 4, 921. [Google Scholar] [CrossRef]
- Xiao, Q.; Li, Z.; Gao, D.; Zhang, H. A novel sandwiched membrane as polymer electrolyte for application in lithium-ion battery. J. Membr. Sci. 2009, 326, 260–264. [Google Scholar] [CrossRef]
- Mahdavi, H.; Moslehi, M. A new thin film composite nanofiltration membrane based on pet nanofiber support and polyamide top layer: Preparation and characterization. J. Polym. Res. 2016, 23, 257. [Google Scholar] [CrossRef]
- Hao, J.; Lei, G.; Li, Z.; Wu, L.; Xiao, Q.; Wang, L. A novel polyethylene terephthalate nonwoven separator based on electrospinning technique for lithium ion battery. J. Membr. Sci. 2013, 428, 11–16. [Google Scholar] [CrossRef]
- Arora, P.; Zhang, Z. Battery separators. Chem. Rev. 2004, 104, 4419–4462. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liu, Y.; Ma, Y.; Yang, W. Improved performance of lithium ion battery separator enabled by co-electrospinnig polyimide/poly(vinylidene fluoride-co-hexafluoropropylene) and the incorporation of tio 2-(2-hydroxyethyl methacrylate). J. Power Sources 2015, 273, 1127–1135. [Google Scholar] [CrossRef]












| Separator | Thickness/μm | Resistance/Ω 1 | Ionic Conductivity 1/mS cm−1 |
|---|---|---|---|
| PP | 20 | 2.15 | 0.503 |
| PP/PET-16% | 29 | 3.17 | 0.495 |
| PP/PET-18% | 30 | 2.83 | 0.573 |
| PP/PET-20% | 31 | 2.52 | 0.665 |
| PP/PET-22% | 32 | 2.21 | 0.782 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, H.; Tong, X.; Chen, K.; Shen, Y.; Wu, J.; Xiang, Y.; Wang, Z.; Li, J. Electrospun Polyethylene Terephthalate Nonwoven Reinforced Polypropylene Separator: Scalable Synthesis and Its Lithium Ion Battery Performance. Polymers 2018, 10, 574. https://doi.org/10.3390/polym10060574
Cai H, Tong X, Chen K, Shen Y, Wu J, Xiang Y, Wang Z, Li J. Electrospun Polyethylene Terephthalate Nonwoven Reinforced Polypropylene Separator: Scalable Synthesis and Its Lithium Ion Battery Performance. Polymers. 2018; 10(6):574. https://doi.org/10.3390/polym10060574
Chicago/Turabian StyleCai, Haopeng, Xing Tong, Kai Chen, Yafei Shen, Jiashun Wu, Yinyu Xiang, Zhao Wang, and Junsheng Li. 2018. "Electrospun Polyethylene Terephthalate Nonwoven Reinforced Polypropylene Separator: Scalable Synthesis and Its Lithium Ion Battery Performance" Polymers 10, no. 6: 574. https://doi.org/10.3390/polym10060574