Preparation, Physicochemical Properties and Hemocompatibility of Biodegradable Chitooligosaccharide-Based Polyurethane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of CPU
2.3. Preparation of CPU Films
2.4. Characterization
3. Results and Discussion
3.1. FT-IR
3.2. Thermal Transition
3.3. Thermal Stability
3.4. Crystallization Behavior
3.5. Mechanical Properties
3.6. Surface Hydrophilicity and Swellability
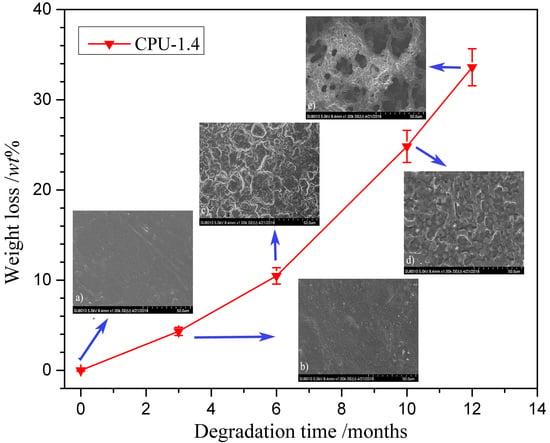
3.7. In Vitro Degradation
3.8. Protein Adsorption
3.9. Platelet Adhesion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Janik, H.; Marzec, M. A review: Fabrication of porous polyurethane scaffolds. Mat. Sci. Eng. C Mater. 2015, 48, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Noreen, A.; Zia, K.M.; Zuber, M.; Tabasum, S.; Zahoor, A.F. Bio-based polyurethane: An efficient and environment friendly coating systems: A review. Prog. Org. Coat. 2016, 91, 25–32. [Google Scholar] [CrossRef]
- John, K.R.S. The use of polyurethane materials in the surgery of the spine: a review. Spine J. 2014, 14, 3038–3047. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Jiang, X.; He, L.; Zhang, T.; Xu, M.; Yu, X. Novel biocompatible waterborne polyurethane using l-lysine as an extender. J. Appl. Polym. Sci. 2002, 84, 2474–2480. [Google Scholar] [CrossRef]
- Spaans, C.J.; Belgraver, V.W.; Rienstra, O.; Groot, J.H.D.; Veth, R.P.; Pennings, A.J. Solvent-free fabrication of micro-porous polyurethane amide and polyurethane-urea scaffolds for repair and replacement of the knee-joint meniscus. Biomaterials 2000, 21, 2453–2460. [Google Scholar] [CrossRef]
- Shen, Z.; Kang, C.; Chen, J.; Ye, D.; Qiu, S.; Guo, S.; Zhu, Y. Surface modification of polyurethane towards promoting the ex vivo cytocompatibility and in vivo biocompatibility for hypopharyngeal tissue engineering. J. Biomater. Appl. 2013, 28, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Bochyńska, A.I.; Hammink, G.; Grijpma, D.W.; Buma, P. Tissue adhesives for meniscus tear repair: An overview of current advances and prospects for future clinical solutions. J. Mater. Sci. Mater. Med. 2016, 27, 85–102. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Chen, H.; Glenn, M.W.; Brash, J.L. Lysine-PEG-modified polyurethane as a fibrinolytic surface: Effect of PEG chain length on protein interactions, platelet interactions and clot lysis. Acta Biomater. 2009, 5, 1864–1871. [Google Scholar] [CrossRef] [PubMed]
- Puskas, J.E.; Chen, Y. Biomedical application of commercial polymers and novel polyisobutylene-based thermoplastic elastomers for soft tissue replacement. Biomacromolecule 2004, 5, 1141–1154. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Hasan, A.; Deeb, G.; Hu, C.; Han, H. Rheological and mechanical behavior of silk fibroin reinforced waterborne polyurethane. Polymers 2016, 8, 94. [Google Scholar] [CrossRef]
- Dong, C.; Lv, Y. Application of collagen scaffold in tissue engineering: Recent advances and new perspectives. Polymers 2016, 8, 42. [Google Scholar] [CrossRef]
- Adipurnama, I.; Yang, M.C.; Ciach, T.; Butruk-Raszeja, B. Surface modification and endothelialization of polyurethane for vascular tissue engineering applications: A review. Biomater. Sci. 2017, 5, 22–37. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.C.; Tseng, C.H.; Yang, M.C. In-vitro hemocompatibility evaluation of a thermoplastic polyurethane membrane with surface-immobilized water-soluble chitosan and heparin. Macromol. Biosci. 2010, 5, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liao, J.F.; Shi, X.H.; Qiu, Y.G.; Chen, H.J. Surface biocompatible construction of polyurethane by heparinization. J. Polym. Res. 2015, 22, 68–79. [Google Scholar] [CrossRef]
- Ren, Z.; Chen, G.; Wei, Z.; Lin, S.; Qi, M. Hemocompatibility evaluation of polyurethane film with surface-grafted poly(ethylene glycol) and carboxymethyl-chitosan. J. Appl. Polym. Sci. 2013, 127, 308–315. [Google Scholar] [CrossRef]
- Xu, D.; Meng, Z.; Han, M.; Xi, K.; Jia, X.; Yu, X.; Chen, Q. Novel blood-compatible waterborne polyurethane using chitosan as an extender. J. Appl. Polym. Sci. 2008, 109, 240–246. [Google Scholar] [CrossRef]
- Tan, A.C.W.; Polo-Cambronell, B.J.; Provaggi, E.; Ardila-Suárez, C.; Ramirez-Caballero, G.E.; Baldovino-Medrano, V.G.; Kalaskar, D.M. Design and development of low cost polyurethane biopolymer based on castor oil and glycerol for biomedical applications. Biopolymers 2018, 109, e23078. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.R.; Singh, U.S.; Momin, M.; Bhavsar, C. Chitosan: Application in tissue engineering and skin grafting. J. Polym. Res. 2017, 24, 125–146. [Google Scholar] [CrossRef]
- Shih, C.; Chen, C.; Huang, K. Adsorption of color dyestuffs on polyurethane-chitosan blends. J. Appl. Polym. Sci. 2004, 91, 3991–3998. [Google Scholar] [CrossRef]
- Shih, C.; Huang, K. Synthesis of a polyurethane-chitosan blended polymer and a compound process for shrink-proof and antimicrobial woolen fabrics. J. Appl. Polym. Sci. 2003, 88, 2356–2363. [Google Scholar] [CrossRef]
- Silva, S.S.; Menezes, S.M.C.; Garcia, R.B. Synthesis and characterization of polyurethane-g-chitosan. Eur. Polym. J. 2003, 39, 1515–1519. [Google Scholar] [CrossRef]
- Zhu, Y.B.; Gao, C.Y.; He, T.; Shen, J.C. Endothelium regeneration on luminal surface of polyurethane vascular scaffold modified with diamine and covalently grafted with gelatin. Biomaterials 2004, 25, 423–430. [Google Scholar] [CrossRef]
- El-Sayed, A.A.; Gabry, L.K.E.; Allam, O.G. Application of prepared waterborne polyurethane extended with chitosan to impart antibacterial properties to acrylic fabrics. J. Mater. Sci. Mater. Med. 2010, 21, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Nikje, M.M.A.; Tehrani, Z.M. Synthesis and characterization of waterborne polyurethane-chitosan nanocomposites. Polym. Plast. Technol. 2010, 49, 812–817. [Google Scholar] [CrossRef]
- Lee, K.Y.; Ha, W.S.; Park, W.H. Blood compatibility and biodegradability of partially N-acylated chitosan derivatives. Biomaterials 1995, 16, 1211–1216. [Google Scholar] [CrossRef]
- Chae, S.Y.; Jang, M.K.; Nah, J.W. Influence of molecular weight on oral absorption of water soluble chitosans. J. Control. Release 2005, 102, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Wang, W.; Yang, W.; Du, Y.; Song, L. Chitosan oligosaccharide inhibits EGF-induced cell growth possibly through blockade of epidermal growth factor receptor/mitogen-activated protein kinase pathway. Int. J. Biol. Macromol. 2017, 98, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.J.; Kim, S.K. Production of chitooligosaccharides using an ultrafiltration membrane reactor and their antibacterial activity. Carbohydr. Polym. 2000, 41, 133–141. [Google Scholar] [CrossRef]
- Kim, K.Y.; Kwon, S.L.; Park, J.H.; Chung, H.; Jeong, S.Y.; Kwon, I.C. Physicochemical characterizations of self-assembled nanoparticles of glycol chitosan–deoxycholic acid conjugates. Biomacromolecules 2005, 6, 1154–1158. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Park, J.H.; Chung, H.; Kwon, I.C.; Jeong, S.Y.; Kim, I.S. Physicochemical characteristics of self-assembled nanoparticles based on glycol chitosan bearing 5β-cholanic acid. Langmuir 2003, 19, 10188–10193. [Google Scholar] [CrossRef]
- Yokasan, R.; Matsusaki, M.; Akashi, M.; Chirachanchai, S. Controlled hydrophobic/hydrophilic chitosan: Colloidal phenomena and nanosphere formation. Colloid Polym. Sci. 2004, 282, 337–342. [Google Scholar] [CrossRef]
- Xu, D.; Wu, K.; Zhang, Q.; Hu, H.; Xi, K.; Chen, Q.; Yu, X.; Chen, J.; Jia, X. Synthesis and biocompatibility of anionic polyurethane nanoparticles coated with adsorbed chitosan. Polymer 2010, 51, 1926–1933. [Google Scholar] [CrossRef]
- Usman, A.; Zia, K.M.; Zuber, M.; Tabasum, S.; Rehman, S.; Zia, F. Chitin and chitosan based polyurethanes: A review of recent advances and prospective biomedical applications. Int. J. Biol. Macromol. 2016, 86, 630–645. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.Q.; Xia, Y.R.; Jiang, L.J.; Zhang, L.W.; Hou, Z.S. Synthesis and characterization of a new biodegradable polyurethanes with good mechanical properties. Chin. Chem. Lett. 2016, 27, 135–138. [Google Scholar] [CrossRef]
- Hou, Z.; Qu, W.; Kan, C. Synthesis and properties of triethoxysilane-terminated anionic polyurethane and its waterborne dispersions. J. Polym. Res. 2015, 22, 111–119. [Google Scholar] [CrossRef]
- Pei, D.; Wang, J.; Mu, Y.; Wan, X. A simple and low-cost synthesis of antibacterial polyurethane with high mechanical and antibacterial properties. Macromol. Chem. Phys. 2017, 218, 1700203. [Google Scholar] [CrossRef]
- Ajitha, P.; Vijayalakshmi, K.; Saranya, M.; Gomathi, T.; Rani, K.; Sudha, P.N.; Anil, S. Removal of toxic heavy metal lead (II) using chitosan oligosaccharide-graft-maleic anhydride/polyvinyl alcohol/silk fibroin composite. Int. J. Biol. Macromol. 2017, 104, 1469–1482. [Google Scholar]
- Barrioni, B.R.; Carvalho, S.M.D.; Oréfice, R.L.; Oliveira, A.A.R.D.; Pereira, M.D.M. Synthesis and characterization of biodegradable polyurethane films based on HDI with hydrolyzable crosslinked bonds and a homogeneous structure for biomedical applications. Mater. Sci. Eng. C 2015, 52, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Teng, H.; Hou, Z.; Gu, C.; Zhu, L. Comb-like polysiloxanes with oligo(oxyethylene) and sulfonate groups in side chains for solvent-free dimethoxysilyl-terminated polypropylene oxide waterborne emulsions. Colloid Polym. Sci. 2018, 296, 157–163. [Google Scholar] [CrossRef]
- Li, F.H.; Sun, Y.; Li, S.X.; Ma, S.J. Synthesis and characterization of thermoplastic biomaterial based on acylated chitosan oligosaccharide. Appl. Mech. Mater. 2012, 117–119, 1433–1436. [Google Scholar] [CrossRef]
- Li, F.H.; Li, S.X.; Jiang, T.; Sun, Y. Syntheses and characterization of chitosan oligosaccharide-graft-polycaprolactone copolymer I thermal and spherulite morphology studies. Adv. Mater. Res. 2011, 183–185, 155–160. [Google Scholar] [CrossRef]
- Król, P. Synthesis methods, chemical structures and phase structures of linear polyurethanes. Properties and applications of linear polyurethanes in polyurethane elastomers, copolymers and ionomers. Prog. Mater. Sci. 2007, 52, 915–1015. [Google Scholar] [CrossRef]
- Yin, S.; Xia, Y.; Jia, Q.; Hou, Z.; Zhang, N. Preparation and properties of biomedical segmented polyurethanes based on poly(ether ester) and uniform-size diurethane diisocyanates. J. Biomater. Sci. Polym. Ed. 2017, 28, 119–138. [Google Scholar] [CrossRef] [PubMed]
- Barikani, M.; Zia, K.M.; Bhatti, I.A.; Zuber, M.; Bhatti, H.N. Molecular engineering and properties of chitin based shape memory polyurethanes. Carbohydr. Polym. 2008, 74, 621–626. [Google Scholar] [CrossRef]
- Liu, X.; Xia, W.; Jiang, Q.; Xu, Y.; Yu, P. Synthesis, characterization, and antimicrobial activity of kojic acid grafted chitosan oligosaccharide. J. Agric. Food Chem. 2014, 62, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Teng, S.H.; Lee, E.J.; Yoon, B.H.; Shin, D.S.; Kim, H.E.; Oh, J.S. Chitosan/nanohydroxyapatite composite membranes via dynamic filtration for guided bone regeneration. J. Biomed. Mater. Res. A 2009, 88, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Haut, R.C. Biomechanics of soft tissue. In Accidental Injury; Nahum, A.M., Melvin, J.W., Eds.; Springer: New York, NY, USA, 2002; pp. 228–253. [Google Scholar]
- Caracciolo, P.C.; Queiroz, A.A.D.; Higa, Q.Z.; Buffa, F.; Abraham, G.A. Segmented poly(esterurethane urea)s from novel urea-diol chain extenders: Synthesis, characterization and in vitro biological properties. Acta Biomater. 2008, 4, 976–988. [Google Scholar] [CrossRef] [PubMed]
- Takami, K.; Matsuno, R.; Ishihara, K. Synthesis of polyurethanes by polyaddition using diol compounds with methacrylate-derived functional groups. Polymer 2011, 52, 5445–5451. [Google Scholar] [CrossRef]
- Brzeska, J.; Morawska, M.; Heimowska, A.; Sikorska, W.; Tercjak, A.; Kowalczuk, M.; Rutkowska, M. Degradability of cross-linked polyurethanes/chitosan composites. Polimery 2017, 62, 567–575. [Google Scholar] [CrossRef]
- Kwon, M.J.; Bae, J.H.; Kim, J.J.; Na, K.; Lee, E.S. Long acting porous microparticle for pulmonary protein delivery. Int. J. Pharm. 2007, 333, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, S.; Chang, Y.; Jiang, S. Surface grafted sulfobetaine polymers via atom transfer radical polymerization as superlow fouling coatings. J. Phys. Chem. B 2006, 110, 10799–10804. [Google Scholar] [CrossRef] [PubMed]
- Tangpasuthadol, V.; Pongchaisirikul, N.; Hoven, V.P. Surface modification of chitosan films. Effects of hydrophobicity on protein adsorption. Carbohydr. Res. 2003, 338, 937–942. [Google Scholar] [CrossRef]
- Yu, S.H.; Mi, F.L.; Shyu, S.S.; Tsai, C.H.; Peng, C.K.; Lai, J.Y. Miscibility, mechanical characteristic and platelet adhesion of 6-O-carboxymethylchitosan/polyurethane semi-IPN membranes. J. Membr. Sci. 2006, 276, 68–80. [Google Scholar] [CrossRef]












| Samples | PCL/g | HBH/g | COS/g | COS Content/wt % * | n-OH:n–NCO:n–NH2 ** |
|---|---|---|---|---|---|
| CPU-1.0 | 8.0 | 1.70 | 0 | 0 | 1:1:0 |
| CPU-1.4 | 8.0 | 2.39 | 1.14 | 9.89 | 1:1.4:0.8 |
| CPU-1.7 | 8.0 | 2.90 | 2.0 | 15.5 | 1:1.7:1.4 |
| CPU-2.0 | 8.0 | 3.41 | 2.86 | 20.0 | 1:2.0:2.0 |
| Samples | COS | PCL | CPU-1.0 | CPU-1.4 | CPU-1.7 | CPU-2.0 |
|---|---|---|---|---|---|---|
| Tg1 (oC) | - | −58.7 | −17.4 | −21.0 | −18.3 | −20.2 |
| Tg2 (oC) | - | - | 48~63 | 46~61 | 46~62.5 | 45.5~63 |
| Tm (oC) | 45~130 | 61.2 | 90~118 | 83~123 | 82~125 | 80~129 |
| ΔHf (J/g) | 52 | 61.8 | 48.2 | 78.3 | 108.9 | 133.4 |
| Films | Strain at Break (%) | Ultimate Stress (MPa) | Yield Stress (MPa) | Yield Strain (%) | Initial Modulus (MPa) |
|---|---|---|---|---|---|
| CPU-1.0 | 774 ± 17 | 24.1 ± 2.2 | 10.6 ± 1.6 | 41.3 ± 3.5 | 25.5 |
| CPU-1.4 | 671 ± 15 | 30.9 ± 1.8 | 9.3 ± 1.1 | 22.6 ± 1.2 | 41.2 |
| CPU-1.7 | 569 ± 15 | 34.9 ± 1.6 | 10.2 ± 1.1 | 19.7 ± 1.0 | 51.7 |
| CPU-2.0 | 462 ± 13 | 35.3 ± 1.6 | 11.9 ± 1.2 | 22.1 ± 1.3 | 53.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Xiao, M.; Yuan, L.; Zhang, J.; Hou, Z. Preparation, Physicochemical Properties and Hemocompatibility of Biodegradable Chitooligosaccharide-Based Polyurethane. Polymers 2018, 10, 580. https://doi.org/10.3390/polym10060580
Xu W, Xiao M, Yuan L, Zhang J, Hou Z. Preparation, Physicochemical Properties and Hemocompatibility of Biodegradable Chitooligosaccharide-Based Polyurethane. Polymers. 2018; 10(6):580. https://doi.org/10.3390/polym10060580
Chicago/Turabian StyleXu, Weiwei, Minghui Xiao, Litong Yuan, Jun Zhang, and Zhaosheng Hou. 2018. "Preparation, Physicochemical Properties and Hemocompatibility of Biodegradable Chitooligosaccharide-Based Polyurethane" Polymers 10, no. 6: 580. https://doi.org/10.3390/polym10060580