Rheology, Non-Isothermal Crystallization Behavior, Mechanical and Thermal Properties of PMMA-Modified Carbon Fiber-Reinforced Poly(Ethylene Terephthalate) Composites
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of the Composites
2.3. Characterization of the Composites
3. Results and Discussion
3.1. Characteristics of Carbon Fiber Surface
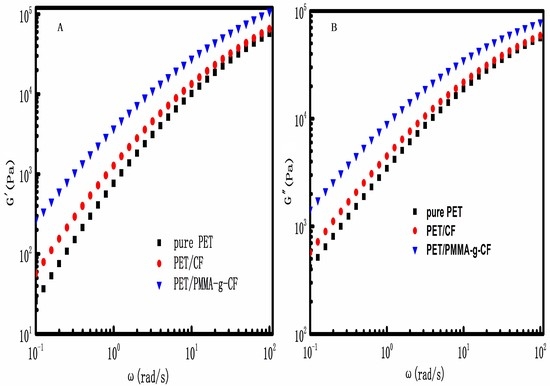
3.2. Rheological Properties
3.3. Thermal Analysis of the Composites
3.3.1. The Non-isothermal Crystallization Behavior and Melting Behavior
3.3.2. Non-Isothermal Crystallization Kinetics
3.3.3. Crystallization Activation Energy
3.4. Morphology of the Composites
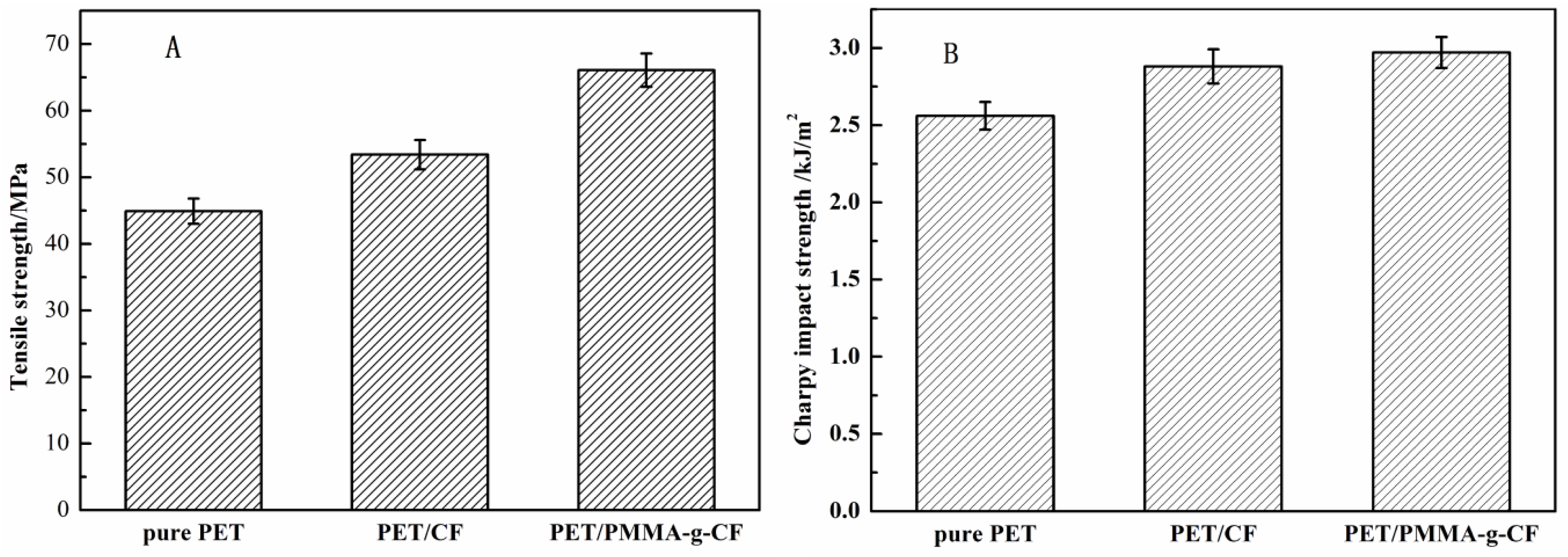
3.5. Mechanical Properties
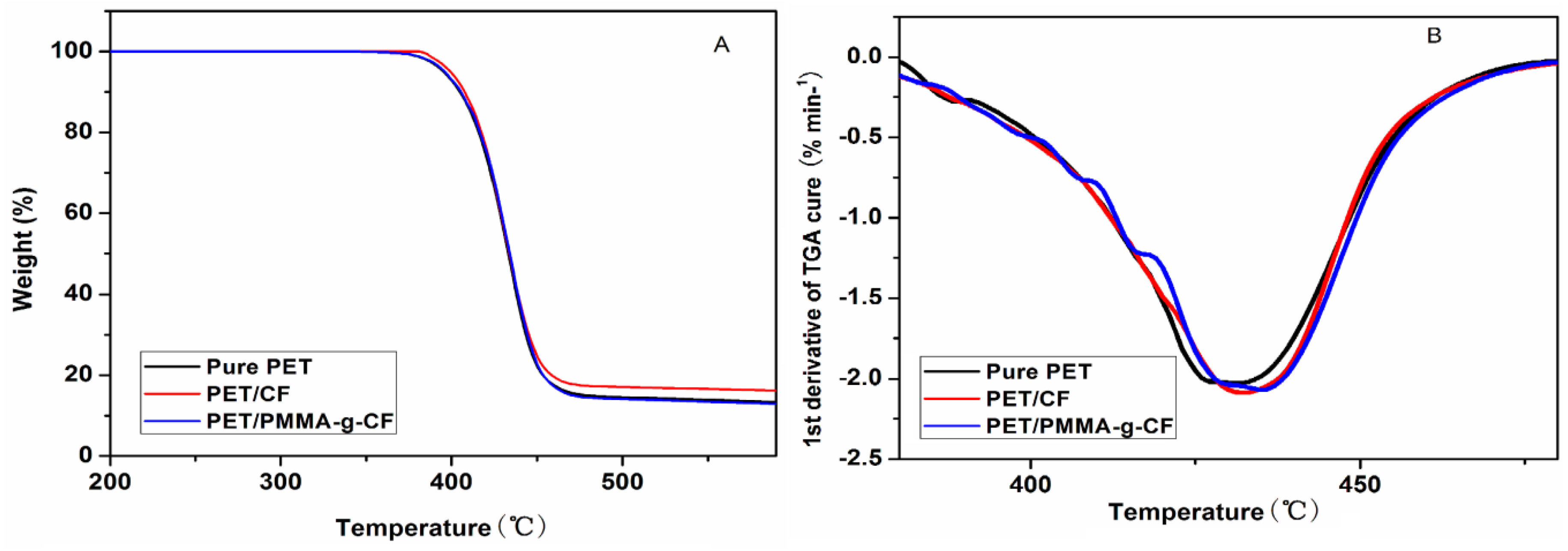
3.6. Thermogravimetric Analysis (TGA)
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Papageorgiou, G.Z.; Karandrea, E.; Giliopoulos, D.; Papageorgiou, G.Z.; Ladavos, A.; Katerinopoulou, A.; Achilias, D.S.; Triantafyllidis, K.S.; Bikiaris, D.N. Effect of clay structure and type of organomodifier on the thermal properties of poly(ethylene terephthalate) based nanocomposites. Thermochim. Acta 2014, 576, 84–96. [Google Scholar] [CrossRef]
- Aoyama, S.; Park, Y.T.; Ougizawa, T.; Macosko, C.W. Melt crystallization of poly(ethylene terephthalate): Comparing addition of graphene vs. carbon nanotubes. Polymer 2014, 55, 2077–2085. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, H.; Ke, M.; Xiao, L.; Zuo, D.; Qian, Q.; Chen, Q. Non-isothermal crystallization kinetics of poly(ethylene terephthalate)/mica composites. Polym. Bull. 2014, 71, 2287–2301. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, R.; Dong, Z.; Huang, X.; Zhang, D. Morphology, crystallization, and mechanical properties of poly(ethylene terephthalate)/multiwalled carbon nanotubes composites. J. Appl. Polym. Sci. 2015, 120, 3460–3468. [Google Scholar] [CrossRef]
- Xie, L.; Xie, Y.; Wu, Q.; Wang, M.; Wu, Q.; Zhou, X.; Ge, X. Effect of poly(acrylic acid)-modified poly(ethylene terephthalate) on improving the integrated mechanical properties of poly(ethylene terephthalate)/elastomer blend. Ind. Eng. Chem. Res. 2015, 54, 4748–4755. [Google Scholar] [CrossRef]
- Unterweger, C.; Duchoslav, J.; Stifter, D.; Fürst, C. Characterization of carbon fiber surfaces and their impact on the mechanical properties of short carbon fiber reinforced polypropylene composites. Compos. Sci. Technol. 2015, 108, 41–47. [Google Scholar] [CrossRef]
- Tian, H.; Zhang, S.; Ge, X.; Xiang, A. Crystallization behaviors and mechanical properties of carbon fiber-reinforced polypropylene composites. J. Therm. Anal. Calorim. 2017, 128, 1495–1504. [Google Scholar] [CrossRef]
- Liang, J.; Xu, Y.; Wei, Z.; Song, P.; Chen, G.; Zhang, W. Mechanical properties, crystallization and melting behaviors of carbon fiber-reinforced PA6 composites. J. Therm. Anal. Calorim. 2014, 115, 209–218. [Google Scholar] [CrossRef]
- Karsli, N.G.; Aytac, A. Tensile and thermomechanical properties of short carbon fiber reinforced polyamide 6 composites. Compos. Part B Eng. 2013, 51, 270–275. [Google Scholar] [CrossRef]
- Karsli, N.G.; Ozkan, C.; Aytac, A.; Deniz, V. Characterization of poly(butylene terephthalate) composites prepared by using various types of sized carbon fibers. Mater. Des. 2015, 87, 318–323. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Wu, D. Recycled carbon fiber reinforced poly(butylene terephthalate) thermoplastic composites: Fabrication, crystallization behaviors and performance evaluation. Polym. Adv. Technol. 2013, 24, 364–375. [Google Scholar] [CrossRef]
- Run, M.; Song, H.; Yao, C.; Wang, Y. Crystal morphology and nonisothermal crystallization kinetics of short carbon fiber/poly(trimethylene terephthalate) composites. J. Appl. Polym. Sci. 2007, 106, 868–877. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, D.; Rodriguez-Millan, M.; Rusinek, A.; Arias, A. Investigation of mechanical impact behavior of short carbon-fiber-reinforced PEEK composites. Compos. Struct. 2015, 133, 1116–1126. [Google Scholar] [CrossRef]
- Luo, H.; Xiong, G.; Yang, Z.; Raman, S.R.; Li, Q.; Ma, C.; Li, D.; Wang, Z.; Wan, Y. Preparation of three-dimensional braided carbon fiber-reinforced PEEK composites for potential load-bearing bone fixations. Part I. Mechanical properties and cytocompatibility. J. Mech. Behav. Biomed. 2014, 29, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Karsli, N.G.; Aytac, A. Effects of maleated polypropylene on the morphology, thermal and mechanical properties of short carbon fiber reinforced polypropylene composites. Mater. Des. 2011, 32, 4069–4073. [Google Scholar] [CrossRef]
- Liu, J.; Tian, Y.; Chen, Y.; Liang, J. Interfacial and mechanical properties of carbon fibers modified by electrochemical oxidation in (NH4HCO3)/(NH4)2C2O4·H2O aqueous compound solution. Appl. Surf. Sci. 2010, 256, 6199–6204. [Google Scholar] [CrossRef]
- Liu, Y.T.; Wu, G.P.; Lu, C.X. Grafting of carbon nanotubes onto carbon fiber surfaces by step-wise reduction of in-situ generated diazonium salts for enhancing carbon/epoxy interfaces. Mater. Lett. 2014, 134, 75–79. [Google Scholar] [CrossRef]
- Wu, G.; Ma, L.; Wang, Y.; Liu, L.; Huang, Y. Interfacial properties and impact toughness of methylphenylsilicone resin composites by chemically grafting POSS and tetraethylenepentamine onto carbon fibers. Compos. Part A-Appl. Sci. Manuf. 2016, 84, 1–8. [Google Scholar] [CrossRef]
- Xie, J.; Xin, D.; Cao, H.; Wang, C.; Zhao, Y.; Yao, L.; Ji, F.; Qiu, Y. Improving carbon fiber adhesion to polyimide with atmospheric pressure plasma treatment. Surf. Coat. Technol. 2011, 206, 191–201. [Google Scholar] [CrossRef]
- Jiang, S.; Li, Q.; Zhao, Y.; Wang, J.; Kang, M. Effect of surface silanization of carbon fiber on mechanical properties of carbon fiber reinforced polyurethane composites. Compos. Sci. Technol. 2015, 110, 87–94. [Google Scholar] [CrossRef]
- Xu, Z.; Huang, Y.; Zhang, C.; Liu, L.; Zhang, Y.; Wang, L. Effect of gamma-ray irradiation grafting on the carbon fibers and interfacial adhesion of epoxy composites. Compos. Sci. Technol. 2007, 67, 3261–3270. [Google Scholar]
- Liu, P.; Su, Z. Surface-initiated atom-transfer radical polymerization (SI-ATRP) of methyl methacrylate from carbon fibre. Polym. Int. 2005, 54, 1508–1511. [Google Scholar] [CrossRef]
- Harrell, E.R.; Nakajima, N. Modified Cole-Cole plot based on viscoelastic properties for characterizing molecular architecture of elastomers. J. Appl. Polym. Sci. 1984, 29, 995–1010. [Google Scholar] [CrossRef]
- Newcomb, B.A.; Gulgunje, P.V.; Liu, Y.; Gupta, K.; Kamath, M.G.; Pramanik, C.; Ghoshal, S.; Chae, H.G.; Kumar, S. Polyacrylonitrile solution homogeneity study by dynamic shear rheology and the effect on the carbon fiber tensile strength. Polym. Eng. Sci. 2016, 56, 361–370. [Google Scholar] [CrossRef]
- Chae, D.W.; Kim, B.C. Thermal and rheological properties of highly concentrated PET composites with ferrite nanoparticles. Compos. Sci. Technol. 2007, 67, 1348–1352. [Google Scholar] [CrossRef]
- Chae, D.W.; Kim, K.J.; Kim, B.C. Effects of silicalite-1 nanoparticles on rheological and physical properties of HDPE. Polymer 2006, 47, 3609–3615. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase change. I General theory. J. Chem. Phys. 1939, 7, 1103–1112. [Google Scholar] [CrossRef]
- Jeziorny, A. Parameters characterizing the kinetics of the non-isothermal crystallization of poly(ethylene terephthalate) determined by DSC. Polymer 1978, 19, 1142–1144. [Google Scholar] [CrossRef]
- Ou, B.; Zhou, Z.; Liu, Q.; Liao, B.; Xiao, Y.; Liu, J.; Zhang, X.; Li, D.; Xiao, Q.; Shen, S. Mechanical properties and nonisothermal crystallization kinetics of polyamide 6/functionalized TiO2 nanocomposites. Polym. Compos. 2014, 35, 294–300. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Sbirrazzuoli, N. Isoconversional kinetic analysis of thermally stimulated processes in polymers. Macromol. Rapid Commun. 2006, 27, 1515–1532. [Google Scholar] [CrossRef]
- Supaphol, P.; Dangseeyun, N.; Srimoaon, P. Non-isothermal melt crystallization kinetics for poly(trimethylene terephthalate)/poly(butylene terephthalate) blends. Polym. Test. 2004, 23, 175–185. [Google Scholar] [CrossRef]













| Samples | Contents of Correlative Functional Groups | |||||||
|---|---|---|---|---|---|---|---|---|
| C–C | C–OH | C=O | O=C–OR | |||||
| BE (eV) | % | BE (eV) | % | BE (eV) | % | BE (eV) | % | |
| Untreated CF | 284.6 | 81.9 | 286.2 | 11.6 | 287.4 | 1.3 | 288.5 | 5.2 |
| PMMA-g-CF | 284.7 | 59.2 | 286.3 | 19.3 | 287.3 | 3.5 | 288.6 | 18.0 |
| Sample | β (°C/min) | Tonset (°C) | Tp (°C) |
|---|---|---|---|
| PET | 5 | 216.29 | 206.87 |
| 10 | 209.71 | 199.87 | |
| 20 | 204.60 | 191.6 | |
| 40 | 194.11 | 177.02 | |
| PET/CF | 5 | 224.48 | 218.87 |
| 10 | 220.11 | 214.76 | |
| 20 | 214.42 | 208.31 | |
| 40 | 207.84 | 200.33 | |
| PET/PMMA-g-CF | 5 | 225.78 | 220.62 |
| 10 | 220.90 | 215.45 | |
| 20 | 215.85 | 209.38 | |
| 40 | 209.01 | 202.06 |
| Sample | Tc (°C) | Tm (°C) | Xc (%) |
|---|---|---|---|
| PET | 199.87 | 247.15 | 33.31 |
| PET/CF | 214.76 | 248.24 | 35.72 |
| PET/PMMA-g-CF | 215.45 | 248.49 | 36.69 |
| Sample | β (°C/min) | n | Zc |
|---|---|---|---|
| PET | 5 | 3.32 | 0.44 |
| 10 | 2.99 | 0.83 | |
| 20 | 3.00 | 0.97 | |
| 40 | 2.76 | 1.26 | |
| PET/CF | 5 | 3.30 | 0.51 |
| 10 | 3.34 | 0.87 | |
| 20 | 3.13 | 1.22 | |
| 40 | 2.88 | 1.78 | |
| PET/PMMA-g-CF | 5 | 3.50 | 0.47 |
| 10 | 2.72 | 0.95 | |
| 20 | 2.93 | 1.29 | |
| 40 | 2.99 | 1.73 |
| Samples | T10% (°C) | Tmax% (°C) |
|---|---|---|
| pure PET | 405 | 431 |
| PET/CF | 408 | 433 |
| PET/PMMA-g-CF | 406 | 435 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, G.; Li, D.; Liu, M.; Zhang, X.; Zheng, Y. Rheology, Non-Isothermal Crystallization Behavior, Mechanical and Thermal Properties of PMMA-Modified Carbon Fiber-Reinforced Poly(Ethylene Terephthalate) Composites. Polymers 2018, 10, 594. https://doi.org/10.3390/polym10060594
Lin G, Li D, Liu M, Zhang X, Zheng Y. Rheology, Non-Isothermal Crystallization Behavior, Mechanical and Thermal Properties of PMMA-Modified Carbon Fiber-Reinforced Poly(Ethylene Terephthalate) Composites. Polymers. 2018; 10(6):594. https://doi.org/10.3390/polym10060594
Chicago/Turabian StyleLin, Guoliang, Dongwei Li, Minyi Liu, Xiaoyi Zhang, and Yuying Zheng. 2018. "Rheology, Non-Isothermal Crystallization Behavior, Mechanical and Thermal Properties of PMMA-Modified Carbon Fiber-Reinforced Poly(Ethylene Terephthalate) Composites" Polymers 10, no. 6: 594. https://doi.org/10.3390/polym10060594