Acetyl Groups in Typha capensis: Fate of Acetates during Organosolv and Ionosolv Pulping
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. MWL Isolation
2.3. Ionic Liquid Mediated Lignin Extraction
2.4. Sulfuric Acid Catalyzed Ethanol Organosolv Lignin Extraction
2.5. Raman Vibrational Spectroscopy of Native TC
2.6. 1H and 2D NMR (HSQC)
2.7. Zemplén Transesterification Reaction for Degree of Acetylation Determination and Back-Titration Methods
3. Results
3.1. Structural Analysis of Native TC by Raman Spectroscopy
3.2. Isolated Lignin Analysis by 1H NMR
3.3. Isolated Lignin and TCextr Analysis by 2D HSQC
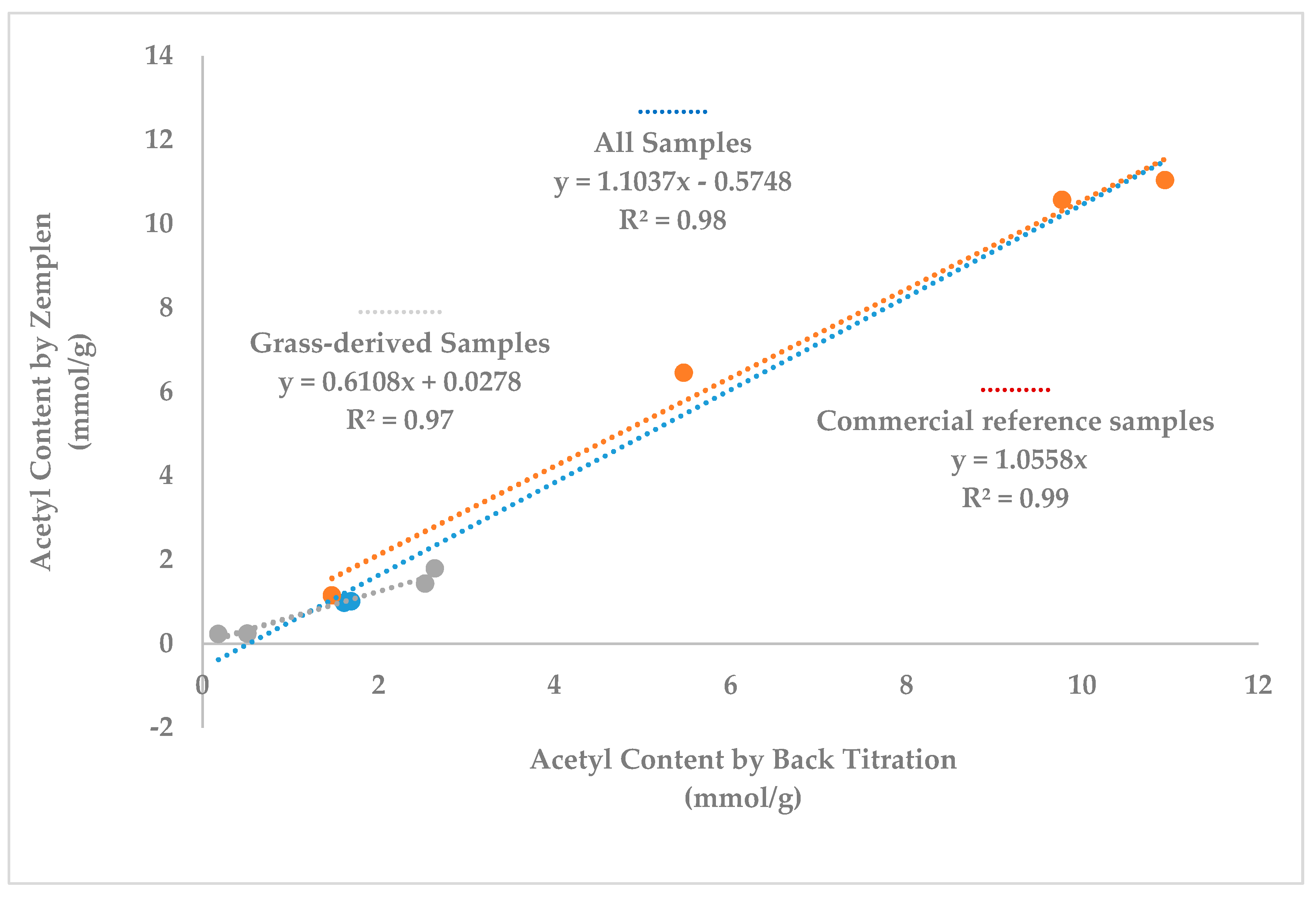
3.4. Degree of Acetylation by Zemplén Transesterification Reaction and by Back-Titration Method
4. Discussion
4.1. Comparison of Acetyl Group Values by Different Methods
4.2. Acetyl Groups in TCextr and MWL
4.3. Changes during EOL Pulping
4.4. Changes during [EMIm][OAc] Pulping
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ralph, J. Hydroxycinnamates in lignification. Phytochem Rev. 2010, 9, 65–83. [Google Scholar] [CrossRef]
- Del Rio, J.C.; Rencoret, J.; Prinsen, P.; Martinez, A.T.; Ralph, J.; Gutierrez, A. Structural Characterization of Wheat Straw Lignin as Revealed by Analytical Pyrolysis, 2D-NMR, and Reductive Cleavage Methods. J. Agric. Food Chem. 2012, 60, 5922–5935. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J. Unique aspects of the grass cell wall. Curr. Opin. Plant Biol. 2008, 11, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Del Río, J.C.; Marques, G.; Rencoret, J.; Martínez, A.T.; Gutiérrez, A. Occurrence of naturally acetylated lignin units. J. Agric. Food Chem. 2007, 55, 5461–5468. [Google Scholar] [CrossRef] [PubMed]
- Romero, I.; Moya, M.; Sanchez, S.; Ruiz, E.; Castro, E.; Bravo, V. Ethanolic fermentation of phosphoric acid hydrolysates from olive tree pruning. Ind. Crops Prod. 2007, 25, 160–168. [Google Scholar] [CrossRef]
- Zweckmair, T.; Becker, M.; Ahn, K.; Hettegger, H.; Kosma, P.; Rosenau, T.; Potthast, A. A novel method to analyze the degree of acetylation in biopolymers. J. Chromatogr. 2014, 1372, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Brandt, A.; Chen, L.; von Dongen, B.; Welton, T.; Hallet, J.P. Structural changes in lignins isolated using an acidic ionic liquid water mixture. Green Chem. 2015, 17, 5019–5034. [Google Scholar] [CrossRef]
- Zweckmair, T.; Hettegger, H.; Abushammala, H.; Bacher, M.; Potthast, A.; Laborie, M.P.; Rosenau, T. On the mechanism of the unwanted acetylation of polysaccharides by 1,3-dialkylimidazolium acetate ionic liquids: Part 1—Analysis, acetylating agent, influence of water, and mechanistic considerations. Cellulose 2015, 22, 3583–3596. [Google Scholar] [CrossRef]
- Abushammala, H.; Hettegger, H.; Bacher, M.; Korntner, P.; Potthast, A.; Rosenau, T.; Laborie, M.P. On the mechanism of the unwanted acetylation of polysaccharides by 1,3-dialkylimidazolium acetate ionic liquids: Part 2—The impact of lignin on the kinetics of cellulose acetylation. Cellulose 2017. [Google Scholar] [CrossRef]
- Cetinko, O.P.; Dibble, D.C.; Cheng, G.; Kent, M.S.; Knierim, B.; Auer, M.; Wemmer, D.E.; Pelton, J.G.; Melnichenko, Y.B.; Ralph, J.; et al. Understanding the Impact of Ionic Liquid Pretreatment on Eucalyptus. Biofuels 2010, 1, 33–46. [Google Scholar] [CrossRef]
- Abushammala, H.; Pontes, J.F.; Gomes, G.H.; Osorio-Madrazo, A.; Thire, R.M.S.M.; Pereiral, V.F.; Laborie, M.P. Swelling, Viscoelastic, and Anatomical Studies on Ionic Liquid-Swollen Norway Spruce as a Screening Tool toward Ionosolv Pulping. Holzforschung 2015, 69, 1059–1067. [Google Scholar] [CrossRef]
- Abushammala, H.; Krossing, I.; Laborie, M.P. Ionic Liquid-Mediated Technology to Produce Cellulose Nanocrystals Directly from Wood. Carbohydr. Polym. 2015, 134, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Abushammala, H.; Goldsztayn, R.; Leao, A.; Laborie, M.P. Combining Steam Explosion with 1-Ethyl-3-Methylimidazlium Acetate Treatment of Wood Yields Lignin-Coated Cellulose Nanocrystals of High Aspect Ratio. Cellulose 2016, 23, 1813–1823. [Google Scholar] [CrossRef]
- Voigt, W.; Porte, H. South Africa National Biodiversity Institute. 2007. Available online: http://www.plantzafrica.com/planttuv/typhacapen.htm (accessed on 19 July 2017).
- Junrungreang, S.; Jutvapornvanit, P. Possibility of Cattail for Waste-Water Treatment; Report of Land Development Department: Bangkok, Thailand, 1996. [Google Scholar]
- Audu, I.G.; Ziegler-Devin, I.; Winter, H.; Bremer, M.; Hoffmann, A.; Fischer, S.; Laborie, M.-P.; Brosse, N. Impact of Ionic Liquid 1-Ethyl-3-Methylimidazolium Acetate Mediated Extraction on Lignin Features. Green Sustain. Chem. 2017, 7, 114–140. [Google Scholar] [CrossRef]
- Ralph, J.; Lu, F. The DFRC Method for Lignin Analysis. 6. A Simple Modification for Identifying Natural Acetates on Lignins. J. Agric. Food Chem. 1998, 46, 4616–4619. [Google Scholar] [CrossRef]
- Del Rio, J.C.; Prinsen, P.; Rencoret, J.; Nieto, L.; Jimenez-Barbero, J.; Ralph, J.; Martinez, A.T.; Gutierrez, A. Structural characterization of lignin in the Cortex and pith of Elephant grass (Pennisetum Purpureum) stems. J. Agric. Food Chem. 2012, 60, 3619–3634. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Ralph, J. The DFRC (Derivatization followed by reductive cleavage) method and its applications for lignin characterization. In Lignin; Lu, F., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2014; pp. 27–64. ISBN 978-63117-452-0. [Google Scholar]
- Schafer, J.; Urbat, F.; Rund, K.; Bunzel, M.J. A Stable-Isotope Dilution GC-MS Approach for the Analysis of DFRC (Derivatization Followed by Reductive Cleavage) Monomers from Low Lignin Plant Materials. J. Agric. Food Chem. 2015, 63, 2668–2673. [Google Scholar] [CrossRef] [PubMed]
- Wurzburg, O.B. Methods in Carbohydrate Chemistry; Volume IV, Acetylation; Academic Press: New York, NY, USA, 1964; pp. 286–288. [Google Scholar]
- Levigne, S.; Thomas, M.; Ralet, M.C.; Quemener, B.; Thibault, T.F. Determination of the degrees of methylation and acetylation of pectins using a C18 column and internal standards. Food Hydrocoll. 2002, 16, 547–550. [Google Scholar] [CrossRef]
- Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, A.; Sluiter, J.; Templeton, D. Preparation of Samples for Compositional Analysis. In Laboratory Analytical Procedure (LAP), National Renewable Energy Laboratory; National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- Obst, J.R.; Kirk, T.K. Isolation of Lignin. Methods Enzymol. 1988, 161, 87–101. [Google Scholar]
- Rencoret, J.; Marques, G.; Gutierrez, A.; Ibarra, D.; Li, J.; Gellerstedt, G.; Santos, J.I.; Jimene-Barbero, J.; Martinez, A.T.; del Rio, J.C. Structural Characterization of Milled Wood Lignins from Different Eucalypt Species. Holzforschung 2008, 62, 514–526. [Google Scholar] [CrossRef]
- Sun, N.; Rahman, M.; Qin, Y.; Maxim, M.L.; Rodriguez, H.; Rogers, R.D. Complete Dissolution and Partial Delignification of Wood in Ionic Liquid 1-Ethyl-3-Methylimidazolium Acetate. Green Chem. 2009, 11, 646–655. [Google Scholar] [CrossRef]
- El Hage, R.; Brosse, N.; Sannigrahi, P.; Ragauskas, A. Effects of Process Severity on the Chemical Structure of Miscanthus Ethanol Organosolv Lignin. Polym. Degrad. Stab. 2010, 95, 997–1003. [Google Scholar] [CrossRef]
- Lu, F.C.; Ralph, J. Non-degradative dissolution and acetylation of balled milled plant cell walls high-resolution solution state NMR. Plant J. 2003, 35, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Rencoret, J.; Marques, G.; Gutierrez, A.; Nieto, L.; Santos, J.I.; Jimene-Barbero, J.; Martinex, A.T.; del Rio, J.C. HSQC-NMR analysis of lignin in woody (Eucalyptus globulus and Picea abies) and non-woody (Agave sisalana) ball milled and plant materials at the gel state. Holzforchung 2009, 63, 691–698. [Google Scholar]
- Guiochon, G.; Guillemin, C.L. Quantitative Analysis by Gas Chromatography: Measurement of Peak Area and Derivation of Sample Composition. In Laboratory Analyses and On-Line Process Control; Elsevier: Amsterdam, The Netherlands, 1988; pp. 629–659. [Google Scholar]
- Kim, D.-Y.; Nishiyama, Y.; Kuga, S. Surface acetylation of bacterial cellulose. Cellulose 2002, 9, 361–367. [Google Scholar] [CrossRef]
- Agarwal, U.P.; Ralph, S.A. FT-Raman Spectroscopy of Wood: Identifying Contributions of Lignin and Carbohydrate Polymers in the Spectrum of Black Spruce (Picea mariana). Appl. Spectrosc. 1997, 51, 1648–1655. [Google Scholar] [CrossRef]
- Lupoi, J.S.; Smith, E.A. Characterization of Woody and Herbaceous Biomasses Lignin Composition with 1064 nm Dispersive Multichannel Raman Spectroscopy. Appl. Spectrosc. 2011, 66, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Willey, J.H.; Atalla, R.H. Band assignments in the Raman spectra of celluloses. Carbohydr. Res. 1987, 160, 113–129. [Google Scholar] [CrossRef]
- Saariaho, A.; Jaaskelainen, A.; Nuopponen, M.; Vuorinen, T. Ultra Violet Raman Spectroscopy in lignin analysis: Determination of characteristic vibrations of pHydroxophenyl, Guaiacyl, and Syringyl lignin structures. Appl. Spectrosc. 2003, 57, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.W.; Lupoi, J.S.; Smith, E.A. 1064 nm dispersive multichannel Raman spectroscopy for the analysis of plant lignin. Anal. Chim. Acta 2011, 706, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Lundquist. Proton (1H) NMR spectroscopy. In Methods in Lignin Chemistry; Lin, S.Y., Dence, C.W., Eds.; Springer Verlag: Heidelberg, Germany, 1992; pp. 242–247. ISBN 3-540-50295-5. [Google Scholar]
- Li, S.; Lundquist, L. A new method for the analysis of phenolic groups in lignin by 1H NMR spectroscopy. Nordic Pulp Pap. Res. J. 1994, 9, 191–195. [Google Scholar] [CrossRef]
- Mansfield, S.D.; Kim, H.; Lu, F.; Ralph, J. Whole plant cell wall characterization using solution-state 2D NMR. Nat. Protoc. 2012, 7, 1579–1589. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Kang, S.; Zhang, X.; Wu, Y.; Sun, R. Comparative Characterization of Lignins Extracted from Cotton Stalk Based on Complete Dissolution in Different Systems. Ind. Eng. Chem. Res. 2012, 51, 9858–9866. [Google Scholar] [CrossRef]
- Kim, H.; Ralph, J.; Akiyama, T. Solution-state 2D NMR of Ball-milled Plant Cell Wall Gels in DMSO-d6. Bioenergy Res. 2008. [Google Scholar] [CrossRef]
- Ralph, J.; Lundquist, K.; Brunow, G.; Lu, F.; Kim, H.; Schatz, P.F.; Marita, J.M.; Hatfield, R.D.; Ralph, S.A.; Christensen, J.H.; et al. Lignins: Natural polymers from oxidative coupling of 4-hydroxyphenyl-propanoids. Phytochem. Rev. 2004, 3, 29–60. [Google Scholar] [CrossRef]
- Rencoret, J.; del Rio, J.C.; Gutierrez, A.; Martinez, A.T.; Li, S.; Parkas, J.; Lundquist, K. Origin of the acetylated structures present in white birch (Betula pendula Roth) milled wood lignin. Wood Sci. Technol. 2011. [Google Scholar] [CrossRef]
- Lapierre, C.; Rolando, C. Thioacidolyses of pre-methylated lignin samples from Pine compression and Poplar woods. Holzforschung: Int. J. Biol. Chem. Phys. Technol. Wood. 1988, 42, 1–4. [Google Scholar] [CrossRef]
- Bethge, P.O.; Lindströnt, K. Determination of O-acetyl groups in wood. Svensk Papperstidning Nr 1973, 17, 645–649. [Google Scholar]
- Mansson, P.; Samuelsson, B. Quantitative Determination of O-Acetyl and Other O-Acyl Groups in Cellulosic Materials. Sven. Papperstidn. 1981, 84, R15–R24. [Google Scholar]
- Balaban, M.; Ucar, G. Estimation of volatile acids in wood and bark. Holz als Roh-und Werkst 2003, 61, 465–468. [Google Scholar] [CrossRef]
- Bacon, J.S.D.; Gordon, A.H.; Morris, E.J. Acetyl Groups in Cell-Wall Preparations from Higher Plants. Biochem. J. 1975, 149, 485–487. [Google Scholar] [CrossRef] [PubMed]
- Nabarlatz, D.; Ebringerová, A.; Montané, D. Autohydrolysis of agricultural by-products for the production of XYLO-oligosaccharides. Carbohyd. Polym. 2007, 69, 20–28. [Google Scholar] [CrossRef]
- Kumar, R.; Mago, G.; Balan, V.; Wyman, C.E. Physical and chemical characterizations of corn stover and poplar solids resulting from leading pretreatment technologies. Bioresour. Technol. 2009, 100, 3948–3962. [Google Scholar] [CrossRef] [PubMed]
- Rémond, C.; Aubry, N.; Crônier, D.; Noël, S.; Martel, F.; Roge, B.; Rakotoarivonina, H.; Debeire, P.; Chabbert, B. Combination of ammonia and xylanase pretreatments: Impact on enzymatic xylan and cellulose recovery from wheat straw. Bioresour. Technol. 2010, 101, 6712–6717. [Google Scholar] [CrossRef] [PubMed]
- Mante, O.D.; Babu, S.P.; Amidon, T.E. A comprehensive study on relating cell-wall components of lignocellulosic biomass to oxygenated species formed during pyrolysis. J. Anal. Appl. Pyrolysis 2014, 108, 56–67. [Google Scholar] [CrossRef]
- Haffner, F.B.; Mitchell, V.D.; Arundale, R.A.; Bauer, S. Compositional analysis of Miscanthus giganteus by near infrared spectroscopy. Cellulose 2013, 20, 1629–1637. [Google Scholar] [CrossRef]
- Nsaful, F.; Collard, F.; Carrier, M.; Görgens, J.F.; Knoetze, J.H. Lignocellulose pyrolysis with condensable volatiles quantification by thermogravimetric analysis—Thermal desorption/gas chromatography–mass spectrometry method. J. Anal. Appl. Pyrolysis 2015, 116, 86–95. [Google Scholar] [CrossRef]
- Garrote, G.; Domínguez, H.; Parajó, J.C. Autohydrolysis of corncob: Study of non-isothermal operation for xylooligosaccharide production. J. Food Eng. 2002, 52, 211–218. [Google Scholar] [CrossRef]
- Fujii, Y.; Azuma, J.I.; Marchessault, R.H.; Morin, F.G.; Aibara, S.; Okamura, K. Chemical composition change of bamboo accompanying its growth. Holzforschung 1993, 47, 109–115. [Google Scholar] [CrossRef]
- Gosselink, R.J.A.; van Dam, J.G.; Zomers, F.H.A. Combined hplc analysis of organic acids and furans formed during organosolv pulping of fiber hemp. J. Wood Chem. Technol. 1995, 15, 1–25. [Google Scholar] [CrossRef]
- De Carvalho, D.M.; Sevastyanova, O.; Penna, L.S.; da Silva, B.P.; Lindström, M.E.; Colodette, J.L. Assessment of chemical transformations in eucalyptus, sugarcane bagasse and straw during hydrothermal, dilute acid, and alkaline pretreatments. Ind. Crops Prod. 2015, 73, 118–126. [Google Scholar] [CrossRef]
- Iiyama, K.; Bach, T.L.T.; Natsuki, K.; Stone, B.A. Rapid and simple determination of o-acetyl groups bound to plant cell walls by acid hydrolysis and 1h NMR measurement. Phytochemistry 1994, 35, 959–961. [Google Scholar] [CrossRef]
- Suzuki, S.; Rodriguez, E.B.; Iiyama, K.; Saito, K.; Shintani, H. Compositional and structural characteristics of residual biomass from tropical plantations. J. Wood Sci. 1998, 44, 40–46. [Google Scholar] [CrossRef]
- Thammasouk, K.; Tandjo, D.; Penner, M.H. Influence of extractives on the analysis of herbaceous biomass. J. Agric. Food Chem. 1997, 45, 437–443. [Google Scholar] [CrossRef]
- Gallacher, J.; Snape, C.E.; Hassan, K.; Jarvis, M.C. Solid-state 13C NMR study of palm trunk cell walls. J. Food Agric. 1994, 64, 487–491. [Google Scholar] [CrossRef]
- Beckers, E.P.J.; Bongers, H.P.M.; van der Zee, M.E.; Sander, C. Acetyl content determination using different analytical techniques. In Proceedings of the First European Conference on Wood Modification, Ghent, Belgium, 3–4 April 2003; pp. 83–101. [Google Scholar]
- Zhang, G.; Huang, K.; Jiang, X.; Huang, D.; Yang, Y. Acetylation of rice straw for thermoplastic applications. Carbohyd. Polym. 2013, 96, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Puls, J. Chemistry and biochemistry of hemicelluloses: Relationship between hemicellulose structure and enzymes required for hydrolysis. Macromol. Symp. 1997, 120, 183–196. [Google Scholar] [CrossRef]
- Pinto, P.C.; Evtuguin, D.V.; Neto, C.P. Structure of hardwood glucuronoxylans: Modifications and impact on pulp retention during wood Kraft pulping. Carbohyd. Polym. 2005, 60, 489–497. [Google Scholar] [CrossRef]





| Acetyl Content | ||||
|---|---|---|---|---|
| Sample | Zemplén | Back-Titration | ||
| (−) | (mmol/g) | (wt %) | (mmol/g) | (wt %) |
| Typha capensis samples | ||||
| TCextr | 1.43 ± 0.02 | 6 ± 0.08 | 2.53 ± 0.05 | 11 ± 0.23 |
| ILcrr a | – b | – b | 0.70 ± 0.02 | 3 ± 0.08 |
| MWL | 1.79 ± 0.07 | 8 ± 0.28 | 2.64 ± 0.07 | 11 ± 0.30 |
| ILL | 0.24 ± 0.04 | 1 ± 0.15 | 0.51 ± 0.01 | 2 ± 0.05 |
| EOL | 0.23 ± 0.04 | 1 ± 0.18 | 0.18 ± 0.01 | 1 ± 0.01 |
| Reference samples | ||||
| Cellulose acetate | 10.57 ± 0.54 | 45 ± 2.33 | 9.77 ± 0.02 | 42 ± 0.08 |
| Ethyl acetate | 11.04 ± 0.18 | 48 ± 0.76 | 10.94 ± 0.08 | 47 ± 0.36 |
| Beech wood | 1.15 ± 0.04 | 5 ± 0.17 | 1.47 ± 0.05 | 6 ± 0.22 |
| Beech wood acetylated | 6.45 ± 0.05 | 28 ± 0.21 | 5.47 ± 0.03 | 24 ± 0.14 |
| Miscanthus x giganteus | 0.97 ± 0.01 | 4 ± 0.05 | 1.61 ± 0.08 | 7 ± 0.36 |
| Coconut trunk | 1.01 ± 0.07 | 4 ± 0.28 | 1.69 ± 0.04 | 7 ± 0.15 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Audu, I.G.; Brosse, N.; Winter, H.; Hoffmann, A.; Bremer, M.; Fischer, S.; Laborie, M.-P. Acetyl Groups in Typha capensis: Fate of Acetates during Organosolv and Ionosolv Pulping. Polymers 2018, 10, 619. https://doi.org/10.3390/polym10060619
Audu IG, Brosse N, Winter H, Hoffmann A, Bremer M, Fischer S, Laborie M-P. Acetyl Groups in Typha capensis: Fate of Acetates during Organosolv and Ionosolv Pulping. Polymers. 2018; 10(6):619. https://doi.org/10.3390/polym10060619
Chicago/Turabian StyleAudu, Idi Guga, Nicolas Brosse, Heiko Winter, Anton Hoffmann, Martina Bremer, Steffen Fischer, and Marie-Pierre Laborie. 2018. "Acetyl Groups in Typha capensis: Fate of Acetates during Organosolv and Ionosolv Pulping" Polymers 10, no. 6: 619. https://doi.org/10.3390/polym10060619
APA StyleAudu, I. G., Brosse, N., Winter, H., Hoffmann, A., Bremer, M., Fischer, S., & Laborie, M.-P. (2018). Acetyl Groups in Typha capensis: Fate of Acetates during Organosolv and Ionosolv Pulping. Polymers, 10(6), 619. https://doi.org/10.3390/polym10060619








