Selective and Efficient Arsenic Recovery from Water through Quaternary Amino-Functionalized Silica
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Silylation
2.3. Graft Polymerization
2.4. Characterization
2.5. Metal Affinity Studies
3. Results and Discussion
3.1. 13C and 29Si NMR Analysis
3.2. FT-IR Analysis
3.3. TGA Analysis
3.4. XRD Analysis
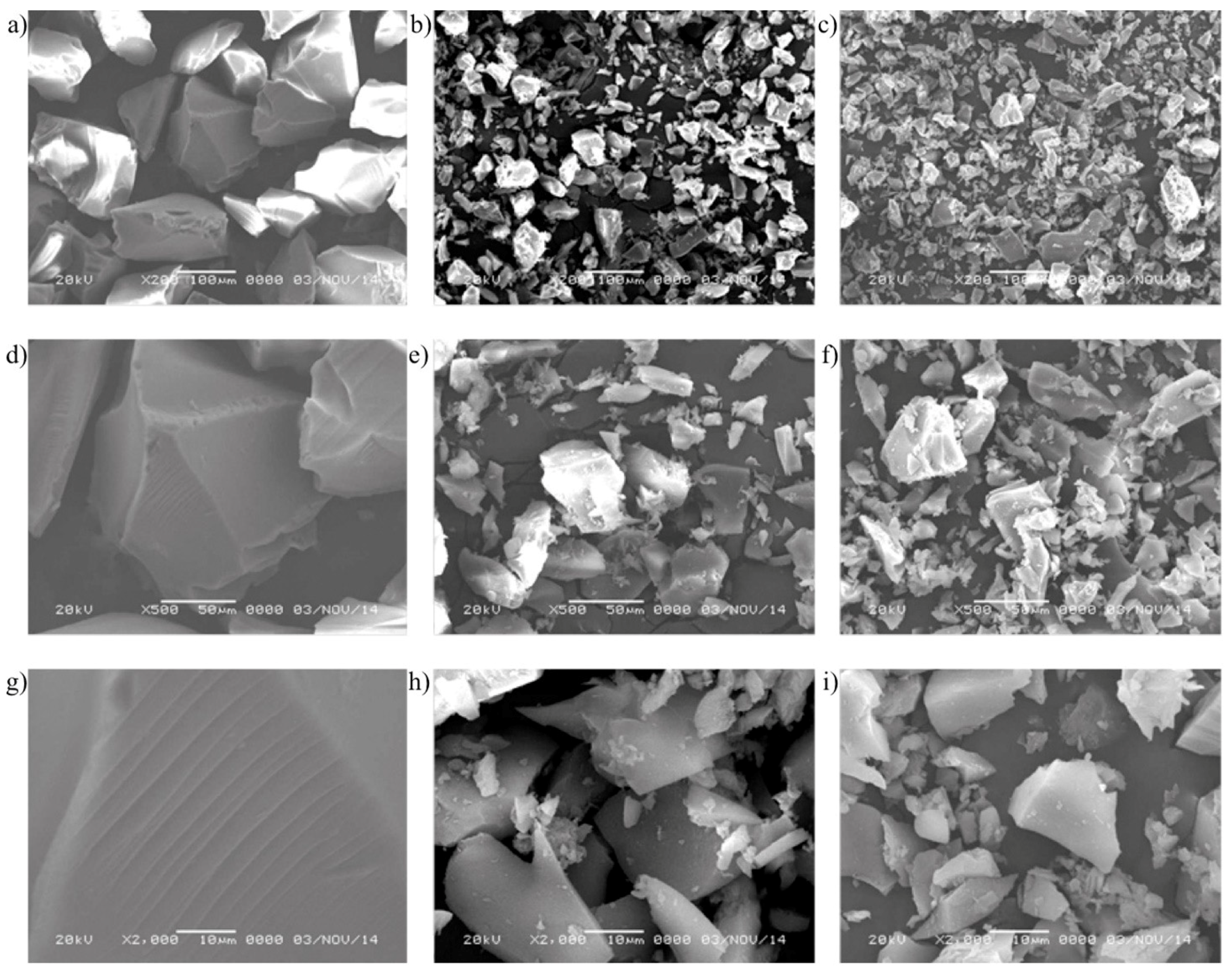
3.5. SEM Analysis
3.6. Arsenic Retention Properties of Q/VTMSO-SiO2
3.7. Metal Affinities of Q/VTMSO-SiO2 Fill and Commercial Water Filter Fill
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Mohan, D.; Pittman, C.U., Jr. Arsenic removal from water/wastewater using adsorbents—A critical review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar] [CrossRef] [PubMed]
- Saha, J.C.; Dikshit, A.K.; Bandyopadhyay, M.; Saha, K.C. A Review of Arsenic Poisoning and its Effects on Human Health. Environ. Sci. Technol. 1999, 29, 281–313. [Google Scholar] [CrossRef]
- Kapaj, S.; Peterson, H.; Liber, K.; Bhattacharya, P. Human Health Effects from Chronic Arsenic Poisoning—A Review. J. Environ. Sci. Health A 2006, 41, 2399–2428. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for Drinking-Water Quality, 3rd ed.; WHO: Geneva, Switzerland, 2008; Volume 1. [Google Scholar]
- Melamed, D. Monitoring Arsenic in the Environment: A Review of Science and Technologies for Field Measurements and Sensors; EPA Report 542/R-04/002; U.S. Environmental Protection Agency: Washington, DC, USA, 2004; pp. 1–23.
- Malik, A.H.; Khan, Z.M.; Mahmood, Q.; Nasreen, S.; Bhatti, Z.A. Perspectives of low cost arsenic remediation of drinking water in Pakistan and other countries. J. Hazard. Mater. 2009, 168, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Villoslada, I.; Rivas, B.L. Competition of Divalent Metal Ions with Monovalent Metal Ions on the Adsorption on Water-Soluble Polymers. J. Phys. Chem. B 2002, 106, 9708–9711. [Google Scholar] [CrossRef]
- Rivas, B.L.; Aguirre, M.D.C.; Pereira, E. Retention Properties of Arsenate Anions of Water-Soluble Polymers by a Liquid-Phase Polymer-Based Retention Technique. J. Appl. Polym. Sci. 2006, 102, 2677–2684. [Google Scholar] [CrossRef]
- Rivas, B.L.; Aguirre, M.D.C.; Pereira, E.; Moutet, J.-C.; Aman, E.S. Capability of Cationic Water-Soluble Polymers in Conjunction With Ultrafiltration Membranes to Remove Arsenate Ions. Polym. Eng. Sci. 2007, 47, 1256–1261. [Google Scholar] [CrossRef]
- Sánchez, J.; Rivas, B.L. Arsenic extraction from aqueous solution: Electrochemical oxidation combined with ultrafiltration membranes and water-soluble polymers. Chem. Eng. J. 2010, 165, 625–632. [Google Scholar] [CrossRef]
- Milner, S.T. Polymer brushes. Science 1991, 251, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.P.; Wang, Y.P.; Wang, R.M.; Zhang, S.C. Preparation of polymer brushes on palygorskite surfaces via RAFT polymerization. React. Funct. Polym. 2008, 68, 643–648. [Google Scholar] [CrossRef]
- Granville, A.M.; Boyes, S.G.; Akgun, B.; Foster, M.D.; Brittain, W.J. Thermo-responsive behavior of semi-fluorinated polymer brushes. Macromolecules 2005, 38, 3263–3270. [Google Scholar] [CrossRef]
- Zhou, Q.; Fan, X.; Xia, C.; Mays, J.; Advincula, R. Living anionic surface initiated polymerization (SIP) of styrene clay surfaces. Chem. Mater. 2001, 13, 2465–2467. [Google Scholar] [CrossRef]
- Fan, X.; Xia, C.; Fulghum, T.; Park, M.K.; Locklin, J.; Advincula, R. Polymer brushes grafted from clay nanoparticles adsorbed on a planar substrate by free radical surface-initiated polymerization. Langmuir 2003, 19, 916–923. [Google Scholar] [CrossRef]
- Rider, D.A.; Chen, J.I.L.; Eloi, J.C. Controlling the morphologies of organometallic block copolymers in the 3-dimensional spatial confinement of colloidal and inverse colloidal crystals. Macromolecules 2008, 41, 2250–2259. [Google Scholar] [CrossRef]
- Norde, W.; Gags, D. Interaction of bovine serum albumin and human blood plasma with PEO-tethered surfaces: Influence of PEO chain length, grafting density, and temperature. Langmuir 2004, 20, 4162–4167. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, J.; Isaacs, L.; Grzybowski, B.; Carbeck, J.D.; Whitesides, G.M. Biospecifîc binding of carbonic anhydrase to mixed SAMs presenting benzenesulfonamide ligands: A model system for studying lateral steric effects. Langmuir 1999, 15, 7186–7198. [Google Scholar] [CrossRef]
- Hucknall, A.; Rangarajan, S.; Chilkoti, A. In pursuit of zero: Polymer brushes that resist the adsorption of proteins. Adv. Mater. 2009, 21, 2441–2446. [Google Scholar] [CrossRef]
- Kataoka, D.E.; Troian, S.M. Patterning liquid flow on the microscopic scale. Nature 1999, 402, 794–797. [Google Scholar] [CrossRef]
- Zhao, B.; Brittain, W.J. Polymer brushes: Surface-immobilized macromolecules. Prog. Polym. Sci. 2000, 25, 677–710. [Google Scholar] [CrossRef]
- Park, J.W.; Thomas, E.L. A surface-reactive rod-coil diblock copolymer: Nano- and micropatterned polymer brushes. J. Am. Chem. Soc. 2002, 124, 514–515. [Google Scholar] [CrossRef] [PubMed]
- Gong, F.; Feng, M.; Zhao, C.; Zhang, S.; Yang, M. Thermal properties of poly(vinyl chloride)/montmorillonite nanocomposites. Polym. Degrad. Stab. 2004, 84, 289–294. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, Y.; Peng, Z.; Zhang, Y. Influence of clay modification on the structure and mechanical properties of EPDM/montmorillonite nanocomposites. Polym. Test. 2004, 23, 217–223. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, H.K. Characterization of organobentonite used for polymer nanocomposites. Mater. Chem. Phys. 2004, 85, 410–415. [Google Scholar] [CrossRef]
- Gong, F.; Feng, M.; Zhao, C.; Zhang, S.; Yang, M. Particle configuration and mechanical properties of poly(vinyl chloride)/montmorillonite nanocomposites via in situ suspension polymerization. Polym. Test. 2004, 23, 847–853. [Google Scholar] [CrossRef]
- Tully, D.C.; Trimble, A.R.; Fréchet, J.M.J.; Wilder, K.; Quate, C.F. Synthesis and preparation of ionically bound dendrimer monolayers and application toward scanning probe lithography. Chem. Mater. 1999, 11, 2892–2898. [Google Scholar] [CrossRef]
- Zhao, B.; Brittain, W.J. Synthesis of polystyrene brushes on silicate substrates via carbocationic polymerization from self-assembled monolayers. Macromolecules 2000, 33, 342–348. [Google Scholar] [CrossRef]
- Tran, Y.; Auroy, P. Synthesis of poly(styrene sulfonate) brushes. J. Am. Chem. Soc. 2001, 123, 3644–3654. [Google Scholar] [CrossRef] [PubMed]
- Mansky, P.; Liu, Y.; Huang, E.; Russell, T.P.; Hawker, C. Controlling polymer-surface interactions with random copolymer brushes. Science 1997, 275, 1458–1460. [Google Scholar] [CrossRef]
- Prucker, O.; Rühe, J. Synthesis of poly(styrene) monolayers attached to high surface area silica gels through self-assembled monolayers of azo initiators. Macromolecules 1998, 31, 592–601. [Google Scholar] [CrossRef]
- Prucker, O.; Rühe, J. Mechanism of radical chain polymerizations initiated by azo compounds covalently bound to the surface of spherical particles. Macromolecules 1998, 3, 602–613. [Google Scholar] [CrossRef]
- Bourgeat-Lami, E.; Lang, J. Encapsulation of inorganic particles by dispersion polymerization in polar media 1. Silica nanoparticles encapsulated by polystyrene. J. Colloid Interface Sci. 1998, 197, 293–308. [Google Scholar] [CrossRef]
- Bartholome, C.; Beyou, E.; Bourgeat-Lami, E.; Chaumont, P.; Zydowicz, N. Nitroxide-Mediated Polymerizations from Silica Nanoparticle Surfaces: “Graft from” Polymerization of Styrene Using a Triethoxysilyl-Terminated Alkoxyamine Initiator. Macromolecules 2003, 36, 7946–7952. [Google Scholar] [CrossRef]
- Guo, Z.X.; Yu, J. Grafting of dendritic polyethers onto nanometer silica surface. J. Mater. Chem. 2002, 12, 468–472. [Google Scholar] [CrossRef]
- Kedong, X.; Lu, C.; Yang, Y.; Zhang, B. Effect of vinyltriethoxysilane addition on the pyrolytic conversion of tetraethoxysilane based silica gel. J. Sol-Gel Sci. 2014, 69, 266–271. [Google Scholar]
- Valdés, O.; Monett, D.; Agüero, L.; Zaldivar, D.; Alexandrova, L.; Katime, I. Synthesis and Characterization of Poly(acryloxyethyltrimethyl-ammonium chloride-co-2-hydroxyethyl methacrylate): A Study of its Interaction with Sodium Alginate. J. Appl. Polym. Sci. 2008, 108, 1680–1688. [Google Scholar] [CrossRef]
- Valdés, O.; Alexandrova, L.; Zaldivar, D.; Katime, I. A comparative study of two polyelectrolyte complexes. J. Appl. Polym. Sci. 2012, 125, 3345–3350. [Google Scholar] [CrossRef]










| Experiment | As concentration [μg·L−1] | Volume [mL] | As captured [μg·L−1] | As capture rate [%] |
|---|---|---|---|---|
| 1 | 16.38 (−) | 100 (−1) | 0.130 | 0.790 |
| 2 | 16.38 (−1) | 500 (1) | 4.52 | 27.6 |
| 3 | 87.42 (−0.64) | 100 (−1) | 3.24 | 3.71 |
| 4 | 87.42 (−0.64) | 500 (1) | 5.27 | 6.03 |
| 5 | 409.2 (1) | 100 (−1) | 26.7 | 6.52 |
| 6 | 409.2 (1) | 500 (1) | 9.20 | 2.25 |
| 7 | 87.42 (−0.64) | 300 (0) | 8.80 | 10.1 |
| 8 | 87.42 (−0.64) | 300 (0) | 10.9 | 12.5 |
| 9 | 87.42 (−0.64) | 300 (0) | 11.2 | 12.8 |
| Experiment | As concentration [μg·L−1] | Volume [mL] | As captured [μg·L−1] | As capture rate [%] |
|---|---|---|---|---|
| 1 | 16.38 (−1.00) | 100 (−1) | 4.31 | 26.30 |
| 2 | 16.38 (−1.00) | 500 (1) | 5.05 | 30.80 |
| 3 | 87.42 (−0.64) | 100 (−1) | 25.20 | 28.80 |
| 4 | 87.42 (−0.64) | 500 (1) | 8.33 | 9.530 |
| 5 | 409.2 (1.00) | 100 (−1) | 156.00 | 38.20 |
| 6 | 409.2 (1.00) | 500 (1) | 80.90 | 19.80 |
| 7 | 87.42 (−0.64) | 300 (0) | 14.90 | 17.00 |
| 8 | 87.42 (−0.64) | 300 (0) | 14.20 | 16.30 |
| 9 | 87.42 (−0.64) | 300 (0) | 14.50 | 16.50 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valdés, O.; Marican, A.; Mirabal-Gallardo, Y.; Santos, L.S. Selective and Efficient Arsenic Recovery from Water through Quaternary Amino-Functionalized Silica. Polymers 2018, 10, 626. https://doi.org/10.3390/polym10060626
Valdés O, Marican A, Mirabal-Gallardo Y, Santos LS. Selective and Efficient Arsenic Recovery from Water through Quaternary Amino-Functionalized Silica. Polymers. 2018; 10(6):626. https://doi.org/10.3390/polym10060626
Chicago/Turabian StyleValdés, Oscar, Adolfo Marican, Yaneris Mirabal-Gallardo, and Leonardo S. Santos. 2018. "Selective and Efficient Arsenic Recovery from Water through Quaternary Amino-Functionalized Silica" Polymers 10, no. 6: 626. https://doi.org/10.3390/polym10060626







