Free-Radical Copolymerization of Dibenzofulvene with (Meth)acrylates Leading to π-Stacked Copolymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
2.3. Determination of Monomeric Unit Ratios of Copolymers
2.4. Synthesis
Radical Copolymerization
2.5. Computational Method
3. Results
3.1. Copolymerization Reaction
3.2. Structrure of Copolymers
3.3. Photophysical Properties of Copolymers
3.4. Electrochemical Properties of Copolymers
3.5. Thermal Properties of Copolymers
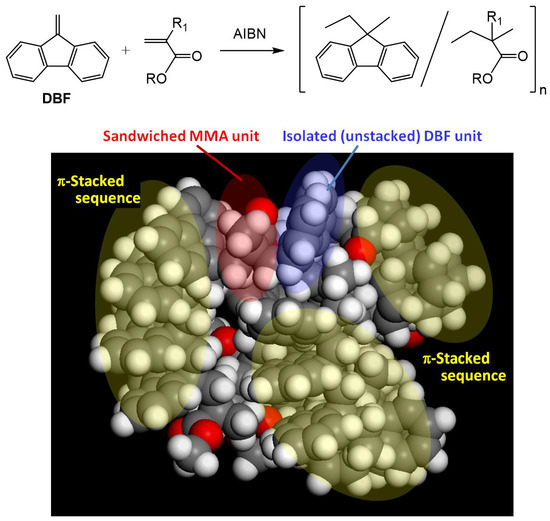
3.6. Proposed Structure of Poly(DBF-co-MMA)
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Appendix A


References
- Yashima, E.; Maeda, K.; Iida, H.; Furusho, Y.; Nagai, K. Helical polymers: Synthesis, structures, and functions. Chem. Rev. 2009, 109, 6102–6211. [Google Scholar] [CrossRef] [PubMed]
- Yashima, E.; Ousaka, N.; Taura, D.; Shimomura, K.; Ikai, T.; Maeda, K. Supramolecular Helical Systems: Helical Assemblies of Small Molecules, Foldamers, and Polymers with Chiral Amplification and Their Functions. Chem. Rev. 2016, 116, 13752–13990. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Okamoto, Y. Synthetic helical polymers: Conformation and function. Chem. Rev. 2001, 101, 4013–4038. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Nakano, T. Asymmetric polymerization. Chem. Rev. 1994, 94, 349–372. [Google Scholar] [CrossRef]
- Nakano, T. Optically active synthetic polymers as chiral stationary phases in HPLC. J. Chromatogr. A 2001, 906, 205–225. [Google Scholar] [CrossRef]
- Nakano, T. Synthesis, structure and function of π-stacked polymers. Polym. J. 2010, 42, 103–123. [Google Scholar] [CrossRef] [Green Version]
- Nakano, T.; Takewaki, K.; Yade, T.; Okamoto, Y. Dibenzofulvene, a 1,1-diphenylethylene analogue, gives a π-stacked polymer by anionic, free-radical, and cationic catalysts. J. Am. Chem. Soc. 2001, 123, 9182–9183. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Yade, T. Synthesis, structure, and photophysical and electrochemical properties of a π-stacked polymer. J. Am. Chem. Soc. 2003, 125, 15474–15484. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Yade, T.; Yokoyama, M.; Nagayama, N. Charge Transport in a π-Stacked Poly(dibenzofulvene) Film. Chem. Lett. 2004, 33, 296–297. [Google Scholar] [CrossRef]
- Nakano, T.; Nakagawa, O.; Tsuji, M.; Tanikawa, M.; Yade, T.; Okamoto, Y. Poly(2,7-di-n-pentyldibenzofulvene) showing chiroptical properties in the solid state based purely on a chiral conformation. Chem. Commun. 2004, 144–145. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Yade, T.; Fukuda, Y.; Yamaguchi, T.; Okumura, S. Free-radical polymerization of dibenzofulvene leading to a π-stacked polymer: Structure and properties of the polymer and proposed reaction mechanism. Macromolecules 2005, 38, 8140–8148. [Google Scholar] [CrossRef]
- Coropceanu, V.; Nakano, T.; Gruhn, N.E.; Kwon, O.; Yade, T.; Katsukawa, K.; Bredas, J.L. Probing charge transport in pi-stacked fluorene-based systems. J. Phys. Chem. B 2006, 110, 9482–9487. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Tanikawa, M.; Nakagawa, O.; Yade, T.; Sakamoto, T. Synthesis and structure of an optically active π-stacked poly(dibenzofulvene) bearing chiral terminal group. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 239–246. [Google Scholar] [CrossRef]
- Nageh, H.; Wang, Y.; Nakano, T. Cationic polymerization of dibenzofulvene leading to a π-stacked polymer. Polymer 2018, 144, 51–56. [Google Scholar] [CrossRef]
- Watanabe, K.; Sakamoto, T.; Taguchi, M.; Fujiki, M.; Nakano, T. A chiral π-stacked vinyl polymer emitting white circularly polarized light. Chem. Commun. 2011, 47, 10996–10998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cappelli, A.; Anzini, M.; Vomero, S.; Donati, A.; Zetta, L.; Mendichi, R.; Casolaro, M.; Lupetti, P.; Salvatici, P.; Giorgi, G. New π-stacked benzofulvene polymer showing thermoreversible polymerization: Studies in macromolecular and aggregate structures and polymerization mechanism. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 3289–3304. [Google Scholar] [CrossRef]
- Cappelli, A.; Razzano, V.; Paolino, M.; Grisci, G.; Giuliani, G.; Donati, A.; Mendichi, R.; Samperi, F.; Battiato, S.; Boccia, A.C.; et al. Bithiophene-based polybenzofulvene derivatives with high stacking and hole mobility. Polym. Chem. 2015, 6, 7377–7388. [Google Scholar] [CrossRef]
- Morisaki, Y.; Chujo, Y. Through-Space Conjugated Polymers Based on Cyclophanes. Angew. Chem. Int. Ed. 2006, 45, 6430–6437. [Google Scholar] [CrossRef] [PubMed]
- Morisaki, Y.; Ishida, T.; Chujo, Y. Synthesis and Properties of Novel Through-Space π-Conjugated Polymers Based on Poly(p-phenylenevinylene)s Having a [2.2]Paracyclophane Skeleton in the Main Chain. Macromolecules 2002, 35, 7872–7877. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Jagtap, S.P.; Coropceanu, V.; Bredas, J.-L.; Collard, D.M. π-Stacked Oligo(phenylene vinylene)s Based on Pseudo-Geminal Substituted [2.2]Paracyclophanes: Impact of Interchain Geometry and Interactions on the Electronic Properties. Angew. Chem. Int. Ed. 2012, 51, 11629–11632. [Google Scholar] [CrossRef] [PubMed]
- Gudeangadi, P.G.; Sakamoto, T.; Shichibu, Y.; Konishi, K.; Nakano, T. Chiral Polyurethane Synthesis Leading to π-Stacked 2/1-Helical Polymer and Cyclic Compounds. ACS Macro Lett. 2015, 4, 901–906. [Google Scholar] [CrossRef]
- Yang, W.; Nakano, T. Synthesis of poly(1,10-phenanthroline-5,6-diyl)s having a π-stacked, helical conformation. Chem. Commun. 2015, 51, 17269–17272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merlani, M.; Koyama, Y.; Sato, H.; Geng, L.; Barbakadze, V.; Chankvetadze, B.; Nakano, T. Ring-opening polymerization of a 2,3-disubstituted oxirane leading to a polyether having a carbonyl–aromatic π-stacked structure. Polym. Chem. 2015, 6, 1932–1936. [Google Scholar] [CrossRef] [Green Version]
- Sugino, H.; Koyama, Y.; Nakano, T. A high triplet-energy polymer: Synthesis and photo-physical properties of a π-stacked vinyl polymer having a xanthone moiety in the side chain. RSC Adv. 2015, 5, 21310–21315. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Ye, X.; Hayama, H.; Sugino, H.; Nakano, H.; Nakano, T. π-Stacked poly(vinyl ketone)s with accumulated push–pull triphenylamine moieties in the side chain. Polym. Chem. 2017, 8, 708–714. [Google Scholar] [CrossRef]
- Sun, H. An ab initio force-field optimized for condensed-phase applications overview with details on alkane and benzene compounds. J. Phys. Chem. 1998, 102, 7338–7364. [Google Scholar] [CrossRef]
- Fletcher, R.; Reeves, C.M. Function minimization by conjugate gradients. Comput. J. 1964, 7, 149–154. [Google Scholar] [CrossRef] [Green Version]
- Berendsen, H.J.C.; Postma, J.P.M.; van Gunsteren, W.F.; DiNola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Bolton, H.; Weiss, J.J. Hypochromism in the ultra-violet absorption of nucleic acids and related structures. Nature 1962, 195, 666–668. [Google Scholar] [CrossRef] [PubMed]
- Tinoco, I., Jr. Hypochromism in Polynucleotides1. J. Am. Chem. Soc. 1960, 82, 4785–4790. [Google Scholar] [CrossRef]
- Rhodes, W. Hypochromism and other spectral properties of helical polynucleotides. J. Am. Chem. Soc. 1961, 83, 3609–3617. [Google Scholar] [CrossRef]
- Horrocks, D.; Brown, W. Solution fluorescence spectrum of highly purified fluorene. Chem. Phys. Lett. 1970, 5, 117–119. [Google Scholar] [CrossRef]







| Run | M2 | [M1]/[M2] in Feed | Conv. (%) b | Hexane-Soluble or Diethyl Ether-Soluble Part c,d | Hexane or Diethyl Ether-Insoluble, THF-Soluble Part | THF-Insoluble Part | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M1 | M2 | Yield e (%) | Mnf | Mw/Mnf | [M1]/[M2] in Polymer g | Yield (%) | Mnf | Mw/Mnf | Yield (%) | |||
| 1 | MMA | 0/100 | 0 | 97 | 2 | 640 | 1.10 | 82 | 3570 | 2.33 | ~0 | |
| 2 | 20/80 | 42 | 8 | 21 | 730 | 1.18 | 82/18 | ~0 | 1120 | 1.11 | ~0 | |
| 3 | 50/50 | 56 | 5 | 10 | 850 | 1.16 | 90/10 | 9 | 1160 | 1.17 | 19 | |
| 4 | 80/20 | 72 | 5 | 8 | 790 | 1.22 | 82/18 | 11 | 1310 | 1.19 | 45 | |
| 5 | HEMA | 0/100 | 0 | >99 | 4 | 410 | 1.51 | 2 | 1540 | 1.29 | 90 | |
| 6 | 20/80 | 56 | 11 | 26 | 670 | 1.52 | 73/27 | 2 | 1280 | 1.15 | 2 | |
| 7 | 50/50 | 60 | 5 | 25 | 940 | 1.40 | 88/12 | 11 | 1490 | 1.14 | 23 | |
| 8 | 79/21 | 71 | 4 | 2 | 830 | 1.26 | 93/7 | 11 | 1470 | 1.19 | 56 | |
| 9 | MA | 0/100 | 0 | 97 | 8 | 400 | 1.18 | 86 | 14,880 | 2.16 | ~0 | |
| 10 | 20/80 | 42 | 17 | 25 | 620 | 1.10 | 87/13 | ~0 | 1620 | 1.52 | ~0 | |
| 11 | 50/50 | 54 | 13 | 18 | 790 | 1.13 | 96/4 | 8 | 1250 | 1.19 | 17 | |
| 12 | 80/20 | 69 | 13 | 5 | 590 | 1.21 | 94/6 | 10 | 1300 | 1.23 | 50 | |
| 13 | HEA | 0/100 | 0 | >99 | 5 | 360 | 1.05 | 87 | 1430 | 1.13 | ~0 | |
| 14 | 20/80 | 48 | 5 | 18 | 700 | 1.19 | 89/11 | 1 | 2750 | 1.43 | ~0 | |
| 15 | 50/50 | 60 | 4 | 12 | 950 | 1.19 | 95/5 | 5 | 1670 | 1.13 | 17 | |
| 16 | 80/20 | 74 | 3 | 3 | 870 | 1.22 | 96/4 | 14 | 1820 | 1.12 | 57 | |
| 17 | None (DBF homo-polymerization) | 100/0 | 78 | 0 | 9 | 790 | 1.14 | 2 | 1490 | 1.13 | 70 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, J.; Wang, Y.; Nakano, T. Free-Radical Copolymerization of Dibenzofulvene with (Meth)acrylates Leading to π-Stacked Copolymers. Polymers 2018, 10, 654. https://doi.org/10.3390/polym10060654
Luo J, Wang Y, Nakano T. Free-Radical Copolymerization of Dibenzofulvene with (Meth)acrylates Leading to π-Stacked Copolymers. Polymers. 2018; 10(6):654. https://doi.org/10.3390/polym10060654
Chicago/Turabian StyleLuo, Jiyue, Yue Wang, and Tamaki Nakano. 2018. "Free-Radical Copolymerization of Dibenzofulvene with (Meth)acrylates Leading to π-Stacked Copolymers" Polymers 10, no. 6: 654. https://doi.org/10.3390/polym10060654