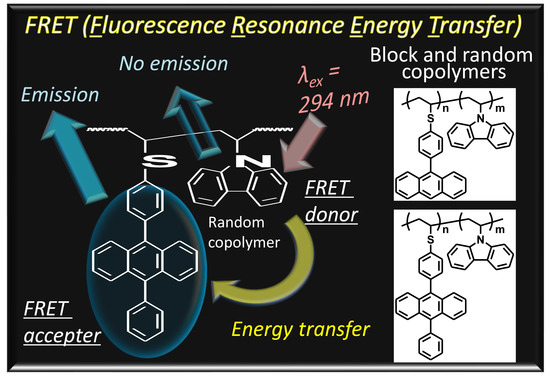
Synthesis and Optoelectronic Properties of Block and Random Copolymers Containing Pendant Carbazole and (Di)phenylanthracene
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of Block Copolymers
2.3. Synthesis of Random Copolymers
2.4. Instrumentation
3. Results and Discussion
3.1. Synthesis of Block and Random Copolymers
3.2. Optoelectronic Properties of Block and Random Copolymers
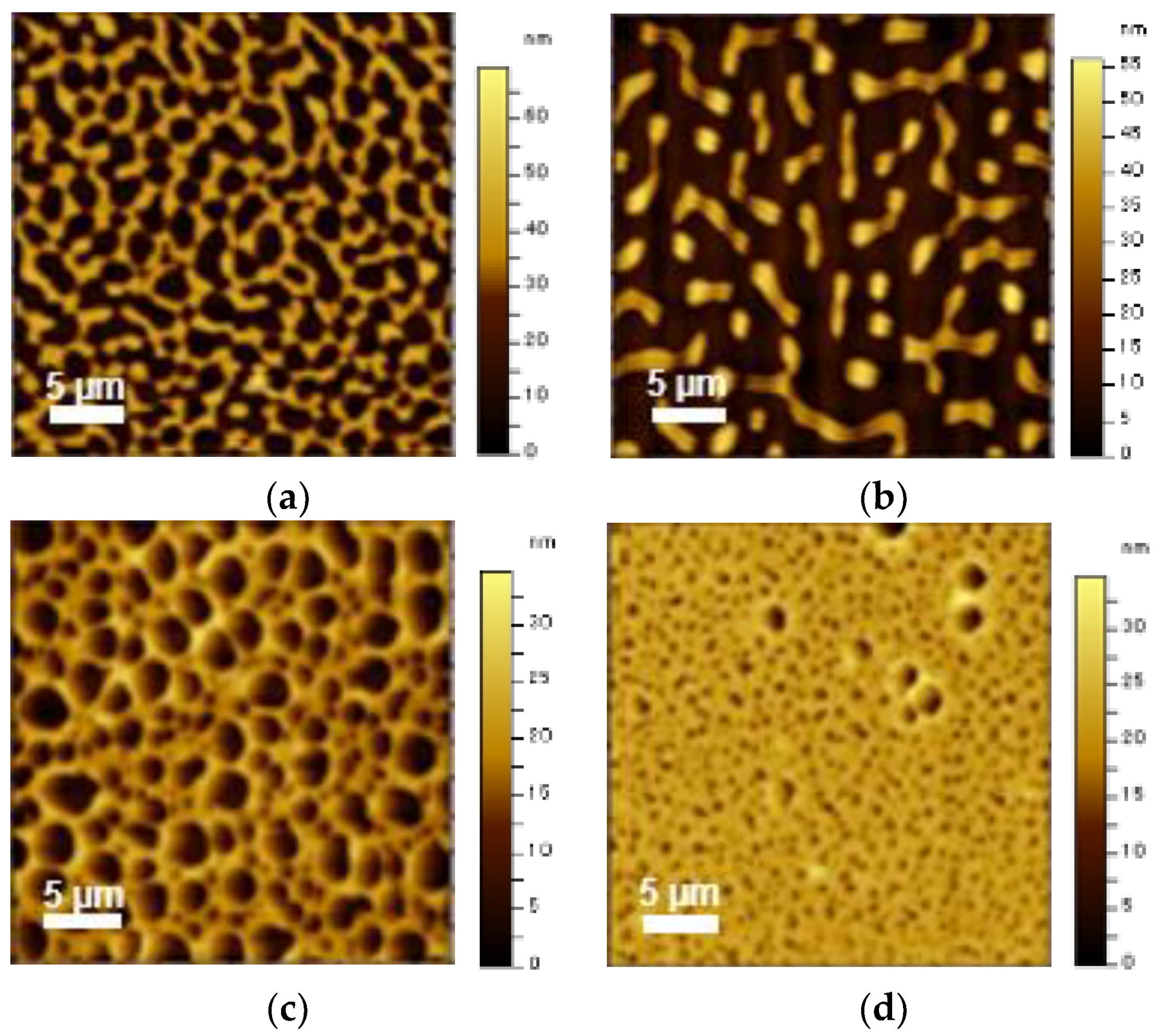
3.3. Assembled Structures of Block and Random Copolymers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Segalman, R.A.; McCulloch, B.; Kirmayer, S.; Urban, J.J. Block copolymers for organic optoelectronics. Macromolecules 2009, 42, 9205–9216. [Google Scholar] [CrossRef]
- Laquai, F.; Park, Y.-S.; Kim, J.-J.; Basche, T. Excitation energy transfer in organic materials: From fundamentals to optoelectronic devices Macromol. Rapid Commun. 2009, 30, 1203–1231. [Google Scholar] [CrossRef] [PubMed]
- Inganas, O.; Zhang, F.; Andersson, M.R. Alternating polyfluorenes collect solar light in polymer photovoltaics. Acc. Chem. Res. 2009, 42, 1731–1739. [Google Scholar] [CrossRef] [PubMed]
- Sonar, P.; Singh, S.P.; Leclere, P.; Surin, M.; Lazzaroni, R.; Lin, T.T.; Dodabalapur, A.; Sellinger, A. Synthesis, characterization and comparative study of thiophene-benzothiadiazole based donor-acceptor-donor (D-A-D) materials. J. Mater. Chem. 2009, 19, 3228–3237. [Google Scholar] [CrossRef]
- Guenes, S.; Neugebauer, H.; Sariciftci, N.S. Conjugated polymer-based organic solar cells. Chem. Rev. 2007, 107, 1324–1338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Z.-C.; Li, K.-B.; Lin, S.; Du, F.-S.; Li, F.-M. Donor/acceptor vinyl monomers and their polymers: Synthesis, photochemical and photophysical behavior. Prog. Polym. Sci. 2006, 31, 893–948. [Google Scholar] [CrossRef]
- Cheng, Y.-J.; Yang, S.-H.; Hsu, C.-S. Synthesis of conjugated polymers for organic solar cell applications. Chem. Rev. 2009, 109, 5868–5923. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Hu, H.; Ghasemi, M.; Wang, T.; Collins, B.A.; Kim, J.-H.; Jiang, K.; Carpenter, J.H.; Li, H.; Li, Z.; et al. Quantitative relations between interaction parameter, miscibility and function in organic solar cells. Nat. Mater. 2018, 17, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Jiao, X.; Zhang, H.; Li, S.; Yao, H.; Ade, H.; Hou, J. 2D-Conjugated Benzodithiophene-Based Polymer Acceptor: Design, Synthesis, Nanomorphology, and Photovoltaic Performance. Macromolecules 2015, 48, 7156–7163. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, S.; Zhao, W.; Yao, H.; Hou, J. Highly Efficient 2D-Conjugated Benzodithiophene-Based Photovoltaic Polymer with Linear Alkylthio Side Chain. Chem. Mater. 2014, 26, 3603–3605. [Google Scholar] [CrossRef]
- Shao, S.; Hu, J.; Wang, X.; Wang, L.; Jing, X.; Wang, F. Blue thermally activated delayed fluorescence polymers with nonconjugated backbone and through-space charge transfer effect. J. Am. Chem. Soc. 2017, 139, 17739–17742. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Luo, J.; Zhou, T.; Chen, T.; Zhou, X.; Wu, K.; Zou, Y.; Xie, G.; Gong, S.; Yang, C. Using ring-opening metathesis polymerization of norbornene to construct thermally activated delayed fluorescence polymers: High-efficiency blue polymer light-emitting diodes. Macromolecules 2018, 51, 1598–1604. [Google Scholar] [CrossRef]
- Demetriou, M.; Krasia-Christoforou, T. Well-defined diblock copolymers possessing fluorescent and metal chelating functionalities as novel macromolecular sensors for amines and metal ions. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 52–60. [Google Scholar] [CrossRef]
- Shenhar, R.; Sanyal, A.; Uzun, O.; Rotello, V.M. Anthracene-functionalized polystyrene random copolymers: Effects of side-chain modification on polymer structure and behavior. Macromolecules 2004, 37, 92–98. [Google Scholar] [CrossRef]
- Howe, D.H.; McDaniel, R.M.; Magenau, A.J.D. From click chemistry to cross-coupling: Designer polymers from one efficient reaction. Macromolecules 2017, 50, 8010–8018. [Google Scholar] [CrossRef]
- Du, J.; Fang, Q.; Bu, D.; Ren, S.; Cao, A.; Chen, X. A new poly(fluorene-co-carbazole) with a large substituent group at the 9-position of the carbazole moiety: An efficient blue emitter. Macromol. Rapid Commun. 2005, 26, 1651–1656. [Google Scholar] [CrossRef]
- Chen, Q.; Han, B.-H. Prepolymerization and postpolymerization functionalization approaches to fluorescent conjugated carbazole-based glycopolymers via click chemistry. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 2948–2957. [Google Scholar] [CrossRef]
- Nakabayashi, K.; Abiko, Y.; Mori, H. Raft polymerization of s-vinyl sulfide derivatives and synthesis of block copolymers having two distinct optoelectronic functionalities. Macromolecules 2013, 46, 5998–6012. [Google Scholar] [CrossRef]
- Mori, H.; Tando, I.; Tanaka, H. Synthesis and optoelectronic properties of alternating copolymers containing anthracene unit in the main chain by radical ring-opening polymerization. Macromolecules 2010, 43, 7011–7020. [Google Scholar] [CrossRef]
- Ray, K.; Misra, T.N. Energy transfer between carbazole and anthracene moieties organised in langmuir–blodgett films J. Phys. Chem. Solids 1999, 60, 401–405. [Google Scholar] [CrossRef]
- Grazulevicius, J.V.; Strohriegl, P.; Pielichowski, J.; Pielichowski, K. Carbazole-containing polymers: Synthesis, properties and applications. Prog. Polym. Sci. 2003, 28, 1297–1401. [Google Scholar] [CrossRef]
- Hoffmann, S.T.; Schrögel, P.; Rothmann, M.; Albuquerque, R.Q.; Strohriegl, P.; Köhler, A. Triplet excimer emission in a series of 4,4′-bis(N-carbazolyl)-2,2′-biphenyl derivatives. J. Phys. Chem. B 2011, 115, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liao, J.-L.; Liang, Y.; Ahmed, M.O.; Tseng, H.-E.; Chen, S.-A. High-efficiency red-light emission from polyfluorenes grafted with cyclometalated iridium complexes and charge transport moiety. J. Am. Chem. Soc. 2002, 125, 636–637. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.R.J.; Lin, J.T.; Tao, Y.-T.; Ko, C.-W. Light-emitting carbazole derivatives: Potential electroluminescent materials. J. Am. Chem. Soc. 2001, 123, 9404–9411. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.J.; Kim, D.Y.; Jeong, M.-Y.; Kim, H.M.; Lee, Y.K.; Fang, X.; Jeon, S.-J.; Cho, B.R. Two-photon absorption properties of 2,6-bis(styryl)anthracene derivatives: Effects of donor–acceptor substituents and the π center. Chem. Eur. J. 2005, 11, 4191–4198. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Jeong, H.C.; Kim, S.H.; Yang, K.; Kwon, S.K. High-purity-blue and high-efficiency electroluminescent devices based on anthracene. Adv. Funct. Mater. 2005, 15, 1799–1805. [Google Scholar] [CrossRef]
- Iida, A.; Yamaguchi, S. Intense solid-state blue emission with a small stokes’ shift: Pi-stacking protection of the diphenylanthracene skeleton. Chem. Commun. 2009, 21, 3002–3004. [Google Scholar] [CrossRef] [PubMed]
- Abiko, Y.; Matsumura, A.; Nakabayashi, K.; Mori, H. Thermoresponsive core–shell nanoparticles with cross-linked π-conjugate core based on amphiphilic block copolymers by raft polymerization and palladium-catalyzed coupling reactions. Polymer 2014, 55, 6025–6035. [Google Scholar] [CrossRef]
- Destarac, M.; Brochon, C.; Catala, J.M.; Wilczewska, A.; Zard, S.Z. Macromolecular design via the interchange of xanthates (madix): Polymerization of styrene with o-ethyl xanthates as controlling agents. Macromol. Chem. Phys. 2002, 203, 2281–2289. [Google Scholar] [CrossRef]
- Maki, Y.; Mori, H.; Endo, T. Controlled raft polymerization of n-vinylphthalimide and its hydrazinolysis to poly(vinyl amine). Macromol. Chem. Phys. 2007, 208, 2589–2599. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]





| Copolymer | Before Postmodification | After Postmodification | ||||
|---|---|---|---|---|---|---|
| Mnb | Mw/Mnb | Composition c (BPVS/NVC) | Reactant d | Yield e | Mnf (Calcd) | |
| poly(BPVS-An)-b-poly(NVC) | 19,400 | 1.28 | 41/59 | An | >99 | 23,000 |
| poly(BPVS-PAn)-b-poly(NVC) | PAn | >99 | 25,800 | |||
| poly(BPVS-An-ran-NVC) | 8200 | 1.40 | 40/60 | An | >99 | 9700 |
| poly(BPVS-PAn-ran-NVC) | PAn | >99 | 10,800 | |||
| Sample | HOMO a,b (eV) | LUMO a,c (eV) | Egel d (eV) |
|---|---|---|---|
| poly(BPVS-An)-b-poly(NVC) | −5.57 | −2.86 | 2.71 |
| poly(BPVS-PAn)-b-poly(NVC) | −5.52 | −2.84 | 2.68 |
| poly(BPVS-An-ran-NVC) | −5.54 | −2.93 | 2.61 |
| poly(BPVS-PAn-ran-NVC) | −5.51 | −2.88 | 2.63 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo, C.-T.; Abiko, Y.; Kosai, J.; Watanabe, Y.; Nakabayashi, K.; Mori, H. Synthesis and Optoelectronic Properties of Block and Random Copolymers Containing Pendant Carbazole and (Di)phenylanthracene. Polymers 2018, 10, 721. https://doi.org/10.3390/polym10070721
Lo C-T, Abiko Y, Kosai J, Watanabe Y, Nakabayashi K, Mori H. Synthesis and Optoelectronic Properties of Block and Random Copolymers Containing Pendant Carbazole and (Di)phenylanthracene. Polymers. 2018; 10(7):721. https://doi.org/10.3390/polym10070721
Chicago/Turabian StyleLo, Chen-Tsyr, Yohei Abiko, Jun Kosai, Yuichiro Watanabe, Kazuhiro Nakabayashi, and Hideharu Mori. 2018. "Synthesis and Optoelectronic Properties of Block and Random Copolymers Containing Pendant Carbazole and (Di)phenylanthracene" Polymers 10, no. 7: 721. https://doi.org/10.3390/polym10070721