Preparation and Characterization of Softwood Kraft Lignin Copolymers as a Paper Strength Additive
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
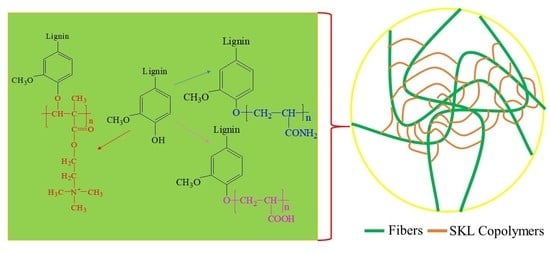
2.2. Preparation of SKL Copolymer
2.3. Analytical Methods
2.3.1. FTIR Analysis
2.3.2. Proton Nuclear Magnetic Resonance Analysis
2.3.3. Thermal Analysis
2.3.4. Molecular Weight Analysis
2.3.5. Elemental Analysis
2.3.6. Charge Density Analysis
2.3.7. Grafting Ratio
2.3.8. Performance Assessments of SKL Copolymer as a Strengthening Agent in Paper-Making
3. Results
3.1. Preparation of SKL Copolymers
3.2. FTIR Analysis
3.3. 1H-NMR Analysis
3.4. Thermogravimetric Analysis
3.5. Properties of SKL and SKL Copolymers
3.6. Application of SKL Copolymers as a Strengthening Agent in Paper Making
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Crestini, C.; Lange, H.; Sette, M.; Argyropoulos, D.S. On the structure of softwood kraft lignin. Green Chem. 2017, 19, 4104–4121. [Google Scholar] [CrossRef]
- Kang, Y.; Chen, Z.; Wang, B.; Yang, Y. Synthesis and mechanical properties of thermoplastic films from lignin, sebacic acid and poly(ethylene glycol). Ind. Crops Prod. 2014, 56, 105–112. [Google Scholar] [CrossRef]
- Ye, D.Z.; Zhang, M.; Gan, L.; Li, Q.; Zhang, X. The influence of hydrogen peroxide initiator concentration on the structure of eucalyptus lignosulfonate. Int. J. Biol. Macromol. 2013, 60, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Luo, W.; Ciesielski, P.N.; Fang, Z.; Zhu, J.Y.; Henriksson, G.; Himmel, M.E.; Hu, L. Wood-derived materials for green electronics, biological devices, and energy applications. Chem. Rev. 2016, 116, 9305–9374. [Google Scholar] [CrossRef] [PubMed]
- Upton, B.M.; Kasko, A.M. Strategies for the conversion of lignin to high-value polymeric materials: Review and perspective. Chem. Rev. 2016, 116, 2275–2306. [Google Scholar] [CrossRef] [PubMed]
- Duval, A.; Lawoko, M. A review on lignin-based polymeric, micro- and nano-structured materials. React. Funct. Polym. 2014, 85, 78–96. [Google Scholar] [CrossRef]
- Wang, J.; Yao, K.; Korich, A.L.; Li, S.; Ma, S.; Ploehn, H.J.; Iovine, P.M.; Wang, C.; Chu, F.; Tang, C. Combining renewable gum rosin and lignin: Towards hydrophobic polymer composites by controlled polymerization. J. Polym. Sci. Polym. Chem. 2011, 49, 3728–3738. [Google Scholar] [CrossRef]
- Konduri, M.K.; Kong, F.; Fatehi, P. Production of carboxymethylated lignin and its application as a dispersant. Eur. Polym. J. 2015, 70, 371–383. [Google Scholar] [CrossRef]
- Fang, R.; Cheng, X.S.; Xu, X.R. Synthesis of lignin-base cationic flocculant and its application in removing anionic azo-dyes from simulated wastewater. Bioresour. Technol. 2010, 101, 7323–7329. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.R.; Benar, P. Hydroxymethylation and oxidation of organosolv lignins and utilization of the products. Bioresour. Technol. 2001, 79, 103–111. [Google Scholar] [CrossRef]
- Kim, S.J. Preparation of a thermoplastic lignin-based biomaterial through atom transfer radical polymerization. J. Wood Chem. Technol. 2015, 35, 251–259. [Google Scholar] [CrossRef]
- Ibrahim, M.N.M.; Azreena, I.N.; Nadiah, M.Y.N.; Saaid, I.M. Lignin graft copolymer as a drilling mud thinner for high temperature well. J. Appl. Sci. 2006, 6, 1808–1813. [Google Scholar]
- Harris, S.B.; Tschirner, U.W.; Lemke, N.; Lierop, J.L.V. Characteristic properties of organosolv lignin/polylactide copolymers. J. Wood Chem. Technol. 2017, 37, 211–224. [Google Scholar] [CrossRef]
- Kong, F.; Wang, S.; Price, J.T.; Konduri, M.K.R.; Fatehi, P. Water soluble kraft lignin–acrylic acid copolymer: Synthesis and characterization. Green Chem. 2015, 17, 4355–4366. [Google Scholar] [CrossRef]
- Ibrahim, M.N.M.; Noorhaida, M.N.; Nadiah, M.Y.N.; Azilawati, M.G. Lignin graft copolymer as mud thinner for deep well drilling operation. J. Appl. Sci. 2006, 6, 2593–2598. [Google Scholar]
- Binh, N.T.T.; Luong, N.D.; Kim, D.O.; Lee, S.H.; Kim, B.J.; Lee, Y.S.; Nam, J.D. Synthesis of lignin-based thermoplastic copolyester using kraft lignin as a macromonomer. Compos. Interfaces 2009, 16, 923–935. [Google Scholar] [CrossRef]
- Wang, S.; Hou, Q.; Kong, F.; Fatehi, P. Production of cationic xylan-metac copolymer as a flocculant for textile industry. Carbohydr. Polym. 2015, 124, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sun, Y.; Kong, F.; Yang, G.; Fatehi, P. Preparation and characterization of lignin-acrylamide copolymer as a paper strength additive. Bioresources 2016, 11, 1765–1783. [Google Scholar] [CrossRef]
- Fang, R.; Cheng, X.S.; Fu, J.; Zheng, Z.B. Research on the graft copolymerization of eh-lignin with acrylamide. Nat. Sci. 2009, 1, 17–22. [Google Scholar] [CrossRef]
- Sc, A.B.D.; Elzairy, M.R.; Hebeish, A. Synthesis and application of new thickerners part i: Preparation of poly (acrylic acid)-starch graft copolymer. Starch-Starke 1989, 41, 233–236. [Google Scholar]
- Yu, C.; Wang, F.; Fu, S.; Liu, H.; Meng, Q. Laccase-assisted grafting of acrylic acid onto lignin for its recovery from wastewater. J. Polym. Environ. 2017, 25, 1072–1079. [Google Scholar] [CrossRef]
- Santos, R.B.; Capanema, E.A.; Balakshin, M.Y.; Chang, H.M.; Jameel, H. Lignin structural variation in hardwood species. J. Agric. Food Chem. 2012, 60, 4923–4930. [Google Scholar] [PubMed]
- Zhang, S.; Liu, L.; Fang, G.; Yan, N.; Ren, S.; Ma, Y. Hydrogenolysis and activation of soda lignin using [bmim]cl as a catalyst and solvent. Polymers 2017, 9, 279. [Google Scholar] [CrossRef]
- Sarma, R.; Islam, M.S.; Running, M.P.; Bhattacharyya, D. Multienzyme immobilized polymeric membrane reactor for the transformation of a lignin model compound. Polymers 2018, 10, 463. [Google Scholar] [CrossRef]
- Ye, D.Z.; Jiang, L.; Ma, C.; Zhang, M.H.; Zhang, X. The graft polymers from different species of lignin and acrylic acid: Synthesis and mechanism study. Int. J. Biol. Macromol. 2014, 63, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, J.; Zhang, S.; Gao, Q.; Li, J.; Zhang, W. Fast curing bio-based phenolic resins via lignin demethylated under mild reaction condition. Polymers 2017, 9, 428. [Google Scholar] [CrossRef]
- Nagy, M.; Kosa, M.; Theliander, H.; Ragauskas, A.J. Characterization of CO2 precipitated kraft lignin to promote its utilization. Green Chem. 2010, 12, 31–34. [Google Scholar] [CrossRef]
- Li, P.H.; Hu, X.X.; Wu, S.L.; Chu, P.; Yeung, K.K.; Xu, Z.S. Cationic lanthanide luminescent copolymer: Design, synthesis and interaction with DNA. J. Macromol. Sci. A 2011, 48, 832–839. [Google Scholar] [CrossRef]
- Hu, L.; Pan, H.; Zhou, Y.; Hse, C.; Liu, C.; Zhang, B.; Xu, B. Chemical groups and structural characterization of lignin via thiol-mediated demethylation. J. Wood Chem. Technol. 2014, 34, 122–134. [Google Scholar] [CrossRef]
- Witono, J.R.; Marsman, J.H.; Noordergraaf, I.W.; Heeres, H.J.; Janssen, L.P. Improved homopolymer separation to enable the application of 1h nmr and hplc for the determination of the reaction parameters of the graft copolymerization of acrylic acid onto starch. Carbohydr. Res. 2013, 370, 38–45. [Google Scholar] [PubMed]
- Zhong, L.Y.; Bao, Y.G.; Li, C.X.; Qin, Y.Y.; Liu, B. Synthesis and characterization of hydrophobically associating cationic polyacrylamide. Chem. Eng. J. 2010, 161, 27–33. [Google Scholar]
- Kong, F.; Parhiala, K.; Wang, S.; Fatehi, P. Preparation of cationic softwood kraft lignin and its application in dye removal. Eur. Polym. J. 2015, 67, 335–345. [Google Scholar] [CrossRef]
- Baniasad, A.; Ghorbani, M. Thermal stability enhancement of modified carboxymethyl cellulose films using sno2 nanoparticles. Int. J. Biol. Macromol. 2016, 86, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, Q.; Shi, Y.; Cha, R. Preparation and application of cationic modified cellulose fibrils as a papermaking additive. Int. J. Ploym. Sci. 2016, 2016, 1–8. [Google Scholar] [CrossRef]
- Kong, F.; Guo, Y.; Liu, Z.; Wang, S.; Lucia, L.A. Synthesis of cationic xylan derivatives and application as strengthening agents in papermaking. BioResources 2018, 13, 2960–2976. [Google Scholar] [CrossRef]







| Ash content (%) | Cellulose content (%) | Pentosan content (%) | Klason lignincontent (%) | Acid dissolved lignin (%) | 1% NaOH extract content (%) | Alcohol–benzene extract content (%) | Beating degree (oSR) |
|---|---|---|---|---|---|---|---|
| 0.43 | 50.38 | 15.27 | 17.35 | 3.56 | 11.47 | 1.28 | 32.5 |
| Samples | SKL | SKL-DMC | SKL-AA | SKL-AM |
|---|---|---|---|---|
| C (wt %) | 63.51 | 51.53 | 58.56 | 55.11 |
| H (wt %) | 6.21 | 6.66 | 5.62 | 6.37 |
| O (wt %) | 30.57 | 23.97 | 31.69 | 24.90 |
| N (wt %) | 0.004 | 3.003 | 0.001 | 8.732 |
| Charge density (mmol/g) | 0 | +1.425 | −6.287 | −3.656 |
| Graft ratio (%) | -- | 80.35 | 82.70 | 79.48 |
| Molecular formula | C9H10.56O3.25 | C9H13.96O3.14N0.45 | C9H10.37O3.85 | C9H12.48O3.05N1.22 |
| Mn (g/mol) | 1.725 × 104 | 4.352 × 105 | 3.267 × 105 | 2.145 × 105 |
| Mw (g/mol) | 2.600 × 104 | 4.965 × 105 | 3.875 × 105 | 3.642 × 105 |
| Mw/Mn | 1.51 | 1.14 | 1.19 | 1.70 |
| Samples | SKL | SKL-DMC | SKL-AA | SKL-AM |
|---|---|---|---|---|
| Internal bonding strength (J/m2) | 185 | 298 | 276 | 287 |
| Brightness (%ISO) | 75.6 | 74.8 | 75.2 | 75.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Xu, D.; Xu, L.; Kong, F.; Wang, S.; Yang, G. Preparation and Characterization of Softwood Kraft Lignin Copolymers as a Paper Strength Additive. Polymers 2018, 10, 743. https://doi.org/10.3390/polym10070743
Liu Z, Xu D, Xu L, Kong F, Wang S, Yang G. Preparation and Characterization of Softwood Kraft Lignin Copolymers as a Paper Strength Additive. Polymers. 2018; 10(7):743. https://doi.org/10.3390/polym10070743
Chicago/Turabian StyleLiu, Zhongming, Dingding Xu, Lei Xu, Fangong Kong, Shoujuan Wang, and Guihua Yang. 2018. "Preparation and Characterization of Softwood Kraft Lignin Copolymers as a Paper Strength Additive" Polymers 10, no. 7: 743. https://doi.org/10.3390/polym10070743