Bioactive and Bioadhesive Catechol Conjugated Polymers for Tissue Regeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization Techniques
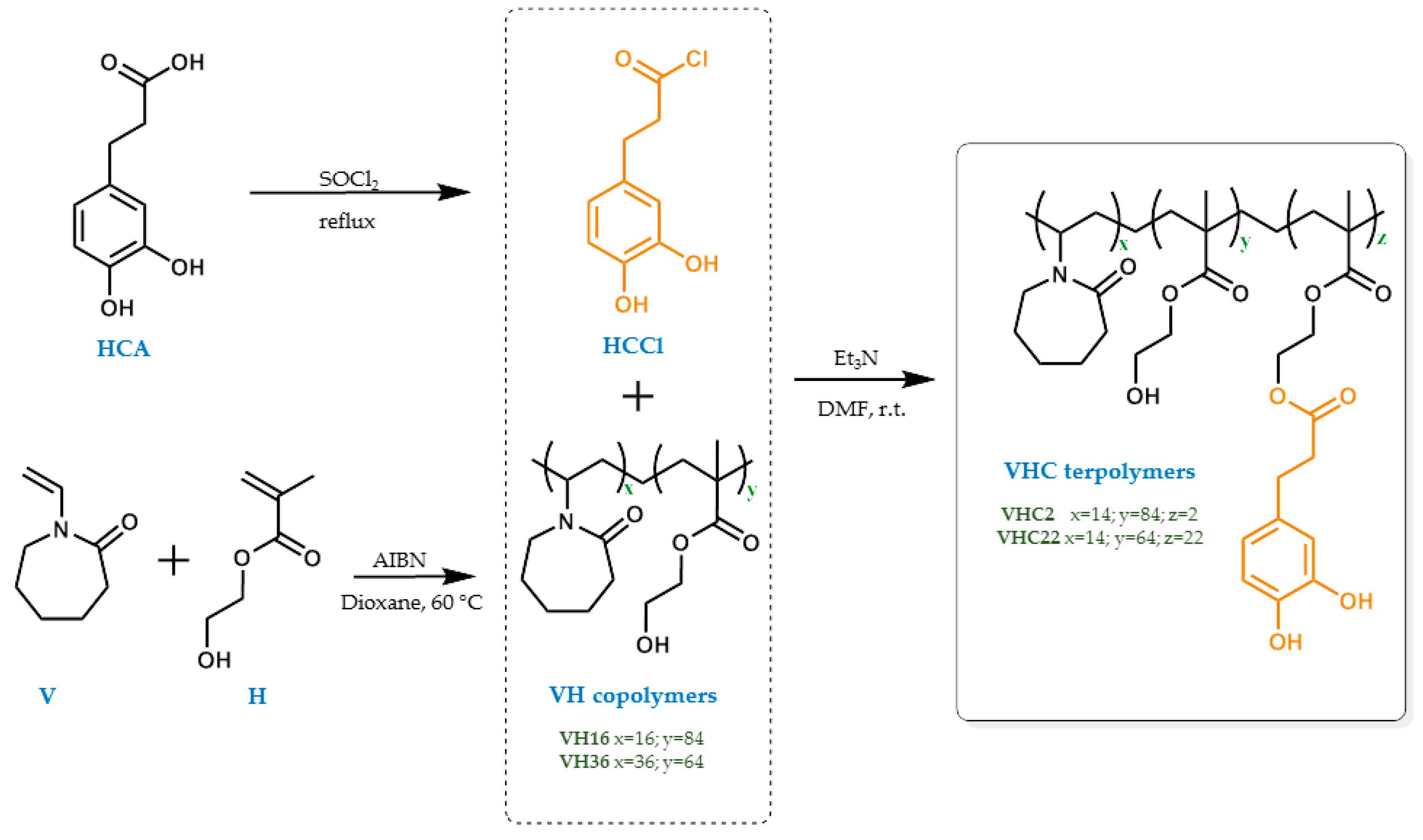
2.3. Synthesis of the VH Copolymers
2.4. Synthesis of the Catechol-Conjugated Polymers VHC
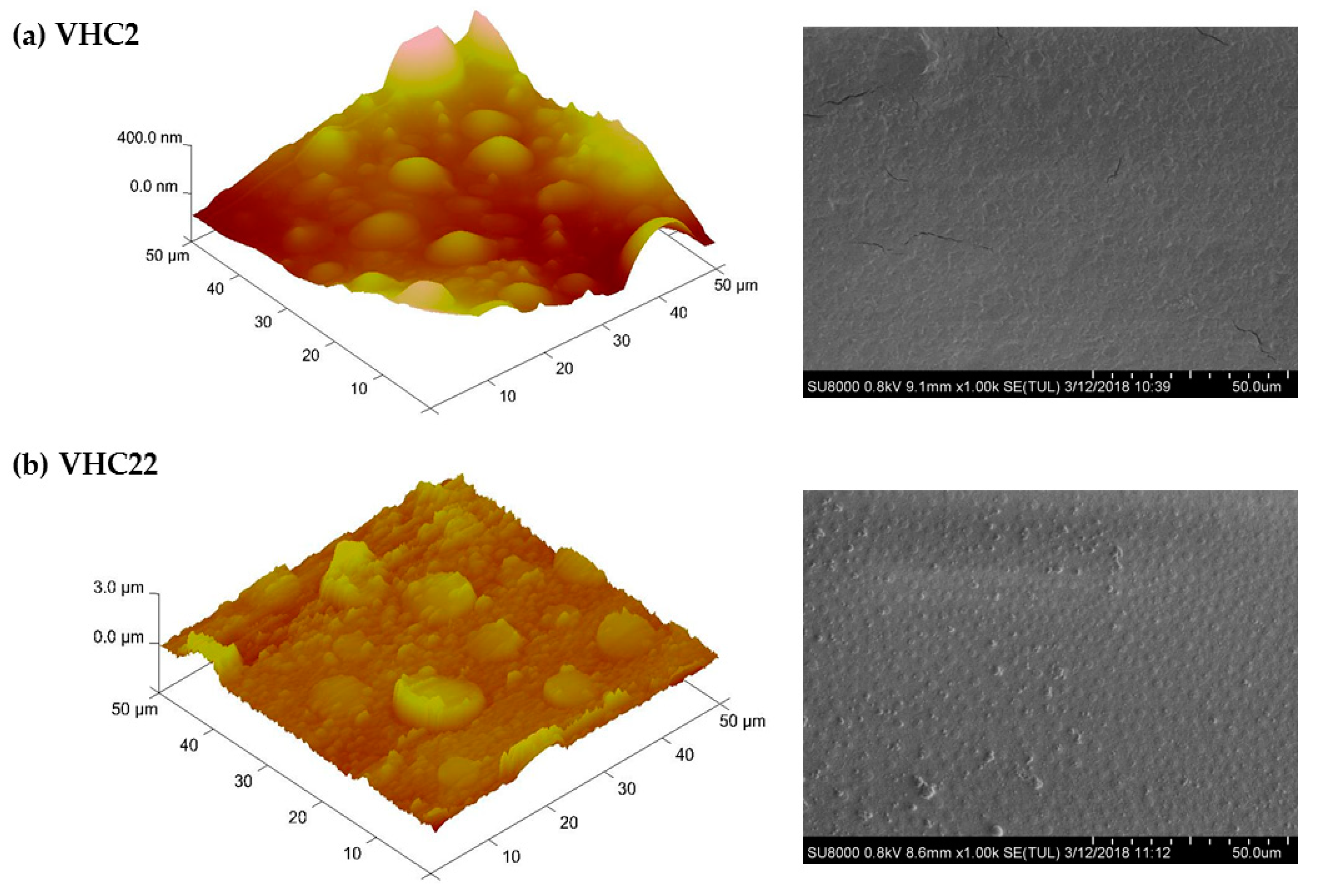
2.5. Films Preparation
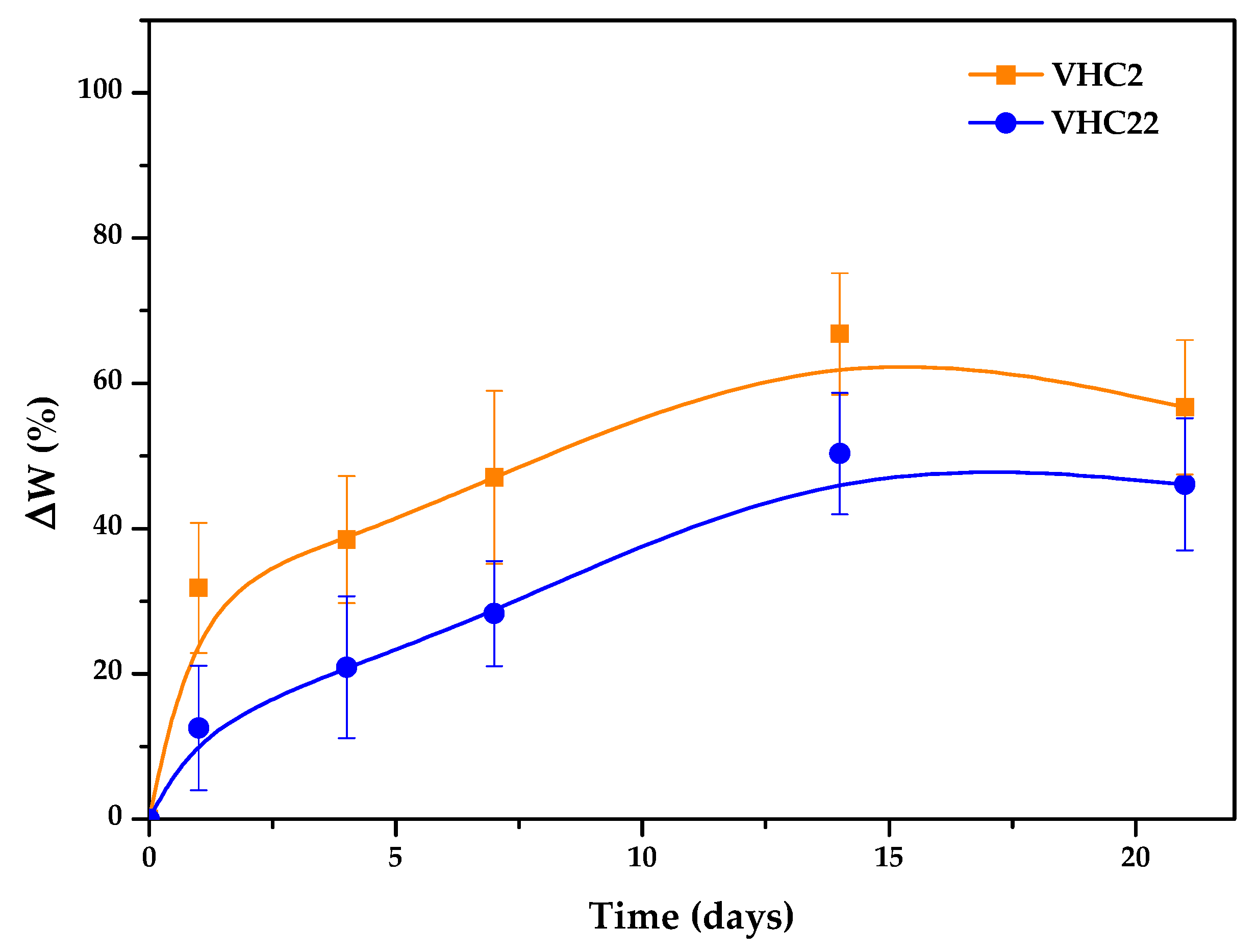
2.6. In Vitro Degradation
2.7. Adhesion Strength Test
2.8. Ultraviolet (UV) Shielding Test
2.9. Cellular Assays
2.9.1. Cell Culture
2.9.2. Cytotoxicity
2.9.3. Reactive Oxygen Species (ROS) Quantification
2.9.4. Anti-inflammatory Activity
3. Results
3.1. Synthesis of the VH Copolymers
3.2. Synthesis of the Catechol Conjugated Polymers VHC
3.3. In Vitro Degradation
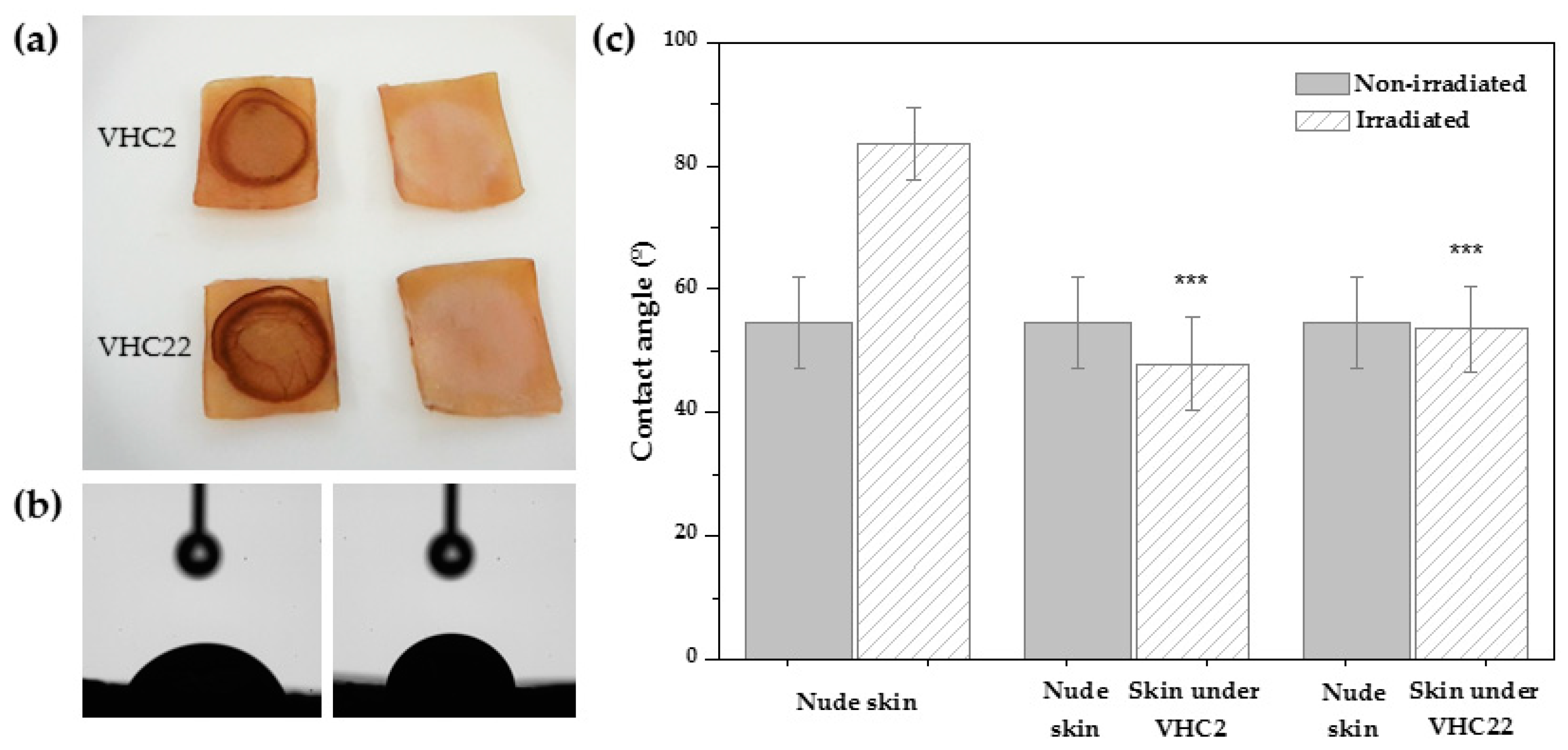
3.4. Adhesion Strength Test
3.5. UV Shielding Test
3.6. Biological Behavior
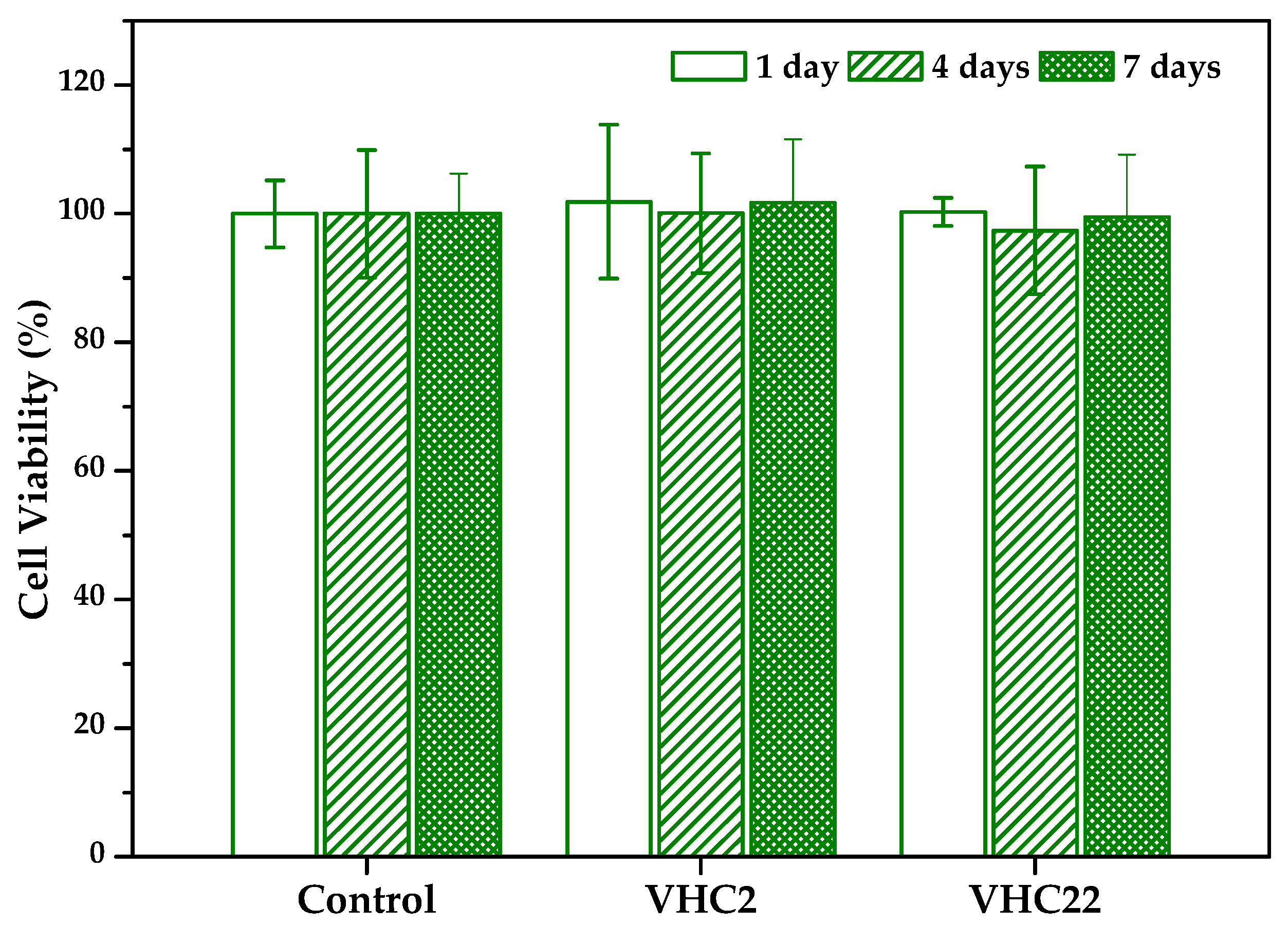
3.6.1. Cytotoxicity
3.6.2. Antioxidant Activity
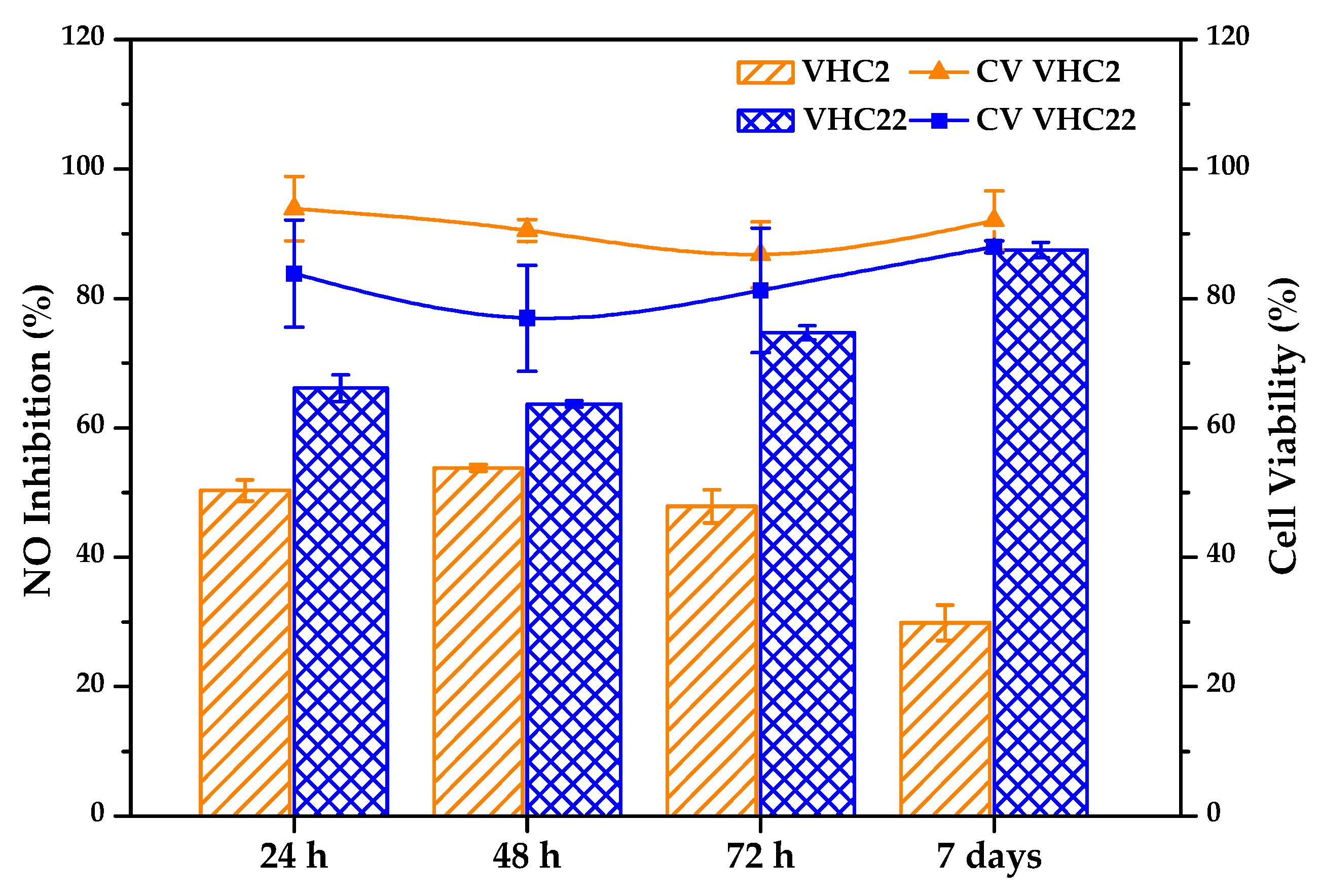
3.6.3. Anti-inflammatory Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Martin, P. Wound healing—Aiming for perfect skin regeneration. Science 1997, 276, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Rahmani Del Bakhshayesh, A.; Annabi, N.; Khalilov, R.; Akbarzadeh, A.; Samiei, M.; Alizadeh, E.; Alizadeh-Ghodsi, M.; Davaran, S.; Montaseri, A. Recent advances on biomedical applications of scaffolds in wound healing and dermal tissue engineering. Artif. Cells Nanomed. Biotechnol. 2018, 46, 691–705. [Google Scholar] [CrossRef] [PubMed]
- Braun, L.R.; Fisk, W.A.; Lev-Tov, H.; Kirsner, R.S.; Isseroff, R.R. Diabetic foot ulcer: An evidence-based treatment update. Am. J. Clin. Dermatol. 2014, 15, 267–281. [Google Scholar] [CrossRef] [PubMed]
- Brem, H.; Stojadinovic, O.; Diegelmann, R.F.; Entero, H.; Lee, B.; Pastar, I.; Golinko, M.; Rosenberg, H.; Tomic-Canic, M. Molecular markers in patients with chronic wounds to guide surgical debridement. Mol. Med. 2007, 13, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D.J.; Schultz, G. Chronic wound diagnostic for matrix metalloproteinase: Chronic wounds. Wound Heal. S. Afr. 2009, 2, 68–70. [Google Scholar]
- Wiegand, C.; Hipler, U.C. Polymer-Based Biomaterials as Dressings for Chronic Stagnating Wounds; Macromolecular Symposia; Wiley Online Library: Hoboken, NJ, USA, 2010; pp. 1–13. [Google Scholar]
- Bjarnsholt, T.; Kirketerp-Møller, K.; Jensen, P.Ø.; Madsen, K.G.; Phipps, R.; Krogfelt, K.; Høiby, N.; Givskov, M. Why chronic wounds will not heal: A novel hypothesis. Wound Repair Regen. 2008, 16, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Novo, E.; Parola, M. Redox mechanisms in hepatic chronic wound healing and fibrogenesis. Fibrogenes. Tissue Repair 2008, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.A.; Krieg, T.; Davidson, J.M. Inflammation in wound repair: Molecular and cellular mechanisms. J. Investig. Dermatol. 2007, 127, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Rojkind, M.; Dominguez-Rosales, J.-A.; Nieto, N.; Greenwel, P. Role of hydrogen peroxide and oxidative stress in healing responses. Cell. Mol. Life Sci. CMLS 2002, 59, 1872–1891. [Google Scholar] [CrossRef] [PubMed]
- Cawston, T.E.; Wilson, A.J. Understanding the role of tissue degrading enzymes and their inhibitors in development and disease. Best Pract. Res. Clin. Rheumatol. 2006, 20, 983–1002. [Google Scholar] [CrossRef] [PubMed]
- Nyanhongo, G.S.; Sygmund, C.; Ludwig, R.; Prasetyo, E.N.; Guebitz, G.M. Synthesis of multifunctional bioresponsive polymers for the management of chronic wounds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, S.; Zhang, B.; Li, M.; Diao, K.; Zhang, Z.; Li, J.; Xu, Y.; Wang, X.; Chen, H. In situ injectable nano-composite hydrogel composed of curcumin, N,O-carboxymethyl chitosan and oxidized alginate for wound healing application. Int. J. Pharm. 2012, 437, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Nan, K.; Li, L.; Zhang, Z.; Chen, H. In vivo evaluation of curcumin nanoformulation loaded methoxy poly(ethylene glycol)-graft-chitosan composite film for wound healing application. Carbohydr. Polym. 2012, 88, 84–90. [Google Scholar] [CrossRef]
- Khil, M.S.; Cha, D.I.; Kim, H.Y.; Kim, I.S.; Bhattarai, N. Electrospun nanofibrous polyurethane membrane as wound dressing. J. Biomed. Mater. Res. Part B Appl. Biomater. 2003, 67, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, V.B.; Thétiot, F.; Ritz, S.; Pütz, S.; Choritz, L.; Lappas, A.; Förch, R.; Landfester, K.; Jonas, U. Antibacterial surface coatings from zinc oxide nanoparticles embedded in poly(N-isopropylacrylamide) hydrogel surface layers. Adv. Funct. Mater. 2012, 22, 2376–2386. [Google Scholar] [CrossRef]
- GhavamiNejad, A.; Park, C.H.; Kim, C.S. In situ synthesis of antimicrobial silver nanoparticles within antifouling zwitterionic hydrogels by catecholic redox chemistry for wound healing application. Biomacromolecules 2016, 17, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
- Sweitzer, S.M.; Fann, S.A.; Borg, T.K.; Baynes, J.W.; Yost, M.J. What is the future of diabetic wound care? Diabetes Educ. 2006, 32, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Grose, R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [CrossRef] [PubMed]
- Dworniczek, E.; Nawrot, U.; Seniuk, A.; Wlodarczyk, K.; Bialynicki-Birula, R. The in vitro effect of a silver-containing dressing on biofilm development. Adv. Clin. Exp. Med. 2009, 18, 277–281. [Google Scholar]
- Jain, R.; Agarwal, A.; Kierski, P.R.; Schurr, M.J.; Murphy, C.J.; McAnulty, J.F.; Abbott, N.L. The use of native chemical functional groups presented by wound beds for the covalent attachment of polymeric microcarriers of bioactive factors. Biomaterials 2013, 34, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Bao, X.; Wang, Z.; Hu, Q. A novel silver-loaded chitosan composite sponge with sustained silver release as a long-lasting antimicrobial dressing. RSC Adv. 2017, 7, 34655–34663. [Google Scholar] [CrossRef]
- Atiyeh, B.S.; Costagliola, M.; Hayek, S.N.; Dibo, S.A. Effect of silver on burn wound infection control and healing: Review of the literature. Burns 2007, 33, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Dunn, K.; Edwards-Jones, V. The role of acticoat™ with nanocrystalline silver in the management of burns. Burns 2004, 30, S1–S9. [Google Scholar] [CrossRef]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.; Eccleston, G.M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Bonilla, A.; Fernández-García, M. Polymeric materials with antimicrobial activity. Prog. Polym. Sci. 2012, 37, 281–339. [Google Scholar] [CrossRef]
- Seaman, S. Dressing selection in chronic wound management. J. Am. Podiatr. Med. Assoc. 2002, 92, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Prasetyo, E.N.; Kudanga, T.; Steiner, W.; Murkovic, M.; Nyanhongo, G.S.; Guebitz, G.M. Antioxidant activity assay based on laccase-generated radicals. Anal. Bioanal. Chem. 2009, 393, 679. [Google Scholar] [CrossRef] [PubMed]
- Roleira, F.M.; Tavares-da-Silva, E.J.; Varela, C.L.; Costa, S.C.; Silva, T.; Garrido, J.; Borges, F. Plant derived and dietary phenolic antioxidants: Anticancer properties. Food Chem. 2015, 183, 235–258. [Google Scholar] [CrossRef] [PubMed]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.; Ferreira, I.C. Bioactivity of phenolic acids: Metabolites versus parent compounds: A review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Fresco, P.; Borges, F.; Diniz, C.; Marques, M. New insights on the anticancer properties of dietary polyphenols. Med. Res. Rev. 2006, 26, 747–766. [Google Scholar] [CrossRef] [PubMed]
- Thiem, B.; Goślińska, O. Antimicrobial activity of rubus chamaemorus leaves. Fitoterapia 2004, 75, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Sakihama, Y.; Cohen, M.F.; Grace, S.C.; Yamasaki, H. Plant phenolic antioxidant and prooxidant activities: Phenolics-induced oxidative damage mediated by metals in plants. Toxicology 2002, 177, 67–80. [Google Scholar] [CrossRef]
- Amaral, S.; Mira, L.; Nogueira, J.; da Silva, A.P.; Florêncio, M.H. Plant extracts with anti-inflammatory properties—A new approach for characterization of their bioactive compounds and establishment of structure–antioxidant activity relationships. Biorgan. Med. Chem. 2009, 17, 1876–1883. [Google Scholar] [CrossRef] [PubMed]
- Landis, S.J. Chronic wound infection and antimicrobial use. Adv. Skin Wound Care 2008, 21, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kawazoe, T.; Han, D.W.; Matsumara, K.; Suzuki, S.; Tsutsumi, S.; Hyon, S.H. Enhanced wound healing by an epigallocatechin gallate-incorporated collagen sponge in diabetic mice. Wound Repair Regen. 2008, 16, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Schweigert, N.; Zehnder, A.J.; Eggen, R.I. Chemical properties of catechols and their molecular modes of toxic action in cells, from microorganisms to mammals. Environ. Microbiol. 2001, 3, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Nagababu, E.; Rifkind, J.M.; Boindala, S.; Nakka, L. Assessment of antioxidant activity of eugenol in vitro and in vivo. In Free Radicals and Antioxidant Protocols; Springer: Berlin/Heidelberg, Germany, 2010; pp. 165–180. [Google Scholar]
- Puertas-Bartolomé, M.; Fernández-Gutiérrez, M.; García-Fernández, L.; Vázquez-Lasa, B.; San Román, J. Biocompatible and bioadhesive low molecular weight polymers containing long-arm catechol-functionalized methacrylate. Eur. Polym. J. 2018, 98, 47–55. [Google Scholar] [CrossRef]
- Huber, D.; Grzelak, A.; Baumann, M.; Borth, N.; Schleining, G.; Nyanhongo, G.S.; Guebitz, G.M. Anti-inflammatory and anti-oxidant properties of laccase-synthesized phenolic-O-carboxymethyl chitosan hydrogels. New Biotechnol. 2018, 40, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Barclay, L.R.C.; Edwards, C.; Vinqvist, M.R. Media effects on antioxidant activities of phenols and catechols. J. Am. Chem. Soc. 1999, 121, 6226–6231. [Google Scholar] [CrossRef]
- Justino, G.C.; Correia, C.F.; Mira, L.; Borges dos Santos, R.M.; Martinho Simões, J.A.; Silva, A.M.; Santos, C.; Gigante, B. Antioxidant activity of a catechol derived from abietic acid. J. Agric. Food Chem. 2006, 54, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.M.; Santos, M.R.; Caroço, G.; Rocha, R.; Justino, G.; Mira, L. Structure-antioxidant activity relationships of flavonoids: A re-examination. Free Radic. Res. 2002, 36, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Heijnen, C.; Haenen, G.; Van Acker, F.; Van der Vijgh, W.; Bast, A. Flavonoids as peroxynitrite scavengers: The role of the hydroxyl groups. Toxicol. In Vitro 2001, 15, 3–6. [Google Scholar] [CrossRef]
- Heijnen, C.G.; Haenen, G.R.; Minou Oostveen, R.; Stalpers, E.M.; Bast, A. Protection of flavonoids against lipid peroxidation: The structure activity relationship revisited. Free Radic. Res. 2002, 36, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Heijnen, C.G.; Haenen, G.R.; Vekemans, J.A.; Bast, A. Peroxynitrite scavenging of flavonoids: Structure activity relationship. Environ. Toxicol. Pharmacol. 2001, 10, 199–206. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.R.; den Hartog, G.J.; Bast, A. Oxidative damage shifts from lipid peroxidation to thiol arylation by catechol-containing antioxidants. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2002, 1583, 279–284. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.P.; Dalsin, J.L.; Messersmith, P.B. Synthesis and gelation of dopa-modified poly(ethylene glycol) hydrogels. Biomacromolecules 2002, 3, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Scognamiglio, F.; Travan, A.; Borgogna, M.; Donati, I.; Marsich, E.; Bosmans, J.W.A.M.; Perge, L.; Foulc, M.P.; Bouvy, N.D.; Paoletti, S. Enhanced bioadhesivity of dopamine-functionalized polysaccharidic membranes for general surgery applications. Acta Biomater. 2016, 44, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Lu, X.; Liu, K.; Wang, K.; Fang, L.; Weng, L.-T.; Zhang, H.; Tang, Y.; Ren, F.; Zhao, C.; et al. Mussel-inspired adhesive and tough hydrogel based on nanoclay confined dopamine polymerization. ACS Nano 2017, 11, 2561–2574. [Google Scholar] [CrossRef] [PubMed]
- GhavamiNejad, A.; Rajan Unnithan, A.; Ramachandra Kurup Sasikala, A.; Samarikhalaj, M.; Thomas, R.G.; Jeong, Y.Y.; Nasseri, S.; Murugesan, P.; Wu, D.; Hee Park, C.; et al. Mussel-inspired electrospun nanofibers functionalized with size-controlled silver nanoparticles for wound dressing application. ACS Appl. Mater. Interfaces 2015, 7, 12176–12183. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Wang, H.; Li, T.E.; Li, Y.; Dou, F.; Sun, S.; Guo, H.; Liao, S.; Yang, Z.; Wei, Q. Mussel-inspired thermoresponsive polypeptide–pluronic copolymers for versatile surgical adhesives and hemostasis. ACS Appl. Mater. Interfaces 2017, 9, 16756–16766. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Aoyagi, T.; Ebara, M.; Auzély-Velty, R. Catechol-modified hyaluronic acid: In situ-forming hydrogels by auto-oxidation of catechol or photo-oxidation using visible light. Polym. Bull. 2017, 74, 4069–4085. [Google Scholar] [CrossRef]
- Jeon, E.Y.; Choi, B.-H.; Jung, D.; Hwang, B.H.; Cha, H.J. Natural healing-inspired collagen-targeting surgical protein glue for accelerated scarless skin regeneration. Biomaterials 2017, 134, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Scognamiglio, F.; Travan, A.; Rustighi, I.; Tarchi, P.; Palmisano, S.; Marsich, E.; Borgogna, M.; Donati, I.; de Manzini, N.; Paoletti, S. Adhesive and sealant interfaces for general surgery applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 626–639. [Google Scholar] [CrossRef] [PubMed]
- Winter, G.D. Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. Nature 1962, 193, 293–294. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Ondrusek, B.A.; Lee, C.; Douglas, W.G.; Chung, H. Synthesis of lightly crosslinked zwitterionic polymer-based bioinspired adhesives for intestinal tissue sealing. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 1564–1573. [Google Scholar] [CrossRef]
- Czuba, U.; Quintana, R.; De Pauw-Gillet, M.C.; Bourguignon, M.; Moreno-Couranjou, M.; Alexandre, M.; Detrembleur, C.; Choquet, P. Atmospheric plasma deposition of methacrylate layers containing catechol/quinone groups: An alternative to polydopamine bioconjugation for biomedical applications. Adv. Healthc. Mater. 2018, 1701059. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Sun, X.; Wu, C.; Hu, J.; Huang, X. Construction of catechol-containing semi-fluorinated asymmetric polymer brush via successive raft polymerization and atrp. Polym. Chem. 2017, 8, 7499–7506. [Google Scholar] [CrossRef]
- Hadjesfandiari, N.; Weinhart, M.; Kizhakkedathu, J.N.; Haag, R.; Brooks, D.E. Development of antifouling and bactericidal coatings for platelet storage bags using dopamine chemistry. Adv. Healthc. Mater. 2018, 7, 1700839. [Google Scholar] [CrossRef] [PubMed]
- Fort, R.; Polyzoidis, T. Intrinsic viscosity-molecular weight relationships for poly(2-hydroxyethyl methacrylate). Eur. Polym. J. 1976, 12, 685–689. [Google Scholar] [CrossRef]
- Xi, P.; Cheng, K.; Sun, X.; Zeng, Z.; Sun, S. Fluorescent magnetic nanoparticles based on a ruthenium complex and Fe3O4. J. Mater. Chem. 2011, 21, 11464–11467. [Google Scholar] [CrossRef]
- Paez, J.I.; Ustahüseyin, O.; Serrano, C.; Ton, X.-A.; Shafiq, Z.; Auernhammer, G.N.K.; d’Ischia, M.; del Campo, A.N. Gauging and tuning cross-linking kinetics of catechol-peg adhesives via catecholamine functionalization. Biomacromolecules 2015, 16, 3811–3818. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Lan, X.Y.; Xiao, J.H.; Yang, J.C.; Kao, Y.T.; Chang, S.T. Antiinflammatory activity of lindera erythrocarpa fruits. Phytother. Res. 2008, 22, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Guevara, I.; Iwanejko, J.; Dembińska-Kieć, A.; Pankiewicz, J.; Wanat, A.; Anna, P.; Goła̧bek, I.; Bartuś, S.; Malczewska-Malec, M.; Szczudlik, A. Determination of nitrite/nitrate in human biological material by the simple griess reaction. Clin. Chim. Acta 1998, 274, 177–188. [Google Scholar] [CrossRef]
- Choi, M.S.; Lee, S.H.; Cho, H.S.; Kim, Y.; Yun, Y.P.; Jung, H.Y.; Jung, J.K.; Lee, B.C.; Pyo, H.B.; Hong, J.T. Inhibitory effect of obovatol on nitric oxide production and activation of nf-κb/map kinases in lipopolysaccharide-treated raw 264.7 cells. Eur. J. Pharmacol. 2007, 556, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Jansen, J.F.; Houben, E.E.; Tummers, P.H.; Wienke, D.; Hoffmann, J. Real-time infrared determination of photoinitiated copolymerization reactivity ratios: Application of the hilbert transform and critical evaluation of data analysis techniques. Macromolecules 2004, 37, 2275–2286. [Google Scholar] [CrossRef]
- Aguilar, M.R.; Gallardo, A.; Fernández, M.D.M.; Román, J.S. In situ quantitative 1 h nmr monitoring of monomer consumption: A simple and fast way of estimating reactivity ratios. Macromolecules 2002, 35, 2036–2041. [Google Scholar] [CrossRef]
- Lopez-Donaire, M.L.; Sussman, E.M.; Fernandez-Gutierrez, M.; Mendez-Vilas, A.; Ratner, B.D.; Vazquez-Lasa, B.; San Roman, J. Amphiphilic self-assembled “polymeric drugs”: Morphology, properties, and biological behavior of nanoparticles. Biomacromolecules 2012, 13, 624–635. [Google Scholar] [CrossRef] [PubMed]
- Lopez Donaire, M.L.; Parra-Caceres, J.; Vazquez-Lasa, B.; Garcia-Alvarez, I.; Fernandez-Mayoralas, A.; Lopez-Bravo, A.; San Roman, J. Polymeric drugs based on bioactive glycosides for the treatment of brain tumours. Biomaterials 2009, 30, 1613–1626. [Google Scholar] [CrossRef] [PubMed]
- García-Fernández, L.; Aguilar, M.A.R.; Fernández, M.A.M.; Lozano, R.M.; Giménez, G.; Román, J.S. Antimitogenic polymer drugs based on amps: Monomer distribution bioactivity relationship of water-soluble macromolecules. Biomacromolecules 2010, 11, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Standardization, I.O.F. Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity, 3rd ed.; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- Huang, J.; de Paulis, T.; May, J.M. Antioxidant effects of dihydrocaffeic acid in human ea. Hy926 endothelial cells. J. Nutr. Biochem. 2004, 15, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.P.; Huang, K.; Nunalee, F.N.; Shull, K.R.; Messersmith, P.B. Synthesis of 3,4-dihydroxyphenylalanine (dopa) containing monomers and their co-polymerization with peg-diacrylate to form hydrogels. J. Biomater. Sci. Polym. Ed. 2004, 15, 449–464. [Google Scholar] [CrossRef] [PubMed]
- Larrosa, M.; Luceri, C.; Vivoli, E.; Pagliuca, C.; Lodovici, M.; Moneti, G.; Dolara, P. Polyphenol metabolites from colonic microbiota exert anti-inflammatory activity on different inflammation models. Mol. Nutr. Food Res. 2009, 53, 1044–1054. [Google Scholar] [CrossRef] [PubMed]
- Odian, G. Radical chain polymerization. In Principles of Polymerization, 4th ed.; John Wiley& Sons: Hoboken, NJ, USA, 2004; pp. 198–349. [Google Scholar]
- Ahn, B.K.; Lee, D.W.; Israelachvili, J.N.; Waite, J.H. Surface-initiated self-healing of polymers in aqueous media. Nat. Mater. 2014, 13, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Glass, P.; Chung, H.; Washburn, N.R.; Sitti, M. Enhanced reversible adhesion of dopamine methacrylamide-coated elastomer microfibrillar structures under wet conditions. Langmuir 2009, 25, 6607–6612. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Quiroz, D.; González-Gómez, Á.; Lizardi-Mendoza, J.; Vázquez-Lasa, B.; Goycoolea, F.M.; San Román, J.; Argüelles-Monal, W.M. Effect of the molecular architecture on the thermosensitive properties of chitosan-g-poly(N-vinylcaprolactam). Carbohydr. Polym. 2015, 134, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Bölgen, N.; Aguilar, M.R.; Fernández, M.D.M.; Gonzalo-Flores, S.; Villar-Rodil, S.; San Román, J.; Pişkin, E. Thermoresponsive biodegradable hema–lactate–dextran-co-nipa cryogels for controlled release of simvastatin. Artif. Cells Nanomed. Biotechnol. 2015, 43, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Rojo, L.; Barcenilla, J.M.; Vázquez, B.; González, R.; San Román, J. Intrinsically antibacterial materials based on polymeric derivatives of eugenol for biomedical applications. Biomacromolecules 2008, 9, 2530–2535. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Pal, A.; Gude, R.; Devi, S. Synthesis and characterization of thermo-responsive copolymeric nanoparticles of poly(methyl methacrylate-co-N-vinylcaprolactam). Eur. Polym. J. 2010, 46, 958–967. [Google Scholar] [CrossRef]
- Qiu, X.; Sukhishvili, S.A. Copolymerization of n-vinylcaprolactam and glycidyl methacrylate: Reactivity ratio and composition control. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 183–191. [Google Scholar] [CrossRef]
- Gallardo, A.; Aguilar, M.R.; Abraham, G.A.; San Román, J. Chain copolymerization reactions: An algorithm to predict the reaction evolution with conversion. J. Chem. Educ. 2004, 81, 1210. [Google Scholar] [CrossRef]
- Akiyoshi, K.; Deguchi, S.; Tajima, H.; Nishikawa, T.; Sunamoto, J. Microscopic structure and thermoresponsiveness of a hydrogel nanoparticle by self-assembly of a hydrophobized polysaccharide. Macromolecules 1997, 30, 857–861. [Google Scholar] [CrossRef]
- Li, L.; Smitthipong, W.; Zeng, H. Mussel-inspired hydrogels for biomedical and environmental applications. Polym. Chem. 2015, 6, 353–358. [Google Scholar] [CrossRef]
- Liu, H.; Qu, X.; Kim, E.; Lei, M.; Dai, K.; Tan, X.; Xu, M.; Li, J.; Liu, Y.; Shi, X. Bio-inspired redox-cycling antimicrobial film for sustained generation of reactive oxygen species. Biomaterials 2018, 162, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Jiang, R.; Zhao, J.; Zhang, J.; Yuan, X.; Yuan, X. In situ formation of adhesive hydrogels based on pl with laterally grafted catechol groups and their bonding efficacy to wet organic substrates. J. Mater. Sci. Mater. Med. 2015, 26, 273. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, C.E.; Messersmith, P.B. Enzymatically degradable mussel-inspired adhesive hydrogel. Biomacromolecules 2011, 12, 4326–4334. [Google Scholar] [CrossRef] [PubMed]
- Cencer, M.; Murley, M.; Liu, Y.; Lee, B.P. Effect of nitro-functionalization on the cross-linking and bioadhesion of biomimetic adhesive moiety. Biomacromolecules 2015, 16, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Strandman, S.; Zhu, J.X.X.; Barralet, J.; Cerruti, M. Genipin-crosslinked catechol-chitosan mucoadhesive hydrogels for buccal drug delivery. Biomaterials 2015, 37, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Chung, H.J.; Yeo, S.; Ahn, C.H.; Lee, H.; Messersmith, P.B.; Park, T.G. Thermo-sensitive, injectable, and tissue adhesive sol-gel transition hyaluronic acid/pluronic composite hydrogels prepared from bio-inspired catechol-thiol reaction. Soft Matter 2010, 6, 977–983. [Google Scholar] [CrossRef]
- Kim, K.; Kim, K.; Ryu, J.H.; Lee, H. Chitosan-catechol: A polymer with long-lasting mucoadhesive properties. Biomaterials 2015, 52, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Bonakdar, S.; Emami, S.H.; Shokrgozar, M.A.; Farhadi, A.; Ahmadi, S.A.H.; Amanzadeh, A. Preparation and characterization of polyvinyl alcohol hydrogels crosslinked by biodegradable polyurethane for tissue engineering of cartilage. Mater. Sci. Eng. C 2010, 30, 636–643. [Google Scholar] [CrossRef]
- Ryu, J.H.; Lee, Y.; Do, M.J.; Jo, S.D.; Kim, J.S.; Kim, B.S.; Im, G.I.; Park, T.G.; Lee, H. Chitosan-g-hematin: Enzyme-mimicking polymeric catalyst for adhesive hydrogels. Acta Biomater. 2014, 10, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Kalyanaraman, B.; Premovic, P.; Sealy, R.C. Semiquinone anion radicals from addition of amino acids, peptides, and proteins to quinones derived from oxidation of catechols and catecholamines. An esr spin stabilization study. J. Biol. Chem. 1987, 262, 11080–11087. [Google Scholar] [PubMed]
- Alegria, A.E.; Sanchez-Cruz, P.; Kumar, A.; Garcia, C.; Gonzalez, F.A.; Orellano, A.; Zayas, B.; Gordaliza, M. Thiols oxidation and covalent binding of bsa by cyclolignanic quinones are enhanced by the magnesium cation. Free Radic. Res. 2008, 42, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Lee, Y.; Kong, W.H.; Kim, T.G.; Park, T.G.; Lee, H. Catechol-functionalized chitosan/pluronic hydrogels for tissue adhesives and hemostatic materials. Biomacromolecules 2011, 12, 2653–2659. [Google Scholar] [CrossRef] [PubMed]
- Cencer, M.; Liu, Y.; Winter, A.; Murley, M.; Meng, H.; Lee, B.P. Effect of ph on the rate of curing and bioadhesive properties of dopamine functionalized poly(ethylene glycol) hydrogels. Biomacromolecules 2014, 15, 2861–2869. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Shen, X.; Hu, Y.; Xu, K.; Ran, Q.; Yu, Y.; Dai, L.; Yuan, Z.; Huang, L.; Shen, T.; et al. Surface functionalization of titanium implants with chitosan-catechol conjugate for suppression of ros-induced cells damage and improvement of osteogenesis. Biomaterials 2017, 114, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-S.; Sun, D.-S.; Lin, Y.-C.; Cheng, C.-L.; Hung, S.-C.; Chen, P.-K.; Yang, J.-H.; Chang, H.-H. Nanodiamonds protect skin from ultraviolet b-induced damage in mice. J. Nanobiotechnol. 2015, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Mencucci, R.; Mercatelli, L.; Fusi, F.; Ponchietti, C.; Monici, M.; Menchini, U. Acrysof natural intraocular lens optical characteristics during and after different doses of ultraviolet-visible light illumination. J. Cataract Refract. Surg. 2006, 32, 1961–1965. [Google Scholar] [CrossRef] [PubMed]
- Gantwerker, E.A.; Hom, D.B. Skin: Histology and physiology of wound healing. Clin. Plast. Surg. 2012, 39, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Hu, N.; Xiong, X.; Lei, Q.; Li, L. A novel promising therapy for skin aging: Dermal multipotent stem cells against photoaged skin by activation of TGF-β/Smad and p38 MAPK signaling pathway. Med. Hypotheses 2011, 76, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Xian, D.; Zhong, J.; Gao, X. A new approach for skin-derived precursors to ameliorate skin photodamage through activation of nrf2 signaling pathway. Curr. Signal Transduct. Ther. 2017, 12, 34–38. [Google Scholar] [CrossRef]
- Pullar, J.M.; Carr, A.C.; Vissers, M. The roles of vitamin C in skin health. Nutrients 2017, 9, 866. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Ma, B.C.; Reinholz, J.; Li, Q.; Wang, J.; Zhang, K.A.; Landfester, K.; Crespy, D. Efficient nanofibrous membranes for antibacterial wound dressing and uv protection. ACS Appl. Mater. Interfaces 2016, 8, 29915–29922. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-S.; Cha, J.-R.; Gong, M.-S. Investigation of the antimicrobial and wound healing properties of silver nanoparticle-loaded cotton prepared using silver carbamate. Text. Res. J. 2017, 88, 766–776. [Google Scholar] [CrossRef]
- Rajwade, J.; Paknikar, K.; Kumbhar, J. Applications of bacterial cellulose and its composites in biomedicine. Appl. Microbiol. Biotechnol. 2015, 99, 2491–2511. [Google Scholar] [CrossRef] [PubMed]
- Araujo, A.; de Melo, M.; Rabelo, T.; Nunes, P.; Santos, S.; Serafini, M.; Santos, M.; Quintans-Júnior, L.; Gelain, D. Review of the biological properties and toxicity of usnic acid. Nat. Prod. Res. 2015, 29, 2167–2180. [Google Scholar] [CrossRef] [PubMed]
- Espada, J.; Matabuena, M.; Salazar, N.; Lucena, S.; Kourani, O.; Carrasco, E.; Calvo, M.; Rodriguez, C.; Reyes, E.; Gonzalez, S. Cryptomphalus aspersa mollusc eggs extract promotes migration and prevents cutaneous ageing in keratinocytes and dermal fibroblasts in vitro. Int. J. Cosmet. Sci. 2015, 37, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-M.; Yoo, J.-H.; Shin, Y.-J. Effects of egg shell membrane hydrolysates on skin whitening, wound healing, and uv-protection. Korean J. Food Sci. Anim. Resour. 2012, 32, 308–315. [Google Scholar] [CrossRef]
- Sohn, M.; Malburet, C.; Baptiste, L.; Prigl, Y. Development of a synthetic substrate for the in vitro performance testing of sunscreens. Skin Pharmacol. Physiol. 2017, 30, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Osterwalder, U.; Sohn, M.; Herzog, B. Global state of sunscreens. Photodermatol. Photoimmunol. Photomed. 2014, 30, 62–80. [Google Scholar] [CrossRef] [PubMed]
- Mavon, A.; Zahouani, H.; Redoules, D.; Agache, P.; Gall, Y.; Humbert, P. Sebum and stratum corneum lipids increase human skin surface free energy as determined from contact angle measurements: A study on two anatomical sites. Colloids Surf. B Biointerfaces 1997, 8, 147–155. [Google Scholar] [CrossRef]
- Ginn, M.; Noyes, C.; Jungermann, E. The contact angle of water on viable human skin. J. Colloid Interface Sci. 1968, 26, 146–151. [Google Scholar] [CrossRef]
- Francesko, A.; da Costa, D.S.; Lisboa, P.; Reis, R.L.; Pashkuleva, I.; Tzanov, T. Gags-thiolated chitosan assemblies for chronic wounds treatment: Control of enzyme activity and cell attachment. J. Mater. Chem. 2012, 22, 19438–19446. [Google Scholar] [CrossRef]
- Perelshtein, I.; Ruderman, E.; Francesko, A.; Fernandes, M.M.; Tzanov, T.; Gedanken, A. Tannic acid nps–synthesis and immobilization onto a solid surface in a one-step process and their antibacterial and anti-inflammatory properties. Ultrason. Sonochem. 2014, 21, 1916–1920. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Joseph, J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader1. Free Radic. Biol. Med. 1999, 27, 612–616. [Google Scholar] [CrossRef]
- Li, Y.F.; Zhang, H.T.; Xin, L. Hyaluronic acid-modified polyamidoamine dendrimer g5-entrapped gold nanoparticles delivering metase gene inhibits gastric tumor growth via targeting cd44+ gastric cancer cells. J. Cancer Res. Clin. Oncol. 2018, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dileep, K.V.; Tintu, I.; Mandal, P.K.; Karthe, P.; Haridas, M.; Sadasivan, C. Binding to pla2 may contribute to the anti-inflammatory activity of catechol. Chem. Biol. Drug Des. 2012, 79, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.T.; Ryu, G.-M.; Kwon, B.-M.; Lee, W.-H.; Suk, K. Anti-inflammatory effects of catechols in lipopolysaccharide-stimulated microglia cells: Inhibition of microglial neurotoxicity. Eur. J. Pharmacol. 2008, 588, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Morisaki, T.; Izuhara, K.; Uchiyama, A.; Matsunari, Y.; Katano, M.; Tanaka, M. Lipopolysaccharide increases cyclo-oxygenase-2 expression in a colon carcinoma cell line through nuclear factor-κb activation. Oncogene 2000, 19, 1225–1231. [Google Scholar] [CrossRef] [PubMed]



| Copolymer | aFH (mol %) | bfH (mol %) | cMn (Da) | d PDI | Yield (%) |
|---|---|---|---|---|---|
| VH16 | 20 | 15.7 | 23,600 | 4.5 | 78 |
| VH36 | 40 | 35.8 | 15,600 | 2.2 | 83 |
| Terpolymer | fV (mol %) | fH (mol %) | fC (mol %) | Yield (%) | aMn (Da) | b PDI |
|---|---|---|---|---|---|---|
| VHC2 | 84 | 13.9 | 2.1 | 13 | 22,200 | 6.7 |
| VHC22 | 64 | 14.2 | 21.8 | 58 | 14,000 | 1.7 |
| Terpolymer | Tmax (°C) main stage | Char yield (%) | Tg (°C) |
|---|---|---|---|
| VHC2 | 439 | 3.9 | 82 |
| VHC22 | 432 | 8.9 | 80 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puertas-Bartolomé, M.; Vázquez-Lasa, B.; San Román, J. Bioactive and Bioadhesive Catechol Conjugated Polymers for Tissue Regeneration. Polymers 2018, 10, 768. https://doi.org/10.3390/polym10070768
Puertas-Bartolomé M, Vázquez-Lasa B, San Román J. Bioactive and Bioadhesive Catechol Conjugated Polymers for Tissue Regeneration. Polymers. 2018; 10(7):768. https://doi.org/10.3390/polym10070768
Chicago/Turabian StylePuertas-Bartolomé, María, Blanca Vázquez-Lasa, and Julio San Román. 2018. "Bioactive and Bioadhesive Catechol Conjugated Polymers for Tissue Regeneration" Polymers 10, no. 7: 768. https://doi.org/10.3390/polym10070768
APA StylePuertas-Bartolomé, M., Vázquez-Lasa, B., & San Román, J. (2018). Bioactive and Bioadhesive Catechol Conjugated Polymers for Tissue Regeneration. Polymers, 10(7), 768. https://doi.org/10.3390/polym10070768