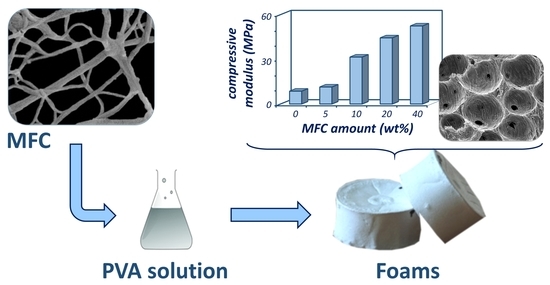
Effect of Microfibrillated Cellulose on Microstructure and Properties of Poly(vinyl alcohol) Foams
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Foam Preparation
2.3. Methods
3. Results and Discussion
3.1. Foams Microstructure
3.2. FTIR Analysis
3.3. Thermal Analysis
3.4. Mechanical Analysis
3.5. Water Vapour Absorption Behaviour
4. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Klempner, D.; Frisch, K.C. Handbook of Polymeric Foams and Foam Technology; Hanser Publishers: Munich, Germany, 1992. [Google Scholar] [CrossRef]
- Shogren, R.L.; Lawton, J.W.; Tiefenbacher, F.K. Baked starch foams: Starch modifications and additives improve process parameters, structure and properties. Ind. Crops Prod. 2002, 16, 69–79. [Google Scholar] [CrossRef]
- Svagan, A.J.; Samir, M.A.S.A.; Berglund, L.A. Biomimetic Foams of High Mechanical Performance Based on Nanostructured Cell Walls Reinforced by Native Cellulose Nanofibrils. Adv. Mater. 2008, 20, 1263–1269. [Google Scholar] [CrossRef]
- Richards, E.; Rizvi, R.; Chow, A.; Naguib, H. Biodegradable Composite Foams of PLA and PHBV Using Subcritical CO2. J. Polym. Environ. 2008, 16, 258–266. [Google Scholar] [CrossRef]
- Koyano, T.; Koshizaki, N.; Umehara, H.; Nagura, M.; Minora, N. Surface states of PVA/chitosan blended hydrogels. Polymer 2000, 41, 4461–4465. [Google Scholar] [CrossRef]
- Bonadies, I.; Renzi, A.I.; Cocca, M.; Avella, M.; Carfagna, C.; Persico, P. Heat Storage and Dimensional Stability of Poly(vinyl alcohol) Based Foams Containing Microencapsulated Phase Change Materials. Ind. Eng. Chem. Res. 2015, 54, 9342–9350. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.; Kolokuris, I.; Nakayama, A.; Yamamoto, N.; Aiba, S.-I. Physico-chemical studies of chitosan-poly (vinyl alcohol) blends plasticized with sorbitol and sucrose. Carbohydr. Polym. 1997, 34, 9–19. [Google Scholar] [CrossRef]
- Song, T.; Tanpichai, S.; Oksman, K. Cross-linked polyvinyl alcohol (PVA) foams reinforced with cellulose nanocrystals (CNCs). Cellulose 2016, 23, 1925–1938. [Google Scholar] [CrossRef]
- Haque, M.U.; Errico, M.E.; Gentile, G.; Avella, M.; Pracella, M. Functionalization and Compatibilization of Poly(e-caprolactone) Composites with Cellulose Microfibres: Morphology, Thermal and Mechanical Properties. Macromol. Mater. Eng. 2012, 297, 985–993. [Google Scholar] [CrossRef]
- Avolio, R.; Bonadies, I.; Capitani, D.; Errico, M.E.; Gentile, G.; Avella, M. A multitechnique approach to assess the effect of ball milling on cellulose. Carbohydr. Polym. 2012, 87, 265–273. [Google Scholar] [CrossRef]
- Lavoine, N.; Desloges, I.; Dufresne, A.; Bras, J. Microfibrillated cellulose—Its barrier properties and applications in cellulosic materials: A review. Carbohydr. Polym. 2012, 90, 735–764. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.P.S.A.; Bhat, A.H.; Yusra, A.F.I. Green composites from sustainable cellulose nanofibrils: A review. Carbohydr. Polym. 2012, 87, 963–979. [Google Scholar] [CrossRef]
- Siró, I.; Plackett, D. Microfibrillated cellulose and new nanocomposite materials: A review. Cellulose 2010, 17, 459–494. [Google Scholar] [CrossRef]
- Volk, N.; He, R.; Magniez, K. Enhanced homogeneity and interfacial compatibility in melt-extruded cellulose nano-fibers reinforced polyethylene via surface adsorption of poly(ethylene glycol)-block-poly(ethylene) amphiphiles. Eur. Polym. J. 2015, 72, 270–281. [Google Scholar] [CrossRef]
- Herzele, S.; Veigel, S.; Liebner, F.; Zimmermann, T.; Gindl-Altmutter, W. Reinforcement of polycaprolactone with microfibrillated lignocellulose. Ind. Crops Prod. 2016, 93, 302–308. [Google Scholar] [CrossRef]
- Adel, A.M.; El-Gendy, A.A.; Diab, M.A.; Abou-Zeid, R.E.; El-Zawawy, W.K.; Dufresne, A. Microfibrillated cellulose from agricultural residues. Part I: Papermaking application. Ind. Crops Prod. 2016, 93, 161–174. [Google Scholar] [CrossRef]
- Ifuku, S.; Yano, H. Effect of a silane coupling agent on the mechanical properties of a microfibrillated cellulose composite. Int. J. Biol. Macromol. 2015, 74, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Avella, M.; Cocca, M.; Errico, M.E.; Gentile, G. Biodegradable PVOH-based foams for packaging applications. J. Cell. Plast. 2011, 47, 271–281. [Google Scholar] [CrossRef]
- Avella, M.; Cocca, M.; Errico, M.E.; Gentile, G. Polyvinyl alcohol Biodegradable Foams Containing Cellulose Fibres. J. Cell. Plast. 2012, 48, 459–470. [Google Scholar] [CrossRef]
- Cocca, M.; Avolio, R.; Gentile, G.; di Pace, E.; Errico, M.E.; Avella, M. Amorphized cellulose as filler in biocomposites based on poly(ε-caprolactone). Carbohydr. Polym. 2014, 118, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Peresin, M.S.; Habibi, Y.; Zoppe, J.O.; Pawlak, J.J.; Rojas, O.J. Nanofiber Composites of Polyvinyl Alcohol and Cellulose Nanocrystals: Manufacture and Characterization. Biomacromolecules 2010, 11, 674–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, M.L.; Su, H.K.; Seon, J.K. Preparation and characteristics of b-chitin and poly(vinyl alcohol) blend. Polymer 1996, 37, 5897–5905. [Google Scholar] [CrossRef]
- Miya, M.; Iwamoto, R.; Mima, S. FT-IR study of intermolecular interactions in polymer blends. J. Polym. Sci. B Polym. Phys. 1984, 22, 1149–1151. [Google Scholar] [CrossRef] [Green Version]
- Lin, F.; Tian, H.; Jia, Q.; Xiang, A.; Li, Y.; Pan, W.; Xu, H. Non-isothermal Crystallization Behaviors of Polyvinyl alcohol/Hydroxyethyl Cellulose Blend Films. J. Polym. Environ. 2013, 21, 343–349. [Google Scholar] [CrossRef]
- Roohani, M.; Habibi, Y.; Belgacem, N.M.; Ebrahim, G.; Karimi, A.N.; Dufresne, A. Cellulose whiskers reinforced polyvinyl alcohol copolymers nanocomposites. Eur. Polym. J. 2008, 44, 2489–2498. [Google Scholar] [CrossRef]









| Tc (°C) | Tg (°C) | Tm (°C) | ΔHm (J/g) | Xc (%) | |
|---|---|---|---|---|---|
| PVA | 119.9 | 42.9 | 176.8 | 25.1 | 15.5 |
| PVA-MFC5 | 154.2 | 58.6 | 202.4 | 31.4 | 19.4 |
| PVA-MFC10 | 149.2 | 56.6 | 200.8 | 30.3 | 18.8 |
| PVA-MFC30 | 138.8 | 61.3 | 183.5 | 17.6 | 10.9 |
| PVA-MFC40 | 127.8 | 63.3 | 166.2 | 11.2 | 6.9 |
| PVA- WPC40 | 133.2 | 58.2 | 173.1 | 14.5 | 9.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gentile, G.; Cocca, M.; Avolio, R.; Errico, M.E.; Avella, M. Effect of Microfibrillated Cellulose on Microstructure and Properties of Poly(vinyl alcohol) Foams. Polymers 2018, 10, 813. https://doi.org/10.3390/polym10080813
Gentile G, Cocca M, Avolio R, Errico ME, Avella M. Effect of Microfibrillated Cellulose on Microstructure and Properties of Poly(vinyl alcohol) Foams. Polymers. 2018; 10(8):813. https://doi.org/10.3390/polym10080813
Chicago/Turabian StyleGentile, Gennaro, Mariacristina Cocca, Roberto Avolio, Maria Emanuela Errico, and Maurizio Avella. 2018. "Effect of Microfibrillated Cellulose on Microstructure and Properties of Poly(vinyl alcohol) Foams" Polymers 10, no. 8: 813. https://doi.org/10.3390/polym10080813