Assignment of Regioirregular Sequences in the 13C NMR Spectrum of Syndiotactic Polypropylene
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
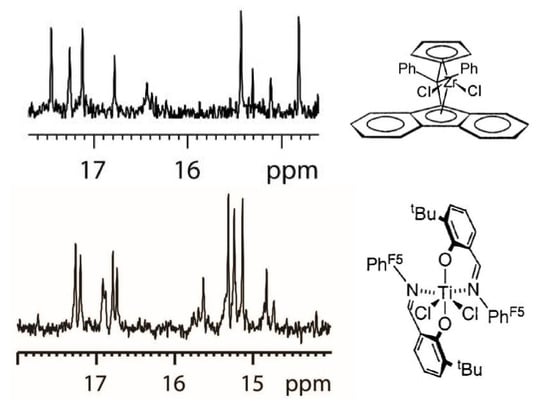
3.1. Assignment of Isolated 2,1 Units in s-PP Made with CAT1
3.2. Assignment of Isolated 2,1 Units in s-PP Made with CAT2
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Busico, V.; Cipullo, R. Microstructure of polypropylene. Prog. Polym. Sci. 2001, 26, 443–533. [Google Scholar] [CrossRef]
- Zhou, Z.; Kümmerle, R.; Stevens, J.C.; Redwine, D.; He, Y.; Qiu, X.; Cong, R.; Klosin, J.; Montañez, N.; Roof, G. 13C NMR of polyolefins with a new high temperature 10 mm cryoprobe. Magn. Reson. 2009, 200, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Stevens, J.C.; Klosin, J.; Kümmerle, R.; Qiu, X.; Redwine, D.; Cong, R.; Taha, A.; Mason, J.; Winniford, B.; et al. NMR study of isolated 2,1-inverse insertion in isotactic polypropylene. Macromolecules 2009, 42, 2291–2294. [Google Scholar] [CrossRef]
- Busico, V.; Cipullo, R.; Mingione, A.; Rongo, L. Accelerating the research approach to Ziegler-Natta catalysts. Ind. Eng. Chem. Res. 2016, 55, 2686–2695. [Google Scholar] [CrossRef]
- Vittoria, A.; Meppelder, A.; Friederichs, N.; Busico, V.; Cipullo, R. Demystifying Ziegler-Natta catalysts: The origin of stereoselectivity. ACS Catal. 2017, 7, 4509–4518. [Google Scholar] [CrossRef]
- Busico, V.; Cipullo, R.; Chadwick, J.C.; Modder, J.F.; Sudmeijer, O. Effects of regiochemical and stereochemical errors on the course of isotactic propene polyinsertion promoted by homogeneous Ziegler-Natta catalysts. Macromolecules 1994, 27, 7538–7543. [Google Scholar] [CrossRef]
- Yu, Y.; Busico, V.; Budzelaar, P.H.M.; Vittoria, A.; Cipullo, R. Of poisons and antidotes in polypropylene catalysis. Angew. Chem. Int. Ed. 2016, 55, 8590–8594. [Google Scholar] [CrossRef] [PubMed]
- Busico, V.; Cipullo, R.; Talarico, G.; Caporaso, L. highly regioselective transition-metal-catalyzed 1-alkene polymerizations: A simple method for the detection and precise determination of regioirregular monomer enchainments. Macromolecules 1998, 31, 2387–2390. [Google Scholar] [CrossRef]
- Busico, V.; Cipullo, R.; Ronca, S. Propene/ethene-[1-13C] copolymerization as a tool for investigating catalyst regioselectivity. 1. Theory and calibration. Macromolecules 2002, 35, 1537–1542. [Google Scholar] [CrossRef]
- Chadwick, J.C.; Van Kessel, G.M.M.; Sudmeijer, O. Regiospecificity and stereospecificity in propene polymerization with MgCl2-supported Ziegler-Natta catalysts—effects of hydrogen and the external donor. Macromol. Chem. Phys. 1995, 196, 1431–1437. [Google Scholar] [CrossRef]
- Busico, V.; Cipullo, R.; Romanelli, V.; Ronca, S.; Togrou, M. Reactivity of secondary metal-alkyls in catalytic propene polymerization: How dormant are “Dormant Chains”? J. Am. Chem. Soc. 2005, 127, 1608–1609. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.P., Jr. (Ed.) Polypropylene Handbook: Polymerization, Characterization, Properties, Processing, Applications; Hanser Publishers: Munich, Germany, 1996. [Google Scholar]
- Chadwick, J.C.; van der Burgt, F.; Rastogi, S.; Busico, V.; Cipullo, R.; Talarico, G.; Heere, J.J.R. Influence of Ziegler-Natta catalyst regioselectivity on polypropylene molecular weight distribution and rheological and crystallization behavior. Macromolecules 2004, 37, 9722–9727. [Google Scholar] [CrossRef]
- Razavi, A. Syndiotactic Polypropylene: Discovery, Development, and Industrialization via Bridged Metallocene Catalysts. In Polyolefins: 50 Years after Ziegler and Natta Ii: Polyolefins by Metallocenes and Other Single-Site Catalysts; Kaminsky, W., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 258, pp. 43–116. [Google Scholar]
- Ewen, J.A.; Elder, M.J.; Jones, R.L.; Haspeslagh, L.; Atwood, J.L.; Bott, S.G.; Robinson, K. Metallocene polypropylene structural relationships—implications on polymerization and stereochemical control mechanisms. Makromol. Chem. Macromol. Symp. 1991, 48–49, 253–295. [Google Scholar] [CrossRef]
- Mitani, M.; Furuyama, R.; Mohri, J.; Saito, J.; Ishii, S.; Terao, H.; Nakano, T.; Tanaka, H.; Fujita, T. Syndiospecific living propylene polymerization catalyzed by titanium complexes having fluorine-containing phenoxy-imine chelate ligands. J. Am. Chem. Soc. 2003, 125, 4293–4305. [Google Scholar] [CrossRef] [PubMed]
- Busico, V.; Cipullo, R.; Cutillo, F.; Friederichs, N.; Ronca, S.; Wang, B. Improving the performance of methylalumoxane: a facile and efficient method to trap “Free” trimethylaluminum. Am. Chem. Soc. 2003, 125, 12402–12403. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.N.; Bennet, M.A. Additive rules for the 13C NMR shifts of methyl-substituted alkanes and ethylene-propylene copolymers. Makromol. Chem. 1987, 188, 135–148. [Google Scholar] [CrossRef]
- Busico, V.; Cipullo, R.; Talarico, G.; Segre, A.L.; Caporaso, L. High-field 13C NMR characterization of ethene-1-13C/propene copolymers prepared with Cs-symmetric ansa-metallocene catalysts: A deeper insight into the regio- and stereoselectivity of syndiotactic propene polymerization. Macromolecules 1998, 31, 8720–8724. [Google Scholar] [CrossRef]
- Busico, V.; Cipullo, R.; Cutillo, F.; Vacatello, M.; Castelli, V.V. Metallocene-catalyzed propene polymerization: from microstructure to kinetics. C-s-Symmetric ansa-Zirconocenes. Macromolecules 2003, 36, 4258–4261. [Google Scholar] [CrossRef]
- Doi, Y. Sequence Distributions of inverted propylene units in polyproplyenes measured by 13C NMR. Macromolecules 1979, 12, 248–251. [Google Scholar] [CrossRef]
- Zambelli, A.; Gatti, G. The carbon-13 nuclear magnetic resonance methyl shift in models of regioirregular polypropylene. Macromolecules 1978, 11, 485–489. [Google Scholar] [CrossRef]
- Borrelli, M.; Busico, V.; Cipullo, R.; Ronca, S.; Budzelaar, P.H.M. Selectivity of metallocene-catalyzed olefin polymerization: A combined experimental and quantum mechanical study. The ansa-Me2Si(ind)2Zr and ansa-Me2C(Cp)(Flu)Zr systems. Macromolecules 2003, 36, 8171–8177. [Google Scholar] [CrossRef]
- Makio, H.; Fujita, T. Development and application of FI catalysts for olefin polymerization: Unique catalysis and distinctive polymer formation. Acc. Chem. Res. 2009, 42, 1532–1544. [Google Scholar] [CrossRef] [PubMed]
- Hustad, P.D.; Tian, J.; Coates, G.W. Mechanism of propylene insertion using bis(Phenoxyimine)-based titanium catalysts: An unusual secondary insertion of propylene in a group IV catalyst system. J. Am. Chem. Soc. 2002, 124, 3614–3621. [Google Scholar] [CrossRef] [PubMed]
- Talarico, G.; Busico, V.; Cavallo, L. Origin of the regiochemistry of propene insertion at Octahedral Column 4 polymerization catalysts: Design or serendipity? J. Am. Chem. Soc. 2003, 125, 7172–7173. [Google Scholar] [CrossRef] [PubMed]
- Ammendola, P.; Shijing, X.; Grassi, A.; Zambelli, A. Statistic model of prevailingly syndiotactic polymerization of propene. Gazz. Chim. Ital. 1988, 118, 769–775. [Google Scholar]
- Cheng, H.N. Probability model treatment of regio- and stereo-irregular polymers. Macromol. Theory Simul. 1994, 3, 979–1004. [Google Scholar] [CrossRef]
- Zambelli, A.; Csok, Z.; Sessa, I. NMR analysis and regiochemical structure of some selectively 13C-enriched poly(propylene)s. Macromol. Rapid Commun. 2005, 26, 519–523. [Google Scholar] [CrossRef]





| 1 | 2 | 2’ | 3 | 3’ | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|---|---|
| ACalc. | 44.13 | 35.11 | 15.76 | 37.68 | 14.85 | 32.5 | 36.1 | 31.26 |
| AExp. | 44.1 | 34.3 | 15.4 | 37.2 | 14.8 | 32.7 | 36.4 | 31.1 (a) |
| BCalc. | 42.32 | 35.83 | 17.1 | 38.53 | 16.76 | 30.77 | 36.70 | 31.30 |
| BExp. | 41.7 | 35.2 | 17.3 | 39.1 | 16.8 | 30.5 | 36.5 | 31.1 (a) |
| CCalc. | 42.33 | 35.84 | 17.63 | 38.53 | 17.14 | 30.46 | 36.70 | 31.30 |
| CExp. | 42.6 | 35.4 | 17.5 | 38.5 | 17.1 | 30.6 | 36.6 | 31.2 |
| DCalc. | 44.12 | 35.10 | 15.25 | 38.08 | 15.34 | 32.32 | 36.12 | 31.25 |
| DExp. | 43.9 | 34.6 | 15.1 | 38.4 | 15.3 | 32.5 | 36.4 | 31.1 (a) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cipullo, R.; Vittoria, A.; Busico, V. Assignment of Regioirregular Sequences in the 13C NMR Spectrum of Syndiotactic Polypropylene. Polymers 2018, 10, 863. https://doi.org/10.3390/polym10080863
Cipullo R, Vittoria A, Busico V. Assignment of Regioirregular Sequences in the 13C NMR Spectrum of Syndiotactic Polypropylene. Polymers. 2018; 10(8):863. https://doi.org/10.3390/polym10080863
Chicago/Turabian StyleCipullo, Roberta, Antonio Vittoria, and Vincenzo Busico. 2018. "Assignment of Regioirregular Sequences in the 13C NMR Spectrum of Syndiotactic Polypropylene" Polymers 10, no. 8: 863. https://doi.org/10.3390/polym10080863