PDDA-Montmorillonite Composites Loaded with Ru Nanoparticles: Synthesis, Characterization, and Catalytic Properties in Hydrogenation of 2-Butanone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
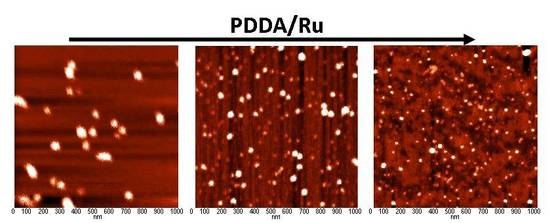
3.1. Physicochemical Characterization
3.2. Catalytic Testing
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- McKenna, K.P. Unique Bonding in Nanoparticles and Powders. In Nanoscale Materials in Chemistry, 2nd ed.; Klabunde, K.J., Richards, R.M., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 15–36. ISBN 978-0-470-22270-6. [Google Scholar]
- Li, Y.; Boone, E.; El-Sayed, M.A. Size Effects of PVP-Pd Nanoparticles on the Catalytic Suzuki Reactions in Aqueous Solution. Langmuir 2002, 18, 4921–4925. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Kulkarni, G.U.; Thomas, P.J.; Edwards, P.P. Size-Dependent Chemistry: Properties of Nanocrystals. Chem. Eur. J. 2002, 8, 28–35. [Google Scholar] [CrossRef]
- Crooks, R.M.; Zhao, M.; Sun, L.; Chechik, V.; Lee, K.Y. Dendrimer-Encapsulated Metal Nanoparticles: Synthesis, Characterization, and Applications to Catalysis. Acc. Chem. Res. 2001, 34, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Bönnemann, H.; Richards, R.M. Nanoscopic Metal Particles—Synthetic Methods and Potential Applications. Eur. J. Inorg. Chem. 2001, 2455–2480. [Google Scholar] [CrossRef]
- Ford, W.T. Inorganic–Organic Composites. In Nanoscale Materials in Chemistry, 2nd ed.; Klabunde, K.J., Richards, R.M., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 369–403. ISBN 978-0-470-22270-6. [Google Scholar]
- Li, Y.; El-Sayed, M.A. The Effect of Stabilizers on the Catalytic Activity and Stability of Pd Colloidal Nanoparticles in the Suzuki Reactions in Aqueous Solution. J. Phys. Chem. B 2001, 105, 8938–8943. [Google Scholar] [CrossRef]
- Králik, M.; Biffis, A.J. Catalysis by metal nanoparticles supported on functional organic polymers. Mol. Catal. A 2001, 177, 113–13810. [Google Scholar] [CrossRef]
- Liu, P. Polymer modified clay minerals: A review. Appl. Clay Sci. 2007, 38, 64–76. [Google Scholar] [CrossRef]
- Chiu, C.W.; Huang, T.K.; Wang, Y.C.; Alamani, B.G.; Lin, J.J. Intercalation strategies in clay/polymer hybrids. Prog. Polym. Sci. 2014, 39, 443–485. [Google Scholar] [CrossRef]
- Zhou, C.H. An overview on strategies towards clay-based designer catalysts for green and sustainable catalysis. Appl. Clay Sci. 2011, 53, 87–96. [Google Scholar] [CrossRef]
- Azeez, A.A.; Rhee, K.Y.; Park, S.J.; Hui, D. Epoxy clay nanocomposites – processing, properties and applications: A review. Compos Part B 2013, 45, 308–320. [Google Scholar] [CrossRef]
- Napruszewska, B.D.; Michalik-Zym, A.; Dula, R.; Bielanska, E.; Rojek, W.; Machej, T.; Socha, R.P.; Lityńska-Dobrzyńska, L.; Bahranowski, K.; Serwicka, E.M. Composites derived from exfoliated Laponite and Mn-Al hydrotalcite prepared in inverse microemulsion: A new strategy for design of robust VOCs combustion catalysts. Appl. Catal. B 2017, 211, 46–56. [Google Scholar] [CrossRef]
- Napruszewska, B.D.; Michalik-Zym, A.; Rogowska, M.; Bielańska, E.; Rojek, W.; Gaweł, A.; Wójcik-Bania, M.; Bahranowski, K.; Serwicka, E.M. Novel Montmorillonite/TiO2/MnAl-Mixed Oxide Composites Prepared from Inverse Microemulsions as Combustion Catalysts. Materials 2017, 10, 1326. [Google Scholar] [CrossRef] [PubMed]
- Papp, S.; Szücs, A.; Dékány, I. Preparation of Pd0 nanoparticles stabilized by polymers and layered silicate. Appl. Clay Sci. 2001, 19, 155–172. [Google Scholar] [CrossRef]
- Papp, S.; Dékány, I.; Szél, J.; Oszkó, A. Synthesis of Polymer-Stabilized Nanosized Rhodium Particles in the Interlayer Space of Layered Silicates. Chem. Mater. 2004, 16, 1674–1685. [Google Scholar] [CrossRef]
- Zhao, X.; Mai, Z.; Kang, X.; Zou, X. Direct electrochemistry and electrocatalysis of horseradish peroxidase based on clay–chitosan-gold nanoparticle nanocomposite. Biosens. Bioelectron. 2008, 23, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Jlassi, K.; Singh, A.; Aswal, D.K.; Losno, R.; Benna-Zayani, M.; Chehimi, M.M. Novel, ternary clay/polypyrrole/silver hybrid materials through in situ photopolymerization. Colloids Surf. A 2013, 439, 193–199. [Google Scholar] [CrossRef]
- Xu, M.; Zhao, J.; Shu, G.; Liu, Q.; Zeng, M. Heterogeneous Catalytic Composites from Palladium Nanoparticles in Montmorillonite Intercalated with Poly (Vinyl Pyrrolidone) Chains. Polymers 2018, 10, 669. [Google Scholar] [CrossRef]
- Fan, M.; Wang, R.; Jia, S. Controllable synthesis of iron nanoparticles on polyethylenimine-modified montmorillonite: Dependence on the amine protonation extent. Appl. Clay Sci. 2018, 162, 418–427. [Google Scholar] [CrossRef]
- Brigatti, M.F.; Galán, E.; Theng, B.K.G. Structure and Mineralogy of Clay Minerals. In Handbook of Clay Science, 2nd ed.; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Part A; pp. 21–81. ISBN 978-0-08-098259-5. [Google Scholar]
- Dautzenberg, H.; Gornitz, E.; Jaeger, W. Synthesis and characterization of poly (diallyldimethylammonium chloride) in a broad range of molecular weight. Macromol. Chem. Phys. 1998, 199, 1561–1571. [Google Scholar] [CrossRef]
- Michel, C.; Gallezot, P. Why Is Ruthenium an Efficient Catalyst for the Aqueous-Phase Hydrogenation of Biosourced Carbonyl Compounds? ACS Catal. 2015, 5, 4130–4132. [Google Scholar] [CrossRef]
- Breen, J.P.; Burch, R.; Griffin, K.; Hardacre, C.; Hayes, M.; Huang, X.; O’Brien, S.D. Bimetallic effects in the liquid-phase hydrogenation of 2-butanone. J. Catal. 2005, 236, 270–281. [Google Scholar] [CrossRef]
- Breen, C.; Rawson, J.O.; Mann, B.E. Adsorption of polycations on clays: An in situ study using 133Cs solution-phase NMR. J. Mater. Chem. 1996, 6, 253–260. [Google Scholar] [CrossRef]
- Churchman, G.J. Formation of complexes between bentonite and different cationic polyelectrolytes and their use as sorbents for non-ionic and anionic pollutants. Appl. Clay Sci. 2002, 21, 177–189. [Google Scholar] [CrossRef]
- Czímerová, A.; Jankovič, L.; Madejová, J.; Čeklovský, A. Unique Photoactive Nanocomposites Based on Rhodamine 6G/Polymer/Montmorillonite Hybrid Systems. J. Polym. Sci. B Polym. Phys. 2013, 51, 1672–1679. [Google Scholar] [CrossRef]
- Meunier, A. Clays; Springer-Verlag: Berlin, Heidelberg, Germany, 2005; pp. 196–198. ISBN 978–3-540-21667-4. [Google Scholar]
- Langier-Kuźniarowa, A. Thermal Analysis of Organoclay Complexes. In Organo Clay Complexes and Interactions; Yariv, S., Cross, H., Eds.; Marcel Dekker: New York, NY, USA, 2001; pp. 273–344. ISBN 0-8247-0586-6. [Google Scholar]
- Yariv, S. The role of charcoal on DTA curves of organo-clay complexes: an overview. Appl. Clay Sci. 2004, 24, 225–236. [Google Scholar] [CrossRef]
- Fajnor, V.Š.; Jesenák, K. Differential thermal analysis of montmorillonite. J. Therm. Anal. 1996, 46, 489–493. [Google Scholar] [CrossRef]
- Kamiuchi, N.; Mitsui, T.; Muroyama, H.; Matsui, T.; Kikuchi, R.; Eguchi, K. Catalytic combustion of ethyl acetate and nano-structural changes of ruthenium catalysts supported on tin oxide. Appl. Catal. B 2010, 97, 120–126. [Google Scholar] [CrossRef]
- Rylander, P.N. Catalytic Hydrogenation in Organic Syntheses; Academic Press: New York, NY, USA, 1979; pp. 82–112. ISBN 978-0-12-605355-5. [Google Scholar]
- Gavnholt, J.; Schiøtz, J. Structure and reactivity of ruthenium nanoparticles. Phys. Rev. B 2008, 77, 035404-1–035404-10. [Google Scholar] [CrossRef]
- Karim, A.M.; Prasad, V.; Mpourmpakis, G.; Lonergan, W.W.; Frenkel, A.I.; Chen, J.G.; Vlachos, D.G. Correlating Particle Size and Shape of Supported Ru/γ-Al2O3 Catalysts with NH3 Decomposition Activity. J. Am. Chem. Soc. 2009, 131, 12230–12239. [Google Scholar] [CrossRef] [PubMed]
- Bu, J.; Liu, J.L.; Chen, X.Y.; Zhuang, J.H.; Yan, S.R.; Qiao, M.H.; He, H.Y.; Fan, K.N. Ru/SBA-15 catalysts for partial hydrogenation of benzene to cyclohexene: Tuning the Ru crystallite size by Ba. Catal. Commun. 2008, 9, 2612–2615. [Google Scholar] [CrossRef]
- Akpa, B.S.; D’Agostino, C.; Gladden, F.L.; Hindle, K.; Manyar, H.; McGregor, J.; Li, R.; Neurock, M.; Sinha, N.; Sitt, E.H; et al. Solvent effects in the hydrogenation of 2-butanone. J. Catal. 2012, 289, 30–41. [Google Scholar] [CrossRef]
- Wan, H.; Vitter, A.; Chaudhari, R.V.; Subramaniam, B. Kinetic investigations of unusual solvent effects during Ru/C catalyzed hydrogenation of model oxygenates. J. Catal. 2014, 309, 174–184. [Google Scholar] [CrossRef]
- Jones, D.R.; Iqbal, S.; Kondrat, S.A.; Lari, G.M.; Miedziak, P.J.; Morgan, D.J.; Parker, S.F.; Hutchings, G.J. An investigation of the effect of carbon support on ruthenium/carbon catalysts for lactic acid and butanone hydrogenation. Phys. Chem. Chem. Phys. 2016, 18, 17259–17264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duraczyńska, D.; Michalik-Zym, A.; Napruszewska, B.D.; Dula, R.; Socha, R.P.; Lityńska-Dobrzyńska, L.; Gaweł, A.; Bahranowski, K.; Serwicka, E.M. Efficient and Versatile Ru/SBA-15 Catalysts for Liquid-hase Hydrogenation of the C=C and C=O Bonds under Mild Conditions. ChemistrySelect 2016, 1, 2148–2155. [Google Scholar] [CrossRef]
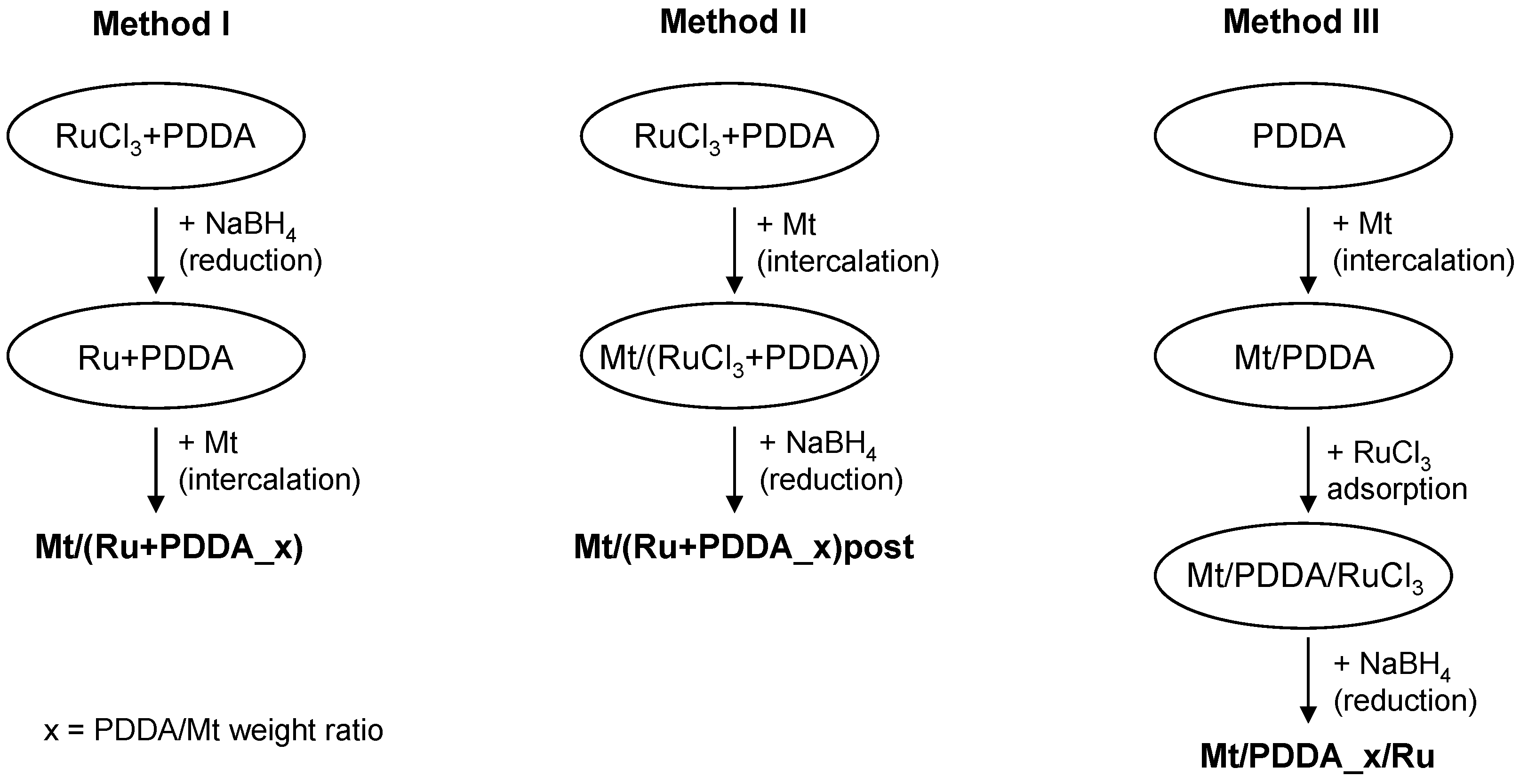








| Sample | d001 (nm) | Na/Si | Ru (wt %) | ΔTG200-1000 (wt %) | 2-Butanone Conversion (%) |
|---|---|---|---|---|---|
| Mt | 1.25 | 0.092 | - | 5.6 | 0 |
| Mt/PDDA_0.01 | 1.46 | 0.021 | - | 7.3 (6.3) 1 | n.d 2 |
| Mt/PDDA_0.025 | 1.49 | 0.012 | - | 8.5 (7.4) | n.d. |
| Mt/PDDA_0.05 | 1.50 | 0.009 | - | 9.7 (9.2) | 0 |
| Mt/PDDA_0.1 | 1.56 | 0.003 | - | 12.3 (12.5) | n.d. |
| Mt/PDDA_0.25 | 1.53 | 0.001 | - | 15.9 (21.3, 15.0 *) | n.d. |
| Mt/(Ru + PDDA_0.01) | 1.27 | 0.080 | 1.92 | 6.2 (6.2) | 63 |
| Mt/(Ru + PDDA_0.025) | 1.30 | 0.062 | 1.85 | 6.8 (7.3) | 82 |
| Mt/(Ru + PDDA_0.05) | 1.45 (1.30) | 0.044 | 1.95 | 8.2 (9.0) | 100 |
| Mt/(Ru + PDDA_0.1) | 1.51 | 0.028 | 1.81 | 12.0 (12.4) | 45 |
| Mt/(Ru + PDDA_0.25) | 1.99 (1.50) | 0.008 | 1.67 | 19.8 (21.3, 14.7 *) | 13 |
| Mt/(Ru + PDDA_0.05)post | 1.49 | 0.046 | 1.74 | 9.6 (9.0) | 36 |
| Mt/(Ru + PDDA_0.25)post | 1.97 (1.56) | 0.002 | 1.61 | 18.9 (21.3, 14.7 *) | 7 |
| Mt/PDDA_0.05/Ru | 1.51 | 0.048 | 1.99 | 9.4 (9.0) | 26 |
| Mt/PDDA_0.25/Ru | 1.57 | 0.001 | 1.74 | 16.3 (21.3, 14.7 *) | 27 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serwicka, E.M.; Zimowska, M.; Duraczyńska, D.; Napruszewska, B.D.; Nattich-Rak, M.; Mordarski, G.; Lityńska-Dobrzyńska, L.; Palkova, H. PDDA-Montmorillonite Composites Loaded with Ru Nanoparticles: Synthesis, Characterization, and Catalytic Properties in Hydrogenation of 2-Butanone. Polymers 2018, 10, 865. https://doi.org/10.3390/polym10080865
Serwicka EM, Zimowska M, Duraczyńska D, Napruszewska BD, Nattich-Rak M, Mordarski G, Lityńska-Dobrzyńska L, Palkova H. PDDA-Montmorillonite Composites Loaded with Ru Nanoparticles: Synthesis, Characterization, and Catalytic Properties in Hydrogenation of 2-Butanone. Polymers. 2018; 10(8):865. https://doi.org/10.3390/polym10080865
Chicago/Turabian StyleSerwicka, Ewa M., Małgorzata Zimowska, Dorota Duraczyńska, Bogna D. Napruszewska, Małgorzata Nattich-Rak, Grzegorz Mordarski, Lidia Lityńska-Dobrzyńska, and Helena Palkova. 2018. "PDDA-Montmorillonite Composites Loaded with Ru Nanoparticles: Synthesis, Characterization, and Catalytic Properties in Hydrogenation of 2-Butanone" Polymers 10, no. 8: 865. https://doi.org/10.3390/polym10080865