Bacteriostatic Activity of LLDPE Nanocomposite Embedded with Sol–Gel Synthesized TiO2/ZnO Coupled Oxides at Various Ratios
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of TiO2/ZnO Nanocomposite
2.3. Fabrication of LLDPE/Metal Oxide Nanocomposite
2.4. Characterization
2.5. Measurement of Antibacterial Ctivity
3. Results and Discussion
3.1. Surface Morphology of LLDPE Nanocomposites
3.2. Crystal Analysis of LLDPE Nanocomposites
3.3. FT-IR
3.4. Mechanical Properties
3.5. Thermal Analysis
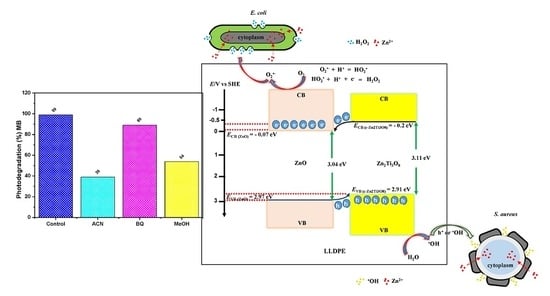
3.6. Relative Production of Reactive Species (Hydroxyl Radicals, Superoxide Anion and Holes)
3.7. Zinc Ion Release
3.8. Antibacterial Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bryant, K.A.; Woods, C.R. Healthcare-acquired infections due to gram-positive bacteria. Pediatr. Infect. Dis. J. 2008, 27, 455–456. [Google Scholar] [CrossRef] [PubMed]
- Peleg, A.Y.; Hooper, D.C. Hospital-acquired infections due to gram-negative bacteria. N. Engl. J. Med. 2010, 362, 1804–1813. [Google Scholar] [CrossRef] [PubMed]
- Fair, R.J.; Tor, Y. Antibiotics and bacterial resistance in the 21st century. Perspect. Med. Chem. 2014, 6. [Google Scholar] [CrossRef] [PubMed]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.; Memic, A. Size-dependent antimicrobial properties of CuO nanoparticles against gram-positive and-negative bacterial strains. Int. J. Nanomed. 2012, 7, 3527–3535. [Google Scholar] [CrossRef] [PubMed]
- Delgado, K.; Quijada, R.; Palma, R.; Palza, H. Polypropylene with embedded copper metal or copper oxide nanoparticles as a novel plastic antimicrobial agent. Lett. Appl. Microbiol. 2011, 53, 50–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Llamazares, S.; Mondaca, M.; Badilla, C.; Maldonado, A. PVC/copper oxide composites and their effect on bacterial adherence. J. Chil. Chem. Soc. 2012, 57, 1163–1165. [Google Scholar] [CrossRef]
- Ren, G.; Hu, D.; Cheng, E.W.; Vargas-Reus, M.A.; Reip, P.; Allaker, R.P. Characterisation of copper oxide nanoparticles for antimicrobial applications. Int. J. Antimicrob. Agents 2009, 33, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Varghese, S.; ElFakhri, S.O.; Sheel, D.W.; Sheel, P.; Bolton, F.J.E.; Foster, H.A. Antimicrobial activity of novel nanostructured Cu-SiO2 coatings prepared by chemical vapour deposition against hospital related pathogens. AMB Express 2013, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Vary, P.S.; Lin, C.-T. Anatase TiO2 nanocomposites for antimicrobial coatings. J. Phys. Chem. B 2005, 109, 8889–8898. [Google Scholar] [CrossRef] [PubMed]
- Sayılkan, F.; Asiltürk, M.; Kiraz, N.; Burunkaya, E.; Arpaç, E.; Sayılkan, H. Photocatalytic antibacterial performance of Sn4+ doped TiO2 thin films on glass substrate. J. Hazard. Mater. 2009, 162, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Chen, J.-H.; Xu, X.-L.; Yang, P.-H.; Hildebrand, H.F. Antibacterial activities of inorganic agents on six bacteria associated with oral infections by two susceptibility tests. Int. J. Antimicrob. Agents 2006, 27, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, N.; Torsi, L.; Ditaranto, N.; Tantillo, G.; Ghibelli, L.; Sabbatini, L.; Bleve-Zacheo, T.; D′Alessio, M.; Zambonin, P.G.; Traversa, E. Copper nanoparticle/polymer composites with antifungal and bacteriostatic properties. Chem. Mater. 2005, 17, 5255–5262. [Google Scholar] [CrossRef]
- Kaali, P.; Pérez-Madrigal, M.; Strömberg, E.; Aune, R.E.; Czél, G.; Karlsson, S. The influence of Ag+, Zn2+ and Cu2+ exchanged zeolite on antimicrobial and long term in vitro stability of medical grade polyether polyurethane. Express Polym. Lett. 2011, 5, 1028–1040. [Google Scholar] [CrossRef] [Green Version]
- Hsu, S.-H.; Tseng, H.-J.; Lin, Y.-C. The biocompatibility and antibacterial properties of waterborne polyurethane-silver nanocomposites. Biomaterials 2010, 31, 6796–6808. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, J.; Xu, Q.; Zhang, J. Fabrication of antibacterial casein-based ZnO nanocomposite for flexible coatings. Mater. Des. 2017, 113, 240–245. [Google Scholar] [CrossRef]
- Raghupathi, K.R.; Koodali, R.T.; Manna, A.C. Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir 2011, 27, 4020–4028. [Google Scholar] [CrossRef] [PubMed]
- Teli, M.D.; Kale, R.D. Polyester nanocomposite fibers with antibacterial properties. Adv. Appl. Sci. Res. 2011, 2, 491–502. [Google Scholar]
- Yañez, D.; Guerrero, S.; Lieberwirth, I.; Ulloa, M.T.; Gomez, T.; Rabagliati, F.M.; Zapata, P.A. Photocatalytic inhibition of bacteria by TiO2 nanotubes-doped polyethylene composites. Appl. Catal. A Gen. 2015, 489, 255–261. [Google Scholar] [CrossRef]
- Yemmireddy, V.K.; Hung, Y.C. Using photocatalyst metal oxides as antimicrobial surface coatings to ensure food safety—Opportunities and challenges. Compr. Rev. Food Sci. Food Saf. 2017, 16, 617–631. [Google Scholar] [CrossRef]
- Khan, S.T.; Al-Khedhairy, A.A.; Musarrat, J. ZnO and TiO2 nanoparticles as novel antimicrobial agents for oral hygiene: A review. J. Nanopart. Res. 2015, 17, 1–16. [Google Scholar] [CrossRef]
- Daou, I.; Moukrad, N.; Zegaoui, O.; Filali, F.R. Antimicrobial activity of ZnO-TiO2 nanomaterials synthesized from three different precursors of ZnO: Influence of ZnO/TiO2 weight ratio. Water Sci. Technol. 2017, 77, 1238–1249. [Google Scholar] [CrossRef] [PubMed]
- Jašková, V.; Hochmannová, L.; Vytřasová, J. TiO2 and ZnO nanoparticles in photocatalytic and hygienic coatings. Int. J. Photoenergy 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Nguyen, V.G.; Thai, H.; Mai, D.H.; Tran, H.T.; Vu, M.T. Effect of titanium dioxide on the properties of polyethylene/TiO2 nanocomposites. Compos. Part B Eng. 2013, 45, 1192–1198. [Google Scholar] [CrossRef]
- Sakthivel, S.; Neppolian, B.; Shankar, M.; Arabindoo, B.; Palanichamy, M.; Murugesan, V. Solar photocatalytic degradation of azo dye: Comparison of photocatalytic efficiency of ZnO and TiO2. Sol. Energy Mater. Sol. Cells 2003, 77, 65–82. [Google Scholar] [CrossRef]
- Color Additives Approved for Use in Drugs, Part 73, Subpart B: Color Additives Exempt from Batch Certification (Zinc Oxide); U.S. Food and Drug Administration, Food and Drugs: Silver Spring, MD, USA, 2015.
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Mondal, K. Recent advances in the synthesis of metal oxide nanofibers and their environmental remediation applications. Inventions 2017, 2, 9. [Google Scholar] [CrossRef]
- Cai, H.; You, Q.; Hu, Z.; Duan, Z.; Cui, Y.; Sun, J.; Xu, N.; Wu, J. Fabrication and correlation between photoluminescence and photoelectrochemical properties of vertically aligned ZnO coated TiO2 nanotube arrays. Energy Mater. Sol. Cells 2014, 123, 233–238. [Google Scholar] [CrossRef]
- Mofokeng, S.; Kumar, V.; Kroon, R.; Ntwaeaborwa, O. Structure and optical properties of Dy3+ activated sol-gel ZnO-TiO2 nanocomposites. J. Alloys Compd. 2017, 711, 121–131. [Google Scholar] [CrossRef]
- Gholami, M.; Shirzad-Siboni, M.; Farzadkia, M.; Yang, J.-K. Synthesis, characterization, and application of ZnO/TiO2 nanocomposite for photocatalysis of a herbicide (bentazon). Desalin. Water Treat. 2016, 57, 13632–13644. [Google Scholar] [CrossRef]
- Rajbongshi, B.M.; Samdarshi, S.; Boro, B. Multiphasic bi-component TiO2–ZnO nanocomposite: Synthesis, characterization and investigation of photocatalytic activity under different wavelengths of light irradiation. J. Mater. Sci. Mater. Electron. 2015, 26, 377–384. [Google Scholar] [CrossRef]
- Long, Y.-M.; Hu, L.-G.; Yan, X.-T.; Zhao, X.-C.; Zhou, Q.-F.; Cai, Y.; Jiang, G.-B. Surface ligand controls silver ion release of nanosilver and its antibacterial activity against escherichia coli. Int. J. Nanomed. 2017, 12, 3193–3206. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-Y.; Choi, H.; Kushida, Y.; Bhayana, B.; Wang, Y.; Hamblin, M.R. Broad-spectrum antimicrobial effects of photocatalysis using titanium dioxide nanoparticles are strongly potentiated by addition of potassium iodide. Antimicrob. Agents Chemother. 2016, 60, 5445–5453. [Google Scholar] [CrossRef] [PubMed]
- Abbaszadegan, A.; Ghahramani, Y.; Gholami, A.; Hemmateenejad, B.; Dorostkar, S.; Nabavizadeh, M.; Sharghi, H. The effect of charge at the surface of silver nanoparticles on antimicrobial activity against gram-positive and gram-negative bacteria: A preliminary study. J. Nanomater. 2015, 16, 1–8. [Google Scholar] [CrossRef]
- Khanam, P.N.; AlMaadeed, M.A.A. Processing and characterization of polyethylene-based composites. Adv. Manuf. Polym. Compos. Sci. 2015, 1, 63–79. [Google Scholar] [CrossRef]
- Chen, W.; Qu, B. LLDPE/ZnAl LDH-exfoliated nanocomposites: Effects of nanolayers on thermal and mechanical properties. J. Mater. Chem. 2004, 14, 1705–1710. [Google Scholar] [CrossRef]
- Fuad, M.; Hanim, H.; Zarina, R.; Ishak, Z.; Hassan, A. Polypropylene/calcium carbonate nanocomposites—Effects of processing techniques and maleated polypropylene compatibiliser. Express Polym. Lett. 2010, 4, 611–620. [Google Scholar] [CrossRef] [Green Version]
- Al-Rawajfeha, A.E.; Al-Salahb, H.A.; Al-Rhaelc, I. Miscibility, crystallinity and morphology of polymer blends of polyamide-6/poly (β-hydroxybutyrate). Jordan J. Chem. 2006, 1, 155–170. [Google Scholar]
- Silvestre, C.; Duraccio, D.; Marra, A.; Strongone, V.; Cimmino, S. Development of antibacterial composite films based on isotactic polypropylene and coated ZnO particles for active food packaging. Coatings 2016, 6, 4. [Google Scholar] [CrossRef]
- Shafiq, M.; Yasin, T.; Saeed, S. Synthesis and characterization of linear low-density polyethylene/sepiolite nanocomposites. J. Appl. Polym. Sci. 2012, 123, 1718–1723. [Google Scholar] [CrossRef]
- Altan, M.; Yildirim, H. Mechanical and morphological properties of polypropylene and high density polyethylene matrix composites reinforced with surface modified nano sized TiO2 particles. World Acad. Sci. Eng. Technol. 2010, 4, 252–257. [Google Scholar]
- Tian, J.; Chen, L.; Yin, Y.; Wang, X.; Dai, J.; Zhu, Z.; Liu, X.; Wu, P. Photocatalyst of TiO2/ZnO nano composite film: Preparation, characterization, and photodegradation activity of methyl orange. Surf. Coat. Technol. 2009, 204, 205–214. [Google Scholar] [CrossRef]
- Radheshkumar, C.; Münstedt, H. Antimicrobial polymers from polypropylene/silver composites—Ag+ release measured by anode stripping voltammetry. React. Funct. Polym. 2006, 66, 780–788. [Google Scholar] [CrossRef]
- Costantini, J.M.; Salvetat, J.P.; Couvreur, F.; Bouffard, S. Carbonization of polyimide by swift heavy ion irradiations: Effects of stopping power and velocity. Nucl. Instrum. Methods Phys. Res. Sect. B 2005, 234, 458–466. [Google Scholar] [CrossRef]
- Campbell, D.; Pethrick, R.A.; White, J.R. Polymer Characterization: Physical Techniques; CRC Press: London, UK, 2000. [Google Scholar]
- Kuila, T.; Bose, S.; Mishra, A.K.; Khanra, P.; Kim, N.H.; Lee, J.H. Effect of functionalized graphene on the physical properties of linear low density polyethylene nanocomposites. Polym. Test. 2012, 31, 31–38. [Google Scholar] [CrossRef]
- Chakradhar, R.P.S.; Kumar, V.D.; Rao, J.L.; Basu, B.J. Fabrication of superhydrophobic surfaces based on ZnO–PDMS nanocomposite coatings and study of its wetting behaviour. Appl. Surf. Sci. 2011, 257, 8569–8575. [Google Scholar] [CrossRef]
- Emamifar, A.; Mohammadizadeh, M. Preparation and application of LDPE/ZnO nanocomposites for extending shelf life of fresh strawberries. Food Technol. Biotechnol. 2015, 53, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.; Arfat, Y.A.; Al-Attar, H.; Auras, R.; Ejaz, M. Rheological, structural, ultraviolet protection and oxygen barrier properties of linear low-density polyethylene films reinforced with zinc oxide (ZnO) nanoparticles. Food Packag. Shelf Life 2017, 13, 20–26. [Google Scholar] [CrossRef]
- Paul, D.; Robeson, L.M. Polymer nanotechnology: Nanocomposites. Polymer 2008, 49, 3187–3204. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Yousef, J.M.; Danial, E.N. In vitro antibacterial activity and minimum inhibitory concentration of zinc oxide and nano-particle zinc oxide against pathogenic strains. J. Health Sci. 2012, 2, 38–42. [Google Scholar] [CrossRef]







| Samples | 2θ (°) (110) | d-Spacing (nm) | 2θ (°) (220) | d-Spacing (nm) | C-Index |
|---|---|---|---|---|---|
| LLDPE | 21.75 | 4.08 | 24.14 | 3.70 | 0.64 |
| LLDPE/100T | 21.88 | 4.10 | 24.88 | 3.72 | 0.49 |
| LLDPE/75T25Z | 21.80 | 4.10 | 24.67 | 3.71 | 0.45 |
| LLDPE/50T50Z | 21.78 | 4.11 | 24.77 | 3.72 | 0.56 |
| LLDPE/25T75Z | 21.83 | 4.08 | 24.81 | 3.71 | 0.36 |
| LLDPE/100Z | 21.81 | 4.11 | 24.90 | 3.71 | 0.43 |
| Films | Tensile Strength (MPa) | Young Modulus (MPa) | Percent Elongation (%) |
|---|---|---|---|
| LLDPE | 5.99 ± 0.14 | 91.93 ± 1.77 | 62.80 ± 18.67 |
| LLDPE/100T | 8.89 ± 0.61 | 122.66 ± 8.17 | 41.50 ± 7.80 |
| LLDPE/75T25Z | 9.21 ± 0.20 | 157.51 ± 4.48 | 24.73 ± 7.62 |
| LLDPE/50T50Z | 8.45 ± 0.42 | 164.03 ± 7.76 | 20.40 ± 1.37 |
| LLDPE/25T75Z | 8.16 ± 1.10 | 131.32 ± 19.46 | 35.20 ± 9.84 |
| LLDPE/100Z | 9.55 ± 0.56 | 181.54 ± 8.12 | 15.97 ± 3.23 |
| Sample | Tc (oC) | Tm (oC) | Apparent Enthalpy H′ (J/g) = Enthalpy of Fusion, Hm − Enthalpy of Crystallisation, Hc | Enthalpy of Melting of 100% Crystalline LLDPE, ∆Ho (J/g) | Percent Crystallinity, Xc (%) |
|---|---|---|---|---|---|
| LLDPE | 36.5 | 106.7 | 52.53 | 276 | 19.03 |
| LLDPE/100T | 36.4 | 108.2 | 37.94 | 276 | 13.74 |
| LLDPE/75T25Z | 39.7 | 107.5 | 33.21 | 276 | 12.26 |
| LLDPE/50T50Z | 38.9 | 107.6 | 50.42 | 276 | 18.24 |
| LLDPE/25T75Z | 38.8 | 108.0 | 44.45 | 276 | 15.59 |
| LLDPE/100Z | 38.9 | 107.6 | 41.97 | 276 | 15.20 |
| Sample | •OH Radical Scavenging Activity (%) | O2•− Radical Scavenging Activity (%) | h+ Scavenging Activity (%) |
|---|---|---|---|
| LLDPE/100T | 15 | - | 50 |
| LLDPE/75T25Z | 10 | - | 50 |
| LLDPE/50T50Z | 5 | - | - |
| LLDPE/25T75Z | 60 | 10 | 40 |
| LLDPE/100Z | 50 | 20 | 25 |
| Time (h) | Samples | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Inoculum (cfu/mL) | Bacterial Reduction (%) | |||||||||
| 100T | 100Z | 25T75Z | 50T50Z | 75T25Z | 100T | 100Z | 25T75Z | 50T50Z | 75T25Z | |
| 1 | (2.43 ± 0.39) × 104 | (2.46 ± 0.47) × 104 | (1.70 ± 0.04) × 104 | (2.35 ± 0.34) × 104 | (1.83 ± 0.10) × 104 | - | - | - | - | - |
| 6 | (0.52 ± 0.10) × 104 | (0.76 ± 0.05) × 104 | (0.37 ± 0.02) × 103 | (1.61 ± 0.01) × 104 | (0.20 ± 0.10) × 103 | 78.68 | 69.11 | 97.82 | 31.49 | 98.91 |
| 12 | (0.53 ± 0.06) × 103 | (0.13 ± 0.02) × 104 | (0.87 ± 0.06) × 103 | (1.06 ± 0.03) × 104 | (1.13 ± 0.76) × 103 | 97.95 | 94.72 | 94.88 | 54.89 | 92.73 |
| 24 | (0.55 ± 0.24) × 104 | (0.6 ± 0.17) × 103 | (0.87 ± 0.21) × 103 | (0.11 ± 0.01) × 104 | (0.90 ± 0.44) × 103 | 77.46 | 97.56 | 94.88 | 95.32 | 95.08 |
| Time (h) | Samples | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Inoculum (cfu/mL) | Bacterial Reduction (%) | |||||||||
| 100T | 100Z | 25T75Z | 50T50Z | 75T25Z | 100T | 100Z | 25T75Z | 50T50Z | 75T25Z | |
| 1 | (3.0 ± 0.0) × 104 | (1.88 ± 0.30) × 104 | (3.0 ± 0.0) × 104 | (2.74 ± 0.28) × 104 | (3.0 ± 0.0) × 104 | - | - | - | - | - |
| 6 | (3.0 ± 0.0) × 104 | (3.0 ± 0.0) × 104 | (1.21 ± 0.09) × 104 | (1.65 ± 0.38) × 104 | (2.34 ± 1.10) × 104 | 0 | 0 | 59.67 | 39.78 | 22.0 |
| 12 | (3.0 ± 0.0) × 104 | (0.89 ± 0.15) × 104 | (0.66 ± 1.15) × 102 | (0.79 ± 0.20) × 104 | (3.0 ± 0.0) × 104 | 0 | 52.66 | 99.78 | 71.17 | 0 |
| 24 | (3.0 ± 0.0) × 104 | (0.33 ± 0.57) × 102 | - | (0.10 ± 0.10) × 103 | (2.90 ± 0.18) × 104 | 0 | 99.82 | 100.0 | 99.64 | 3.33 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saharudin, K.A.; Sreekantan, S.; Basiron, N.; Khor, Y.L.; Harun, N.H.; S. M. N. Mydin, R.B.; Md Akil, H.; Seeni, A.; Vignesh, K. Bacteriostatic Activity of LLDPE Nanocomposite Embedded with Sol–Gel Synthesized TiO2/ZnO Coupled Oxides at Various Ratios. Polymers 2018, 10, 878. https://doi.org/10.3390/polym10080878
Saharudin KA, Sreekantan S, Basiron N, Khor YL, Harun NH, S. M. N. Mydin RB, Md Akil H, Seeni A, Vignesh K. Bacteriostatic Activity of LLDPE Nanocomposite Embedded with Sol–Gel Synthesized TiO2/ZnO Coupled Oxides at Various Ratios. Polymers. 2018; 10(8):878. https://doi.org/10.3390/polym10080878
Chicago/Turabian StyleSaharudin, Khairul Arifah, Srimala Sreekantan, Norfatehah Basiron, Yong Ling Khor, Nor Hazliana Harun, Rabiatul Basria S. M. N. Mydin, Hazizan Md Akil, Azman Seeni, and Kumaravel Vignesh. 2018. "Bacteriostatic Activity of LLDPE Nanocomposite Embedded with Sol–Gel Synthesized TiO2/ZnO Coupled Oxides at Various Ratios" Polymers 10, no. 8: 878. https://doi.org/10.3390/polym10080878
APA StyleSaharudin, K. A., Sreekantan, S., Basiron, N., Khor, Y. L., Harun, N. H., S. M. N. Mydin, R. B., Md Akil, H., Seeni, A., & Vignesh, K. (2018). Bacteriostatic Activity of LLDPE Nanocomposite Embedded with Sol–Gel Synthesized TiO2/ZnO Coupled Oxides at Various Ratios. Polymers, 10(8), 878. https://doi.org/10.3390/polym10080878