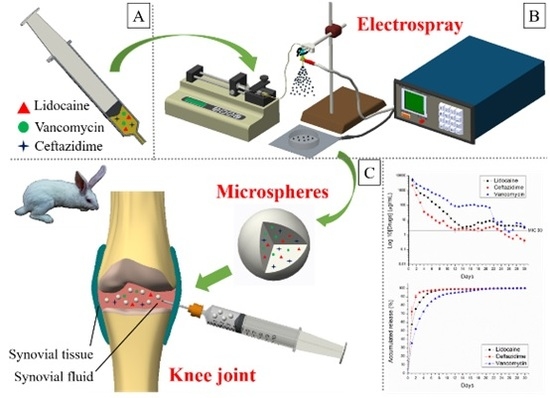
Sustained Delivery of Analgesic and Antimicrobial Agents to Knee Joint by Direct Injections of Electrosprayed Multipharmaceutical-Loaded Nano/Microparticles
Abstract
:1. Introduction
2. Materials and Methods
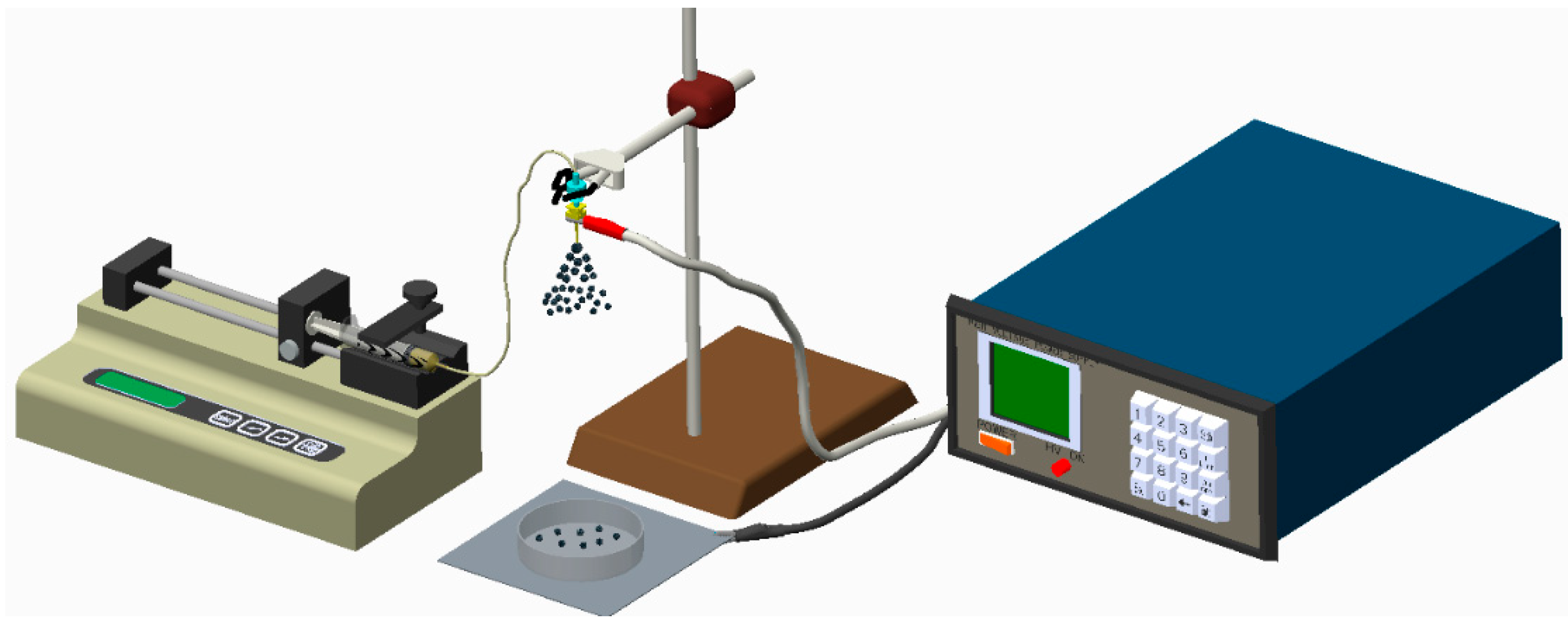
2.1. Fabrication of Biodegradable Drug-Eluting Microparticles
2.2. Scanning Electron Microscopy
2.3. Infrared Spectrometry
2.4. In Vitro Liberation of Antibiotics and Analgesic
2.5. In Vivo Study
3. Results
3.1. Characterization of Electrosprayed Particles
3.2. In Vitro Release of Drug-Eluting Nano/Microparticles
3.3. In Vivo Release
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mathews, C.J.; Weston, V.C.; Jones, A.; Field, M.; Coakley, G. Bacterial septic arthritis in adults. Lancet 2010, 375, 846–855. [Google Scholar] [CrossRef]
- Kaandorp, C.J.; Dinant, H.J.; van de Laar, M.A.; Moens, H.J.; Prins, A.P.; Dijkmans, B.A. Incidence and sources of native and prosthetic joint infection: A community based prospective survey. Ann. Rheum. Dis. 1997, 56, 470–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clerc, O.; Prod’hom, G.; Greub, G.; Zanetti, G.; Senn, L. Adult native septic arthritis: A review of 10 years of experience and lessons for empirical antibiotic therapy. J. Antimicrob. Chemother. 2011, 66, 1168–1173. [Google Scholar] [CrossRef] [PubMed]
- Geirsson, A.J.; Statkevicius, S.; Vikingsson, A. Septic arthritis in Iceland 1990–2002: Increasing incidence due to iatrogenic infections. Ann. Rheum. Dis. 2008, 67, 638–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coakley, G.; Mathews, C.; Field, M.; Jones, A.; Kingsley, G.; Walker, D.; Phillips, M.; Bradish, C.; McLachlan, A.; Mohammed, R.; et al. On behalf of the British Society for Rheumatology Standards, Guidelines and Audit Working Group. BSR & BHPR, BOA, RCGP and BSAC guidelines for management of the hot swollen joint in adults. Rheumatology 2006, 45, 1039–1041. [Google Scholar] [PubMed]
- Dubost, J.J.; Soubrier, M.; De Champs, C.; Ristori, J.M.; Bussiere, J.L.; Sauvezie, B. No changes in the distribution of organisms responsible for septic arthritis over a 20 year period. Ann. Rheum. Dis. 2002, 61, 267–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johns, B.P.; Loewenthal, M.R.; Dewar, D.C. Open compared with arthroscopic treatment of acute septic arthritis of the aative knee. J. Bone Joint Surg. Am. 2017, 99, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, V.; Logan, I.; Brian, E. Bourke Medical vs surgical treatment for the native joint in septic arthritis: A 6-year, single UK academic centre experience. Rheumatology 2009, 48, 1320–1322. [Google Scholar] [CrossRef] [PubMed]
- Gaskell, S.M. Electrospray: Principles and Practice. J. Mass Spectrom. 1997, 32, 677–688. [Google Scholar] [CrossRef]
- Nguyen, D.N.; Clasen, C.; Van den Mooter, G. Pharmaceutical applications of electrospraying. J. Pharm. Sci. 2016, 105, 2601–2620. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Wang, Y.; Wang, B.; Deng, J.; Liu, X.; Liu, J. Rapid preparation of pH-sensitive polymeric nanoparticle with high loading capacity using electrospray for oral drug delivery. Mater. Sci. Eng. C 2013, 33, 4562–4567. [Google Scholar] [CrossRef] [PubMed]
- Enayati, M.; Chang, M.-W.; Bragman, F.; Edirisinghe, M.; Stride, E. Electrohydrodynamic preparation of particles, capsules and bubbles for biomedical engineering applications. Colloids Surf. A Physicochem. Eng. Asp. 2011, 382, 154–164. [Google Scholar] [CrossRef]
- Bock, N.; Woodruff, M.A.; Hutmacher, D.W.; Dargaville, T.R. Electrospraying, a reproducible method for production of polymeric microspheres for biomedical applications. Polymers 2011, 3, 131–149. [Google Scholar] [CrossRef] [Green Version]
- Prabhakaran, M.P.; Zamani, M.; Felice, B.; Ramakrishna, S. Electrospraying technique for the fabrication of metronidazole contained PLGA particles and their release profile. Mater. Sci. Eng. C 2015, 56, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Wang, Y.; Wang, B.; Deng, J.; Zhu, L.; Cao, Y. Formulation of porous poly(lactic-co-glycolic acid) microparticles by electrospray deposition method for controlled drug release. Mater. Sci. Eng. C 2014, 39, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Songsurang, K.; Praphairaksit, N.; Siraleartmukul, K.; Muangsin, N. Electrospray fabrication of doxorubicin-chitosan-tripolyphosphate nanoparticles for delivery of doxorubicin. Arch. Pharm. Res. 2011, 34, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.A.; Ng, W.H.; Bowen, J.; Tang, J.; Gomez, A.; Kenyon, A.J.; Richard, M.; Day, R.M. Electrospray synthesis and properties of hierarchically structured PLGA TIPS microspheres for use as controlled release technologies. J. Colloid Interface Sci. 2016, 467, 220–229. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Wang, S.; Lei, L.; Hou, Z.; Shang, P.; He, X.; Nie, H. Core-shell particles for controllable release of drug. Chem. Eng. Sci. 2015, 125, 108–120. [Google Scholar] [CrossRef]
- Davoodi, P.; Feng, F.; Xua, Q.; Yan, W.-C.; Tong, Y.W.; Srinivasan, M.P.; Sharma, V.K.; Wang, C.-H. Coaxial electrohydrodynamic atomization: Microparticles for drug delivery applications. J. Control. Release 2015, 205, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Luo, J.; Tu, K.; Wang, L.-Q.; Jianga, H. Generation of nano-sized core–shell particles using a coaxialtri-capillary electrospray-template removal method. Colloids Surf. B Biointerfaces 2014, 115, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhao, D.; Chang, M.-W.; Ahmad, Z.; Li, X.; Suo, H.; Li, J.-S. Morphology control of electrosprayed core-shell particles via collection media variation. Mater. Lett. 2015, 146, 59–64. [Google Scholar] [CrossRef]
- Kim, W.; Kim, S.S. Synthesis of biodegradable triple-layered capsules using a triaxial electrospray method. Polymer 2011, 52, 3325–3336. [Google Scholar] [CrossRef]
- Amass, W.; Amass, A.; Tighe, B. A review of biodegradable polymers: Uses, current developments in the synthesis and characterization of biodegradable polyesters, blends of biodegradable polymers and recent advances in biodegradation studies. Polym. Int. 1998, 47, 89–144. [Google Scholar] [CrossRef]
- Doppalapudi, S.; Jain, A.; Khan, W.; Domb, A.J. Biodegradable polymers-an overview. Polym. Adv. Technol. 2014, 25, 427–435. [Google Scholar] [CrossRef]
- Chou, Y.C.; Cheng, Y.S.; Hsu, Y.H.; Yu, Y.H.; Liu, S.J. A bio-artificial poly([d,l]-lactide-co-glycolide) drug-eluting nanofibrous periosteum for segmental long bone open fractures with significant periosteal stripping injuries: In vitro and in vivo studies. Int. J. Nanomed. 2016, 11, 941–953. [Google Scholar] [CrossRef] [PubMed]
- Fraceto, L.F.; de Matos Alves, P.L.; Franzoni, L.; Braga, A.A.; Spisni, A.; Schreier, S.; de Paula, E. Spectroscopic evidence for a preferential location of lidocaine inside phospholipid bilayers. Biophys. Chem. 2002, 99, 229–243. [Google Scholar] [CrossRef]
- Zarif, M.S.; Afidah, A.R.; Abdullah, J.M.; Shariza, A.R. Physicochemical characterization of vancomycin and its complexes with β-cyclodextrin. Biomed. Res. 2012, 23, 513–520. [Google Scholar]
- Moreno, A.D.H.; Salgado, H.R.N. Development and validation of the quantitative analysis of ceftazidime in powder for injection by infrared spectroscopy. Phys. Chem. 2012, 2, 6–11. [Google Scholar] [CrossRef]
- Schulz, M.; Schmoldt, A. Therapeutic and toxic blood concentrations of more than 800 drugs and other xenobiotics. Pharmazie 2003, 58, 447–474. [Google Scholar] [PubMed]
- Mader, J.T.; Calhoun, J.; Cobos, J. In vitro evaluation of antibiotic diffusion from antibiotic-impregnated biodegradable beads and polymethylmethacrylate beads. Antimicrob. Agents Chemother. 1997, 41, 415–418. [Google Scholar] [PubMed]
- Hsu, Y.H.; Hu, C.C.; Hsieh, P.H.; Shih, H.N.; Ueng, S.W.N.; Chang, Y. Vancomycin and ceftazidime in bone cement as a potentially effective treatment for knee periprosthetic Joint Infection. J. Bone Joint Surg. Am. 2017, 99, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Middleton, J.C.; Tipton, A.J. Synthetic biodegradable polymers as orthopedic devices. Biomaterials 2000, 21, 2335–2346. [Google Scholar] [CrossRef]
- Springer, B.D.; Lee, G.C.; Osmon, D.; Haidukewych, G.J.; Hanssen, A.D.; Jacofsky, D.J. Systemic safety of high-dose antibiotic-loaded cement spacers after resection of an infected total knee arthroplasty. Clin. Orthop. Relat. Res. 2004, 427, 47–51. [Google Scholar] [CrossRef]
- Hsu, Y.H.; Chen, D.W.; Tai, C.D.; Chou, Y.C.; Liu, S.J.; Ueng, S.W.N.; Chan, E.C. Biodegradable drug-eluting nanofiber-enveloped implants for sustained release of high bactericidal concentrations of vancomycin and ceftazidime: In vitro and in vivo studies. Int. J. Nanomed. 2014, 9, 4347–4355. [Google Scholar] [CrossRef] [PubMed]











© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, Y.-H.; Chen, D.W.-C.; Li, M.-J.; Yu, Y.-H.; Chou, Y.-C.; Liu, S.-J. Sustained Delivery of Analgesic and Antimicrobial Agents to Knee Joint by Direct Injections of Electrosprayed Multipharmaceutical-Loaded Nano/Microparticles. Polymers 2018, 10, 890. https://doi.org/10.3390/polym10080890
Hsu Y-H, Chen DW-C, Li M-J, Yu Y-H, Chou Y-C, Liu S-J. Sustained Delivery of Analgesic and Antimicrobial Agents to Knee Joint by Direct Injections of Electrosprayed Multipharmaceutical-Loaded Nano/Microparticles. Polymers. 2018; 10(8):890. https://doi.org/10.3390/polym10080890
Chicago/Turabian StyleHsu, Yung-Heng, Dave Wei-Chih Chen, Min-Jhan Li, Yi-Hsun Yu, Ying-Chao Chou, and Shih-Jung Liu. 2018. "Sustained Delivery of Analgesic and Antimicrobial Agents to Knee Joint by Direct Injections of Electrosprayed Multipharmaceutical-Loaded Nano/Microparticles" Polymers 10, no. 8: 890. https://doi.org/10.3390/polym10080890