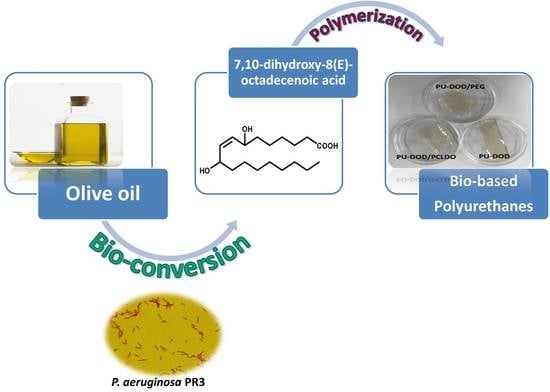
Microbial Conversion of Vegetable Oil to Hydroxy Fatty Acid and Its Application to Bio-Based Polyurethane Synthesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Media and Cultivation Conditions
2.3. Production of DOD from Olive Oil
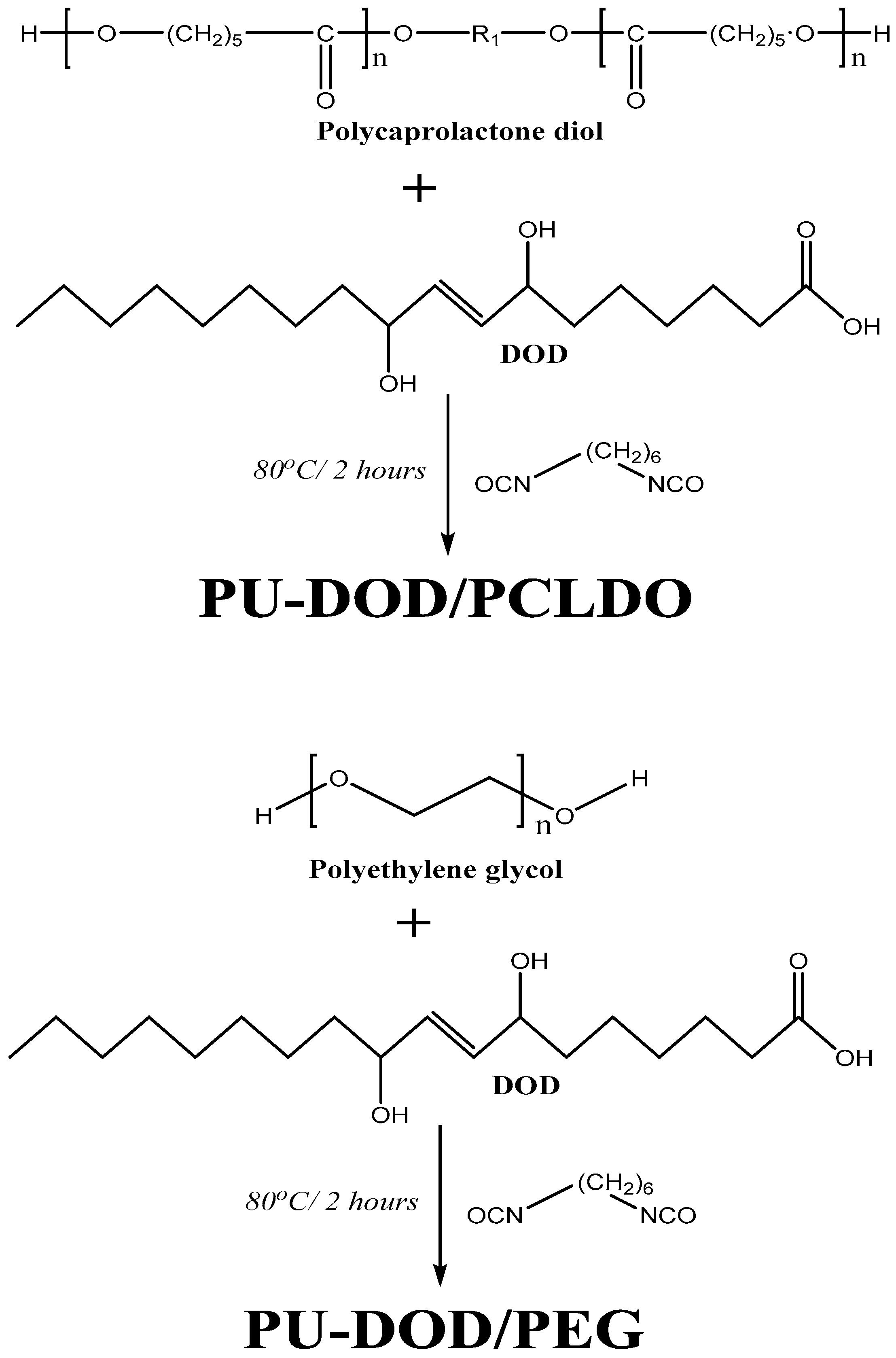
2.4. Preparation of PUs Based on DOD
2.5. Preparation of PUs Based on DOD and PEG or PCLDO
2.6. Analytical Methods
2.6.1. GC/MS
2.6.2. FTIR
2.6.3. DSC and TGA
2.6.4. Tensile Properties
3. Results and Discussion
3.1. Production of DOD from Olive Oil
3.2. Synthesis of PUs from DOD (PU-DOD)
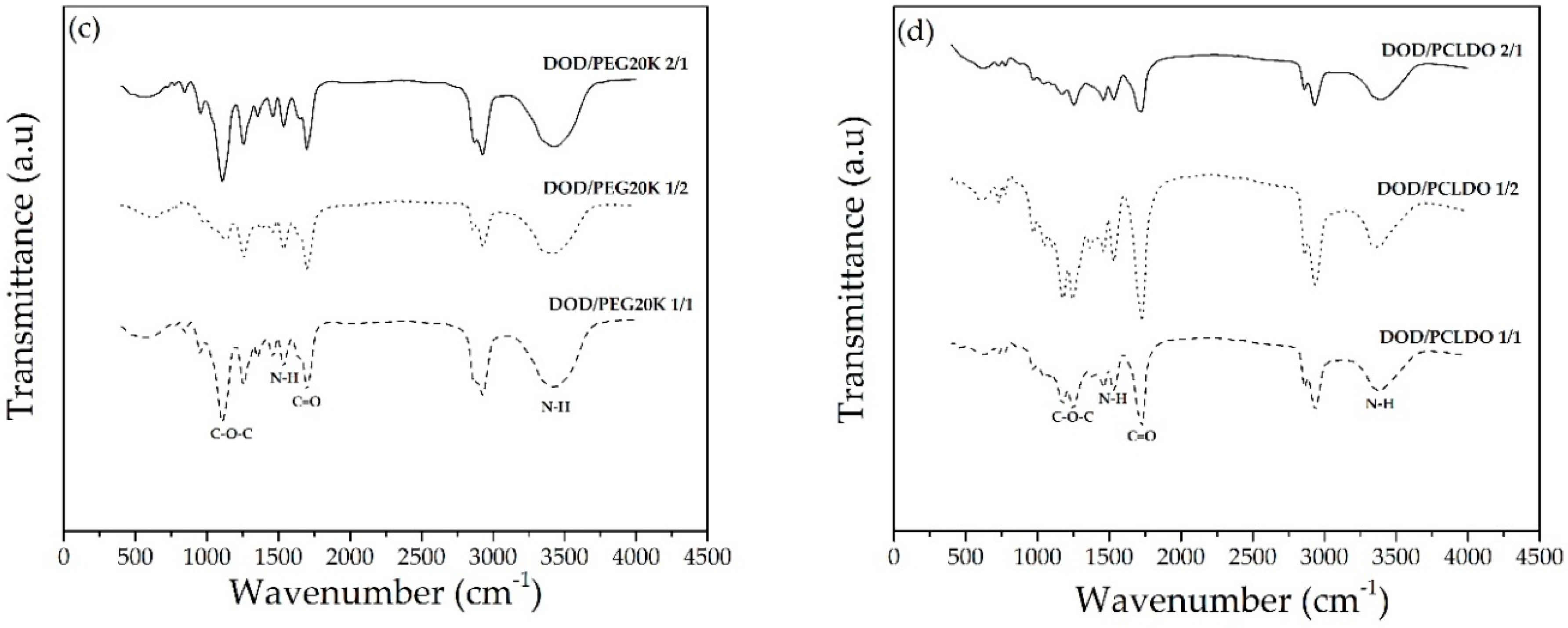
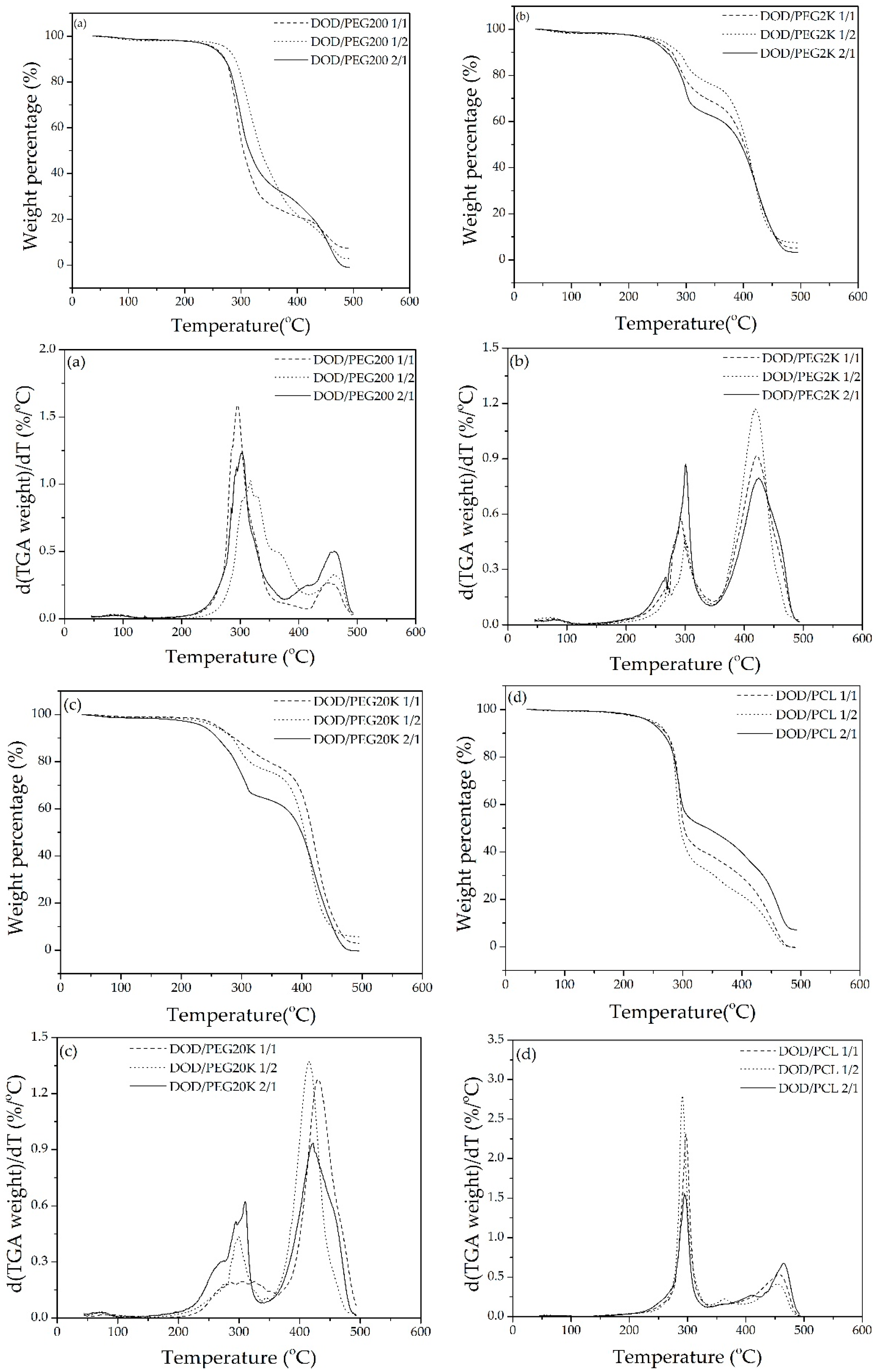
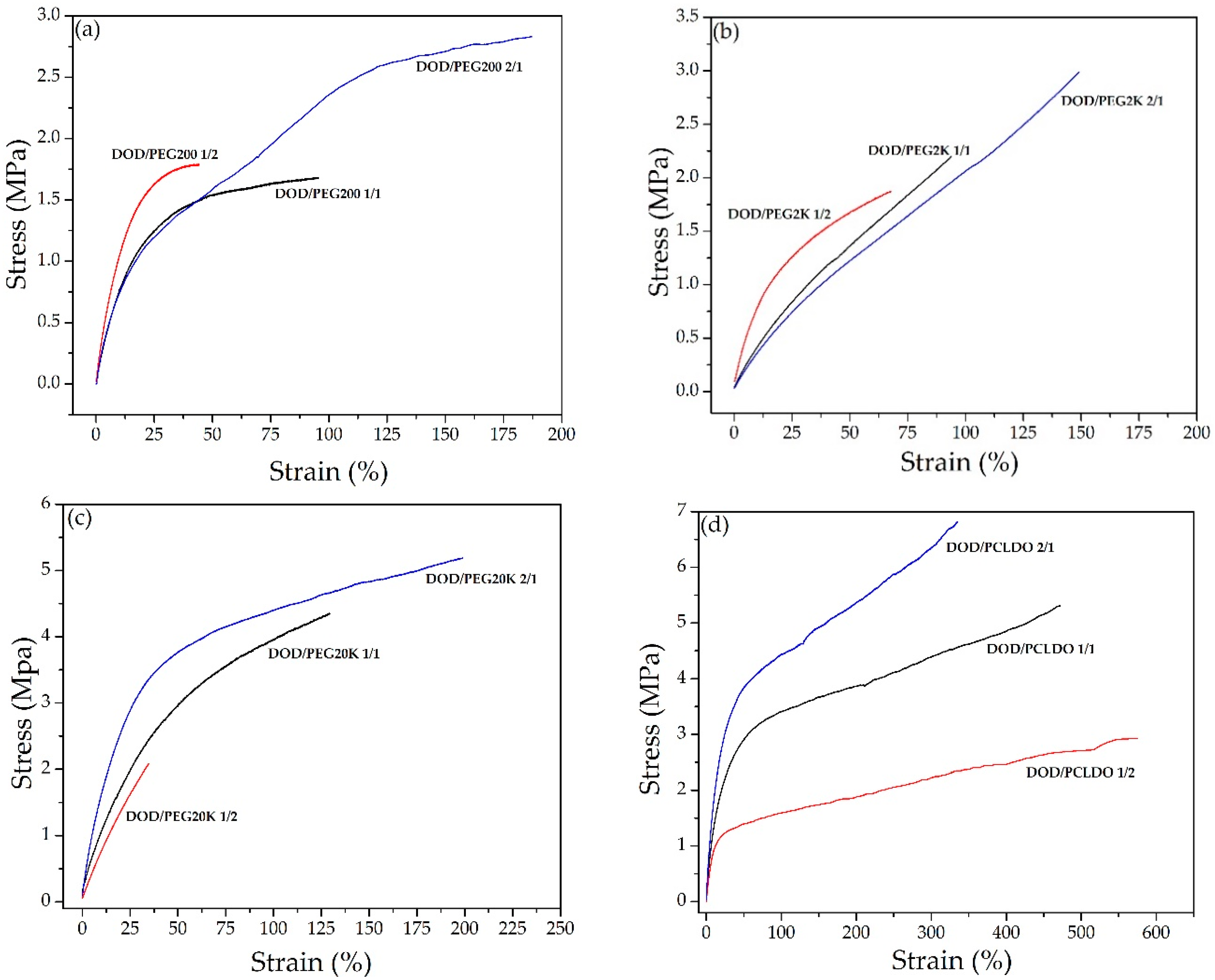
3.3. Synthesis of PUs from DOD and PEG or PCLDO (PU-DOD/PEG or PU-DOD/PCLDO)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Islam, M.R.; Jeyaratnam, N.; Yuvaraj, A.R. Polyurethane types, synthesis and applications—A review. RSC Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef]
- Ding, H.; Xia, C.; Wang, J.; Wang, C.; Chu, F. Inherently flame-retardant flexible bio-based polyurethane sealant with phosphorus and nitrogen-containing polyurethane prepolymer. J. Mater. Sci. 2016, 51, 5008–5018. [Google Scholar] [CrossRef]
- Hu, S.; Wan, C.; Li, Y. Production and characterization of biopolyols and polyurethane foams from crude glycerol based liquefaction of soybean straw. Bioresour. Technol. 2012, 103, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Abraham, T.; Ference, D.; Macosko, C.W. Rigid polyurethane foams from a soybean oil-based Polyol. Polymer 2011, 52, 2840–2846. [Google Scholar] [CrossRef]
- Wu, C.; Huang, X.; Wang, G.; Wu, X.; Yang, K.; Li, S.; Jiang, P. Hyperbranched-polymer functionalization of graphene sheets for enhanced mechanical and dielectric properties of polyurethane composites. J. Mater. Chem. 2012, 22, 7010–7019. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, R.; Moon, K.S.; Liu, Y.; Hansen, K.; Le, T.; Wong, C.P. Highly conductive, flexible, polyurethane-based adhesives for flexible and printed electronics. Adv. Funct. Mater. 2013, 23, 1459–1465. [Google Scholar] [CrossRef]
- Pfister, D.P.; Xia, Y.; Larock, R.C. Recent advances in vegetable oil-based polyurethanes. Chem. Sus. Chem. 2011, 4, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Miao, S.; Zhang, S.; Su, Z.; Wang, P. A novel vegetable oil-lactate hybrid monomer for synthesis of high-Tg polyurethanes. J. Polym. Sci. Pol. Chem. 2010, 48, 243–250. [Google Scholar] [CrossRef]
- Hojabri, L.; Kong, X.; Narine, S.S. Fatty acid-derived diisocyanate and bio-based polyurethane produced from vegetable oil: Synthesis, polymerization, and characterization. Biomacromolecules 2009, 10, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Miao, S.; Zhang, S.; Su, Z.; Wang, P. Synthesis of bio-based polyurethanes from epoxidized soybean oil and isopropanolamine. J. Appl. Polym. Sci. 2013, 127, 1929–1936. [Google Scholar] [CrossRef]
- Feng, Y.; Liang, H.; Yang, Z.; Yuan, T.; Luo, Y.; Li, P.; Yang, Z.; Zhang, C. A solvent-free and scalable method to prepare soybean-oil-based polyols by thiol-ene photo-click reaction and bio-based polyurethanes therefrom. ACS Sustain. Chem. Eng. 2017, 5, 7365–7373. [Google Scholar] [CrossRef]
- Zhang, C.; Ding, R.; Kessler, M.R. Reduction of epoxidized vegetable oils: A novel method to prepare bio-based polyols for polyurethanes. Macromol. Rapid Commun. 2014, 35, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, Y.; Chen, R.; Kessler, M.R. Polyurethanes from solvent-free vegetable oil-based polyols. ACS Sustain. Chem. Eng. 2014, 2, 2465–2476. [Google Scholar] [CrossRef]
- Lligadas, G.; Ronda, J.C.; Galià, M.; Cádiz, V. Renewable polymeric materials from vegetable oils: A perspective. Mater. Today 2013, 16, 337–343. [Google Scholar] [CrossRef]
- Hou, C.T.; Brown, W.; Labeda, D.P.; Abbott, T.P.; Weisleder, D. Microbial production of a novel trihydroxy unsaturated fatty acid from linoleic acid. J. Ind. Microbiol. Biotechnol. 1997, 19, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Gardner, H.W.; Hou, C.T. 10(S)-Hydroxy-8(E)-octadecenoic acid, an intermediate in the conversion of oleic acid to 7,10-dihydroxy-8(E)-octadecenoic acid. J. Am. Oil Chem. Soc. 2000, 77, 95–99. [Google Scholar] [CrossRef]
- Kim, H.; Gardner, H.W.; Hou, C.T. Production of isomeric 9,10,13 (9,12,13)–trihydroxy–11E (10E)–octadecenoic acid from linoleic acid by Pseudomonas aeruginosa PR3. J. Ind. Microbiol. Biotechnol. 2000, 25, 109–115. [Google Scholar] [CrossRef]
- Kuo, T.M.; Manthey, L.K.; Hou, C.T. Fatty acid bioconversions by Pseudomonas aeruginosa PR3. J. Am. Oil Chem. Soc. 1998, 75, 875–879. [Google Scholar] [CrossRef]
- Bae, J.-H.; Suh, M.-J.; Lee, N.-Y.; Hou, C.T.; Kim, H.-R. Production of a value-added hydroxy fatty acid, 7,10-dihydroxy-8(E)-octadecenoic acid, from high oleic safflower oil by Pseudomonas aeruginosa PR3. Biotechnol. Bioprocess Eng. 2010, 15, 953–958. [Google Scholar] [CrossRef]
- Chang, I.-A.; Kim, I.-H.; Kang, S.-C.; Hou, C.T.; Kim, H.-R. Production of 7, 10-dihydroxy-8(E)-octadecenoic acid from triolein via lipase induction by Pseudomonas aeruginosa PR3. Appl. Microbiol. Biotechnol. 2007, 74, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.M.; Ray, K.J.; Manthey, L.K. A facile reactor process for producing 7,10-dihydroxy-8(E)-octadecenoic acid from oleic acid conversion by Pseudomonas aeruginosa. Biotechnol. Lett. 2003, 25, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.T.; Bagby, M.O. Production of a new compound, 7,10-dihydroxy-8-(E)-octadecenoic acid from oleic acid by Pseudomonas sp. PR3. J. Ind. Microbiol. 1991, 7, 123–129. [Google Scholar] [CrossRef]
- Suh, M.-J.; Baek, K.-Y.; Kim, B.-S.; Hou, C.T.; Kim, H.-R. Production of 7,10-dihydroxy-8(E)-octadecenoic acid from olive oil by Pseudomonas aeruginosa PR3. Appl. Microbiol. Biotechnol. 2011, 89, 1721–1727. [Google Scholar] [CrossRef] [PubMed]
- Sohn, H.-R.; Hou, C.T.; Soo Kim, B.; Kim, H.-R. Evaluation of environmental parameters for production of 7, 10-dihydroxy-8(E)-octadecenoic acid from olive oil by Pseudomonas aeruginosa PR3. Biocatal. Agric. Biotechnol. 2013, 2, 352–356. [Google Scholar] [CrossRef]
- Kumar, P.; Jun, H.-B.; Kim, B.S. Co-production of polyhydroxyalkanoates and carotenoids through bioconversion of glycerol by Paracoccus sp. strain LL1. Int. J. Biol. Macromol. 2018, 107, 2552–2558. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-M.; Kim, H.-R.; Hou, C.T.; Kim, B.S. Biodegradable photo-crosslinked thin polymer networks based on vegetable oil hydroxy fatty acids. J. Am. Oil Chem. Soc. 2010, 87, 1451–1459. [Google Scholar] [CrossRef]
- Kim, H.-M.; Kim, H.-R.; Kim, B.S. Soybean oil-based photo-crosslinked polymer networks. J. Polym. Environ. 2010, 18, 291–297. [Google Scholar] [CrossRef]
- Ellamar, J.B.; Dasangrandhi, C.; Kim, Y.S.; Kim, I.H.; Kim, H.R. Microbial bioconversion of natural Philippine nut oils into a value-added hydroxy fatty acid, 7, 10-dihydroxy-8(E)-octadecenoic acid. Korean J. Food Sci. Technol. 2017, 49, 28–34. [Google Scholar]
- Leventis, N.; Chidambareswarapattar, C.; Mohite, D.P.; Larimore, Z.J.; Lu, H.; Sotiriou-Leventis, C. Multifunctional porous aramids (aerogels) by efficient reaction of carboxylic acids and isocyanates. J. Mater. Chem. 2011, 21, 11981–11986. [Google Scholar] [CrossRef]
- Semsarzadeh, M.A.; Navarchian, A.H. Effects of NCO/OH ratio and catalyst concentration on structure, thermal stability, and crosslink density of poly(urethane-isocyanurate). J. Appl. Polym. Sci. 2003, 90, 963–972. [Google Scholar] [CrossRef]
- Corcuera, M.A.; Rueda, L.; Fernandez d’Arlas, B.; Arbelaiz, A.; Marieta, C.; Mondragon, I.; Eceiza, A. Microstructure and properties of polyurethanes derived from castor oil. Polym. Degrad. Stab. 2010, 95, 2175–2184. [Google Scholar] [CrossRef]
- Hablot, E.; Zheng, D.; Bouquey, M.; Avérous, L. Polyurethanes based on castor oil: Kinetics, chemical, mechanical and thermal properties. Macromol. Mater. Eng. 2008, 293, 922–929. [Google Scholar] [CrossRef]
- Wang, T.-L.; Hsieh, T.-H. Effect of polyol structure and molecular weight on the thermal stability of segmented poly(urethaneureas). Polym. Degrad. Stab. 1997, 55, 95–102. [Google Scholar] [CrossRef]


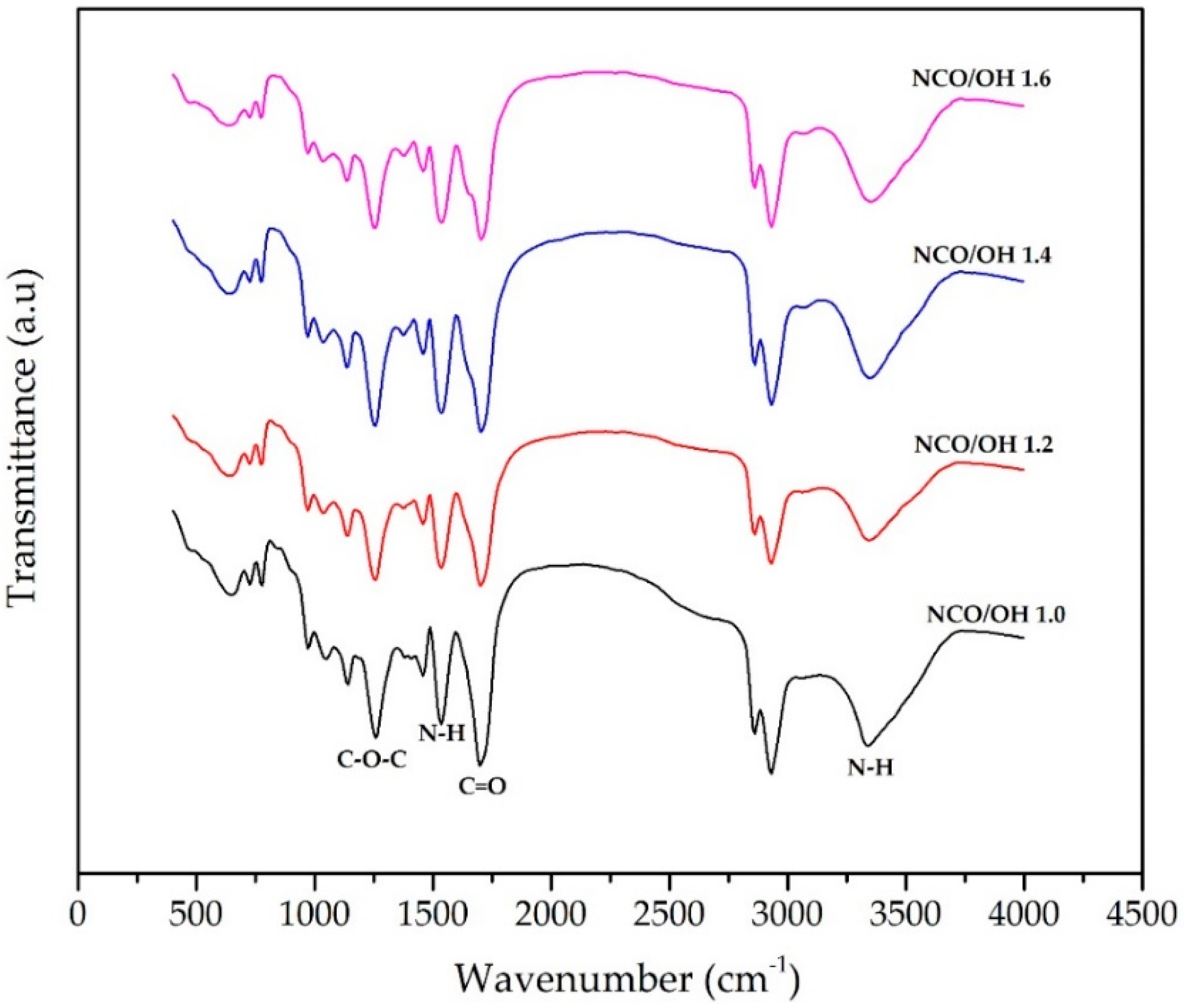



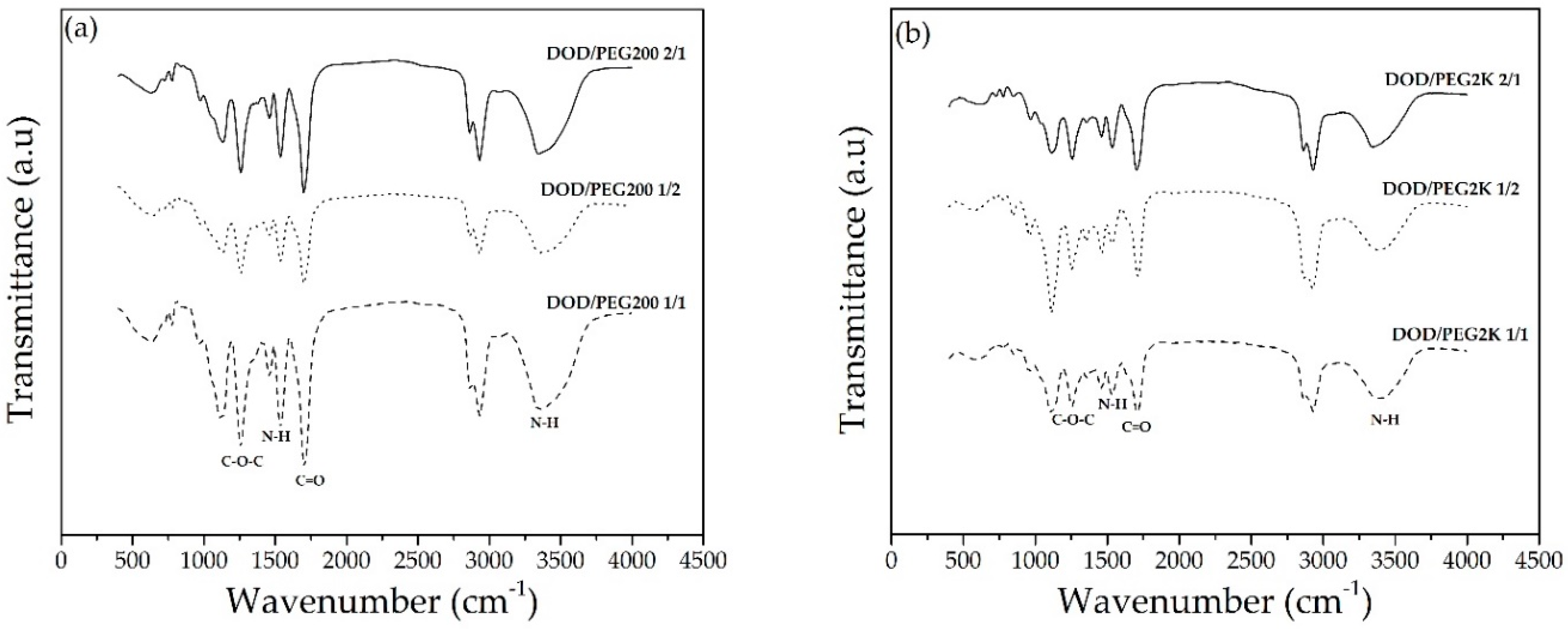
| NCO/OH Ratio | Tg (°C) | TGA in Nitrogen (°C) | Elongation at Break (%) | Tensile Strength (MPa) | ||
|---|---|---|---|---|---|---|
| T10% | T50% | Tmax (first/second) | ||||
| 1.0 | 11.3 | 248 | 398 | 298/458 | 85.6 ± 3.77 | 10.1 ± 1.58 |
| 1.2 | 12.0 | 260 | 407 | 298/452 | 60.8 ± 1.12 | 10.3 ± 0.14 |
| 1.4 | 13.6 | 262 | 414 | 304/463 | 59.3 ± 4.01 | 37.9 ± 4.40 |
| 1.6 | 12.3 | 272 | 400 | 315/461 | 31.2 ± 1.12 | 16.9 ± 1.36 |
| Weight Ratio of DOD to PEG or PCLDO | TGA in Nitrogen (°C) | Elongation at Break (%) | Tensile Strength (MPa) | ||
|---|---|---|---|---|---|
| T10% | T50% | Tmax (first/second) | |||
| PU-DOD/PEG200 | |||||
| 2/1 | 266 | 313 | 303/461 | 187 ± 5.22 | 2.83 ± 0.19 |
| 1/1 | 272 | 304 | 296/449 | 95.4 ± 9.74 | 1.68 ± 0.12 |
| 1/2 | 285 | 336 | 318/460 | 45.5 ± 3.59 | 1.79 ± 0.23 |
| PU-DOD/PEG2K | |||||
| 2/1 | 270 | 395 | 301/425 | 149 ± 5.06 | 2.99 ± 0.15 |
| 1/1 | 276 | 401 | 293/421 | 94.0 ± 5.07 | 2.21 ± 0.28 |
| 1/2 | 287 | 405 | 299/419 | 68.9 ± 5.92 | 1.89 ± 0.38 |
| PU-DOD/PEG20K | |||||
| 2/1 | 273 | 401 | 310/422 | 200 ± 1.80 | 5.18 ± 0.82 |
| 1/1 | 285 | 417 | 299/425 | 129 ± 3.82 | 4.38 ± 0.33 |
| 1/2 | 290 | 409 | 300/415 | 34.7 ± 0.68 | 2.11 ± 0.18 |
| PU-DOD/PCLDO | |||||
| 2/1 | 266 | 342 | 295/465 | 335 ± 5.22 | 6.83 ± 0.24 |
| 1/1 | 272 | 302 | 297/457 | 472 ± 10.30 | 5.32 ± 0.68 |
| 1/2 | 273 | 296 | 291/453 | 576 ± 13.30 | 2.92 ± 0.41 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, T.K.; Kumar, P.; Kim, H.-R.; Hou, C.T.; Kim, B.S. Microbial Conversion of Vegetable Oil to Hydroxy Fatty Acid and Its Application to Bio-Based Polyurethane Synthesis. Polymers 2018, 10, 927. https://doi.org/10.3390/polym10080927
Tran TK, Kumar P, Kim H-R, Hou CT, Kim BS. Microbial Conversion of Vegetable Oil to Hydroxy Fatty Acid and Its Application to Bio-Based Polyurethane Synthesis. Polymers. 2018; 10(8):927. https://doi.org/10.3390/polym10080927
Chicago/Turabian StyleTran, Tuan Kiet, Prasun Kumar, Hak-Ryul Kim, Ching T. Hou, and Beom Soo Kim. 2018. "Microbial Conversion of Vegetable Oil to Hydroxy Fatty Acid and Its Application to Bio-Based Polyurethane Synthesis" Polymers 10, no. 8: 927. https://doi.org/10.3390/polym10080927