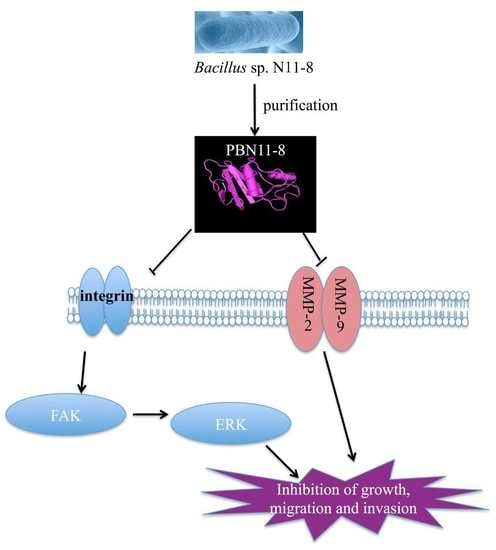
PBN11-8, a Cytotoxic Polypeptide Purified from Marine Bacillus, Suppresses Invasion and Migration of Human Hepatocellular Carcinoma Cells by Targeting Focal Adhesion Kinase Pathways
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Materials
2.3. Microorganism and Fermentation
2.4. Purification and Identification of Polypeptide
2.5. Cloning, Sequence Analysis of PBN11-8
2.6. Measurement of Protease Activity
2.7. Cytotoxic Activity Assay
2.8. Crystal Violet Adhesion Assay
2.9. Migration and Invasion Assay on BEL-7402 Cells
2.10. Immunoblotting Assay
2.11. Quantitative Real-Time Reverse Transcription Polymerase Chain Reaction (qRT-PCR) Assay
- GAPDH forward: 5′-AAGTTCAACGGCACAGTCAAGG-3′,
- GAPDH reverse: 5′-CATACTCAGCACCAGCATCACC-3′;
- Integrin β1forward: 5′-TTCGATGCCATCATGCAAGTTG-3′,
- Integrin β1 reverse: 5′-CCATCTCCAGCAAAGTGAAACC-3′,
- FAK-forward: 5′-ACTCATCGAGAGATCGAGATGG-3′,
- FAK reverse: 5′-GCCCTAGCATTTTCAGTCTTGC-3′.
2.12. Statistical Analysis
3. Results
3.1. Preparation of Crude Extract
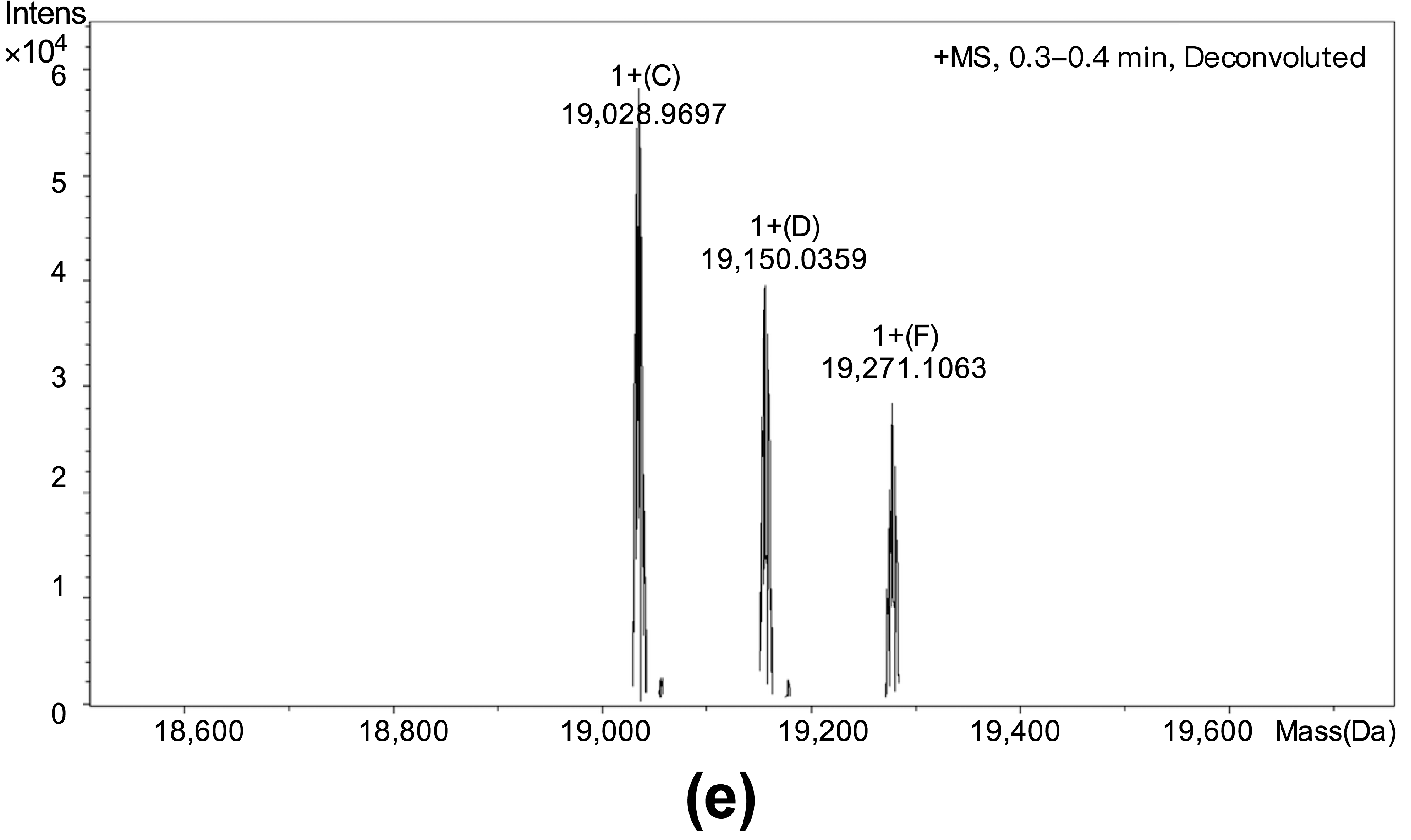
3.2. Purification and Identification of Cytotoxic Polypeptide
3.3. Cloning, Sequence Analysis and the Protease Activity of Polypeptide PBN11-8
3.4. PBN11-8 Displayed Cytotoxicity toward Different Cells
3.5. PBN11-8 Affects the Migration and Invasion of BEL-7402 Cells
3.6. PBN11-8 Inhibited the Activation of FAK in BEL-7402 Cells
3.7. PBN11-8 Inhibited the Activation of ERK in BEL-7402 Cells
3.8. Expression of MMP-2 and MMP-9 Was Reduced by PBN11-8 in BEL-7402 Cells
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Wu, Y.-H.; Yu, P.-S.; Zhou, Y.-D.; Xu, L.; Wang, C.-S.; Wu, M.; Oren, A.; Xu, X.-W. Muricauda antarctica sp. nov., a marine member of the Flavobacteriaceae isolated from Antarctic seawater. Int. J. Syst. Evol. Microbiol. 2013, 63, 3451–3456. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Chen, B.; Zou, Y.; Zheng, T. Polar microorganisms, a potential source for new natural medicines—A review. Wei Sheng Wu Xue Bao 2008, 48, 695–700. [Google Scholar] [PubMed]
- Xu, Y.; Kersten, R.D.; Nam, S.-J.; Lu, L.; Al-Suwailem, A.M.; Zheng, H.; Fenical, W.; Dorrestein, P.C.; Moore, B.S.; Qian, P.-Y. Bacterial biosynthesis and maturation of the didemnin anti-cancer agents. J. Am. Chem. Soc. 2012, 134, 8625–8632. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Yi, Y.; Liu, J.; Lin, X.; Yang, K.; Lv, M.; Zhou, X.; Hao, J.; Liu, J.; Zheng, Y.; et al. Isolation and characterization of marine Brevibacillus sp. S-1 collected from South China Sea and a novel antitumor peptide produced by the strain. PLoS ONE 2014, 9, e111270. [Google Scholar] [CrossRef] [PubMed]
- Singh, Y.; Gundampati, R.K.; Jagannadham, M.V.; Srivastava, S.K. Extracellular l-asparaginase from a protease-deficient Bacillus aryabhattai ITBHU02: purification, biochemical characterization, and evaluation of antineoplastic activity in vitro. Appl. Biochem. Biotechnol. 2013, 171, 1759–1774. [Google Scholar] [CrossRef] [PubMed]
- Bauvois, B. New facets of matrix metalloproteinases MMP-2 and MMP-9 as cell surface transducers: outside-in signaling and relationship to tumor progression. Biochim. Biophys. Acta 2012, 1825, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.-L.; Jeng, L.-B.; Lai, H.-C.; Liao, P.-Y.; Chang, C. Androgen receptor enhances cell adhesion and decreases cell migration via modulating β1-integrin-AKT signaling in hepatocellular carcinoma cells. Cancer Lett. 2014, 351, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.K.; Schlaepfer, D.D. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr. Opin. Cell Biol. 2006, 18, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.-L.; Ko, B.-S.; Liu, T.-A.; Liang, S.-M.; Liu, C.-C.; Lu, Y.-J.; Tzean, S.-S.; Shen, T.-L.; Liou, J.-Y. Cordycepin suppresses integrin/FAK signaling and epithelial-mesenchymal transition in hepatocellular carcinoma. Anticancer. Agents Med. Chem. 2014, 14, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Eke, I.; Deuse, Y.; Hehlgans, S.; Gurtner, K.; Krause, M.; Baumann, M.; Shevchenko, A.; Sandfort, V.; Cordes, N. β(1)Integrin/FAK/cortactin signaling is essential for human head and neck cancer resistance to radiotherapy. J. Clin. Investig. 2012, 122, 1529–1540. [Google Scholar] [CrossRef] [PubMed]
- Saleem, S.; Li, J.; Yee, S.-P.; Fellows, G.F.; Goodyer, C.G.; Wang, R. β1 integrin/FAK/ERK signalling pathway is essential for human fetal islet cell differentiation and survival. J. Pathol. 2009, 219, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, V.; Harnois, C.; Demers, M.-J.; Thibodeau, S.; Laquerre, V.; Gauthier, R.; Vezina, A.; Noel, D.; Fujita, N.; Tsuruo, T.; et al. β1 integrin/Fak/Src signaling in intestinal epithelial crypt cell survival: integration of complex regulatory mechanisms. Apoptosis 2008, 13, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Yang, K.; Liu, J.; Sun, M.; Zhu, J.; Lv, M.; Kang, D.; Wang, W.; Xing, M.; Li, Z. Screening of microorganisms from Antarctic surface water and cytotoxicity metabolites from Antarctic microorganisms. Food Sci. Nutr. 2016, 4, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.D.; Joyce, J.A. Proteolytic networks in cancer. Trends Cell Biol. 2011, 21, 228–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christakopoulos, P.; Hatzinikolaou, D.G.; Fountoukidis, G.; Kekos, D.; Claeyssens, M.; Macris, B.J. Purification and mode of action of an alkali-resistant endo-1, 4-β-glucanase from Bacillus pumilus. Arch. Biochem. Biophys. 1999, 364, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Zhang, Y.; Liu, F. Purification and characterization of a approximately 43 kDa antioxidant protein with antitumor activity from Pholiota nameko. J. Sci. Food Agric. 2016, 96, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Dong, Y.; Chen, G.; Hu, Q. Extraction, purification, characterization and antitumor activity of polysaccharides from Ganoderma lucidum. Carbohydr. Polym. 2010, 80, 783–789. [Google Scholar] [CrossRef]
- Lv, S.; Gao, J.; Liu, T.; Zhu, J.; Xu, J.; Song, L.; Liang, J.; Yu, R. Purification and Partial Characterization of a New Antitumor Protein from Tegillarca granosa. Mar. Drugs 2015, 13, 1466–1480. [Google Scholar] [CrossRef] [PubMed]
- Montsko, G.; Tarjanyi, Z.; Mezosi, E.; Kovacs, G.L. A validated method for measurement of serum total, serum free, and salivary cortisol, using high-performance liquid chromatography coupled with high-resolution ESI-TOF mass spectrometry. Anal. Bioanal. Chem. 2014, 406, 2333–2341. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Ling, P.; Wang, Z.; Niu, R.; Hu, C.; Zhang, T.; Lin, X. A novel polypeptide from shark cartilage with potent anti-angiogenic activity. Cancer Biol. Ther. 2007, 6, 775–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luesch, H.; Yoshida, W.Y.; Moore, R.E.; Paul, V.J.; Corbett, T.H. Total structure determination of apratoxin A, a potent novel cytotoxin from the marine cyanobacterium Lyngbya majuscula. J. Am. Chem. Soc. 2001, 123, 5418–5423. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Luo, J.; Jiang, B.; Wang, L.; Wang, S.; Wang, C.; Fu, C.; Li, J.; Shi, D. Marine bromophenol bis (2,3-dibromo-4,5-dihydroxy-phenyl)-methane inhibits the proliferation, migration, and invasion of hepatocellular carcinoma cells via modulating β1-integrin/FAK signaling. Mar. Drugs 2015, 13, 1010–1025. [Google Scholar] [CrossRef] [PubMed]
- Oberholzer, A.E.; Bumann, M.; Hege, T.; Russo, S.; Baumann, U. Metzincin’s canonical methionine is responsible for the structural integrity of the zinc-binding site. Biol. Chem. 2009, 390, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-L.; Hung, J.-Y.; Liang, Y.-Y.; Lin, Y.-S.; Tsai, M.-J.; Chou, S.-H.; Lu, C.-Y.; Kuo, P.-L. S100P interacts with integrin α7 and increases cancer cell migration and invasion in lung cancer. Oncotarget 2015, 6, 29585–29598. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.Y.; Timpson, P.; Horvath, L.G.; Daly, R.J. FAK signaling in human cancer as a target for therapeutics. Pharmacol. Ther. 2015, 146, 132–149. [Google Scholar] [CrossRef] [PubMed]
- Sarode, S.C.; Sarode, G.S.; Choudhary, S.; Patil, S. FAK is overexpressed in keratocystic odontogenic tumor: A preliminary study. J. Oral. Pathol. Med. 2017, 46, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Rudakova, N.L.; Balaban, N.P.; Danilova, Y.V.; Rudenskaya, G.N.; Sharipova, M.R. Characteristics of a novel secreted zinc-dependent endopeptidase of Bacillus intermedius. Biochemistry 2010, 75, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Sabirova, A.R.; Rudakova, N.L.; Balaban, N.P.; Ilyinskaya, O.N.; Demidyuk, I.V.; Kostrov, S.V.; Rudenskaya, G.N.; Sharipova, M.R. A novel secreted metzincin metalloproteinase from Bacillus intermedius. FEBS Lett. 2010, 584, 4419–4425. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.-Q.; Liu, X.-F.; Yao, L.; Chen, C.-Q.; Lin, J.-F.; Gu, Z.-D.; Ni, P.-H.; Zheng, X.-M.; Fan, Q.-S. Focal adhesion kinase regulates the phosphorylation protein tyrosine phosphatase-alpha at Tyr789 in breast cancer cells. Mol. Med. Rep. 2015, 11, 4303–4308. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; O’Brien, L.E.; Kwon, S.-H.; Mostov, K.E. STAT1 is required for redifferentiation during Madin-Darby canine kidney tubulogenesis. Mol. Biol. Cell 2010, 21, 3926–3933. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hochwald, S.N. The role of FAK in tumor metabolism and therapy. Pharmacol. Ther. 2014, 142, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Montagut, C.; Settleman, J. Targeting the RAF-MEK-ERK pathway in cancer therapy. Cancer Lett. 2009, 283, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.-L.; Hsieh, M.-J.; Yang, J.-S.; Lin, C.-W.; Lue, K.-H.; Lu, K.-H.; Yang, S.-F. Nobiletin inhibits human osteosarcoma cells metastasis by blocking ERK and JNK-mediated MMPs expression. Oncotarget 2016, 7, 35208–35223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jablonska-Trypuc, A.; Matejczyk, M.; Rosochacki, S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J. Enzym. Inhib. Med. Chem. 2016, 31, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Lustosa, S.A.S.; de Viana, L.S.; Affonso, R.J.J.; Silva, S.R.M.; Denadai, M.V.A.; de Toledo, S.R.C.; Oliveira, I.D.; Matos, D. Expression profiling using a cDNA array and immunohistochemistry for the extracellular matrix genes FN-1, ITGA-3, ITGB-5, MMP-2, and MMP-9 in colorectal carcinoma progression and dissemination. Sci. World J. 2014, 2014, 102541. [Google Scholar] [CrossRef] [PubMed]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, L.; Zhu, X.; Yang, K.; Zhu, M.; Farooqi, A.A.; Kang, D.; Sun, M.; Xu, Y.; Lin, X.; Feng, Y.; et al. PBN11-8, a Cytotoxic Polypeptide Purified from Marine Bacillus, Suppresses Invasion and Migration of Human Hepatocellular Carcinoma Cells by Targeting Focal Adhesion Kinase Pathways. Polymers 2018, 10, 1043. https://doi.org/10.3390/polym10091043
Zheng L, Zhu X, Yang K, Zhu M, Farooqi AA, Kang D, Sun M, Xu Y, Lin X, Feng Y, et al. PBN11-8, a Cytotoxic Polypeptide Purified from Marine Bacillus, Suppresses Invasion and Migration of Human Hepatocellular Carcinoma Cells by Targeting Focal Adhesion Kinase Pathways. Polymers. 2018; 10(9):1043. https://doi.org/10.3390/polym10091043
Chicago/Turabian StyleZheng, Lanhong, Xiangjie Zhu, Kangli Yang, Meihong Zhu, Ammad Ahmad Farooqi, Daole Kang, Mi Sun, Yixin Xu, Xiukun Lin, Yingang Feng, and et al. 2018. "PBN11-8, a Cytotoxic Polypeptide Purified from Marine Bacillus, Suppresses Invasion and Migration of Human Hepatocellular Carcinoma Cells by Targeting Focal Adhesion Kinase Pathways" Polymers 10, no. 9: 1043. https://doi.org/10.3390/polym10091043