Synthesis and Characterization of In-Situ-Prepared Nanocomposites Based on Poly(Propylene 2,5-Furan Dicarboxylate) and Aluminosilicate Clays
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PPF Nanocomposites
2.3. Characterization
2.3.1. Intrinsic Viscosity
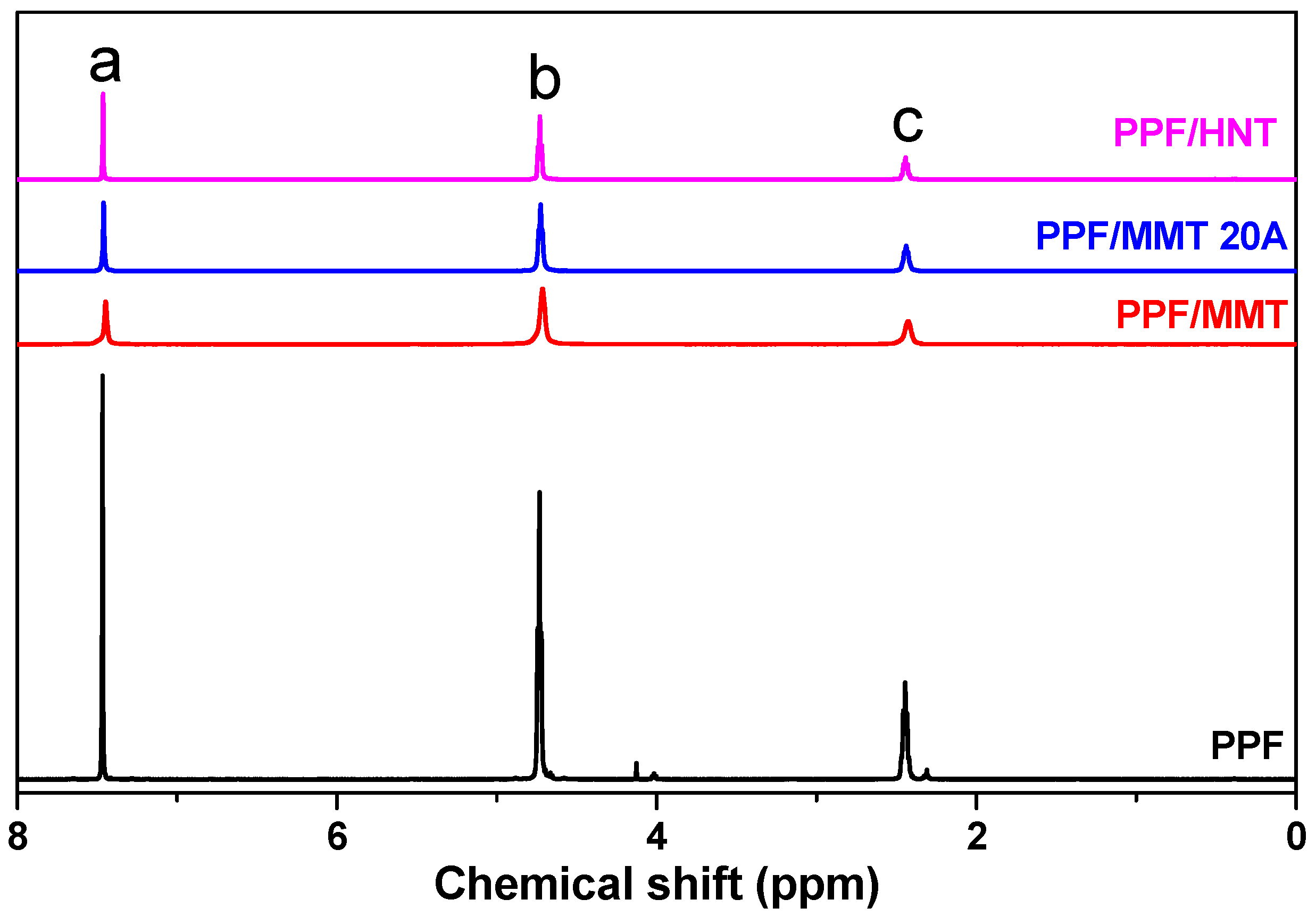
2.3.2. Nuclear Magnetic Resonance Spectroscopy (NMR)
2.3.3. Scanning Electron Microscopy (SEM)
2.3.4. Fourier-Transform Infrared Spectroscopy (FTIR)
2.3.5. X-ray Diffraction (XRD)
2.3.6. Differential Scanning Calorimetry (DSC)
2.3.7. Thermogravimetric Analysis (TGA)
2.3.8. Pyrolysis-Gas Chromatography/Mass Spectroscopy
3. Results and Discussion
3.1. Preparation of PPF Nanocomposites
3.2. Morphological and Structural Characterization
3.3. Crystallization Study
3.3.1. Isothermal Crystallization
3.3.2. Application of the Avrami Theory
3.3.3. Melting Behaviour
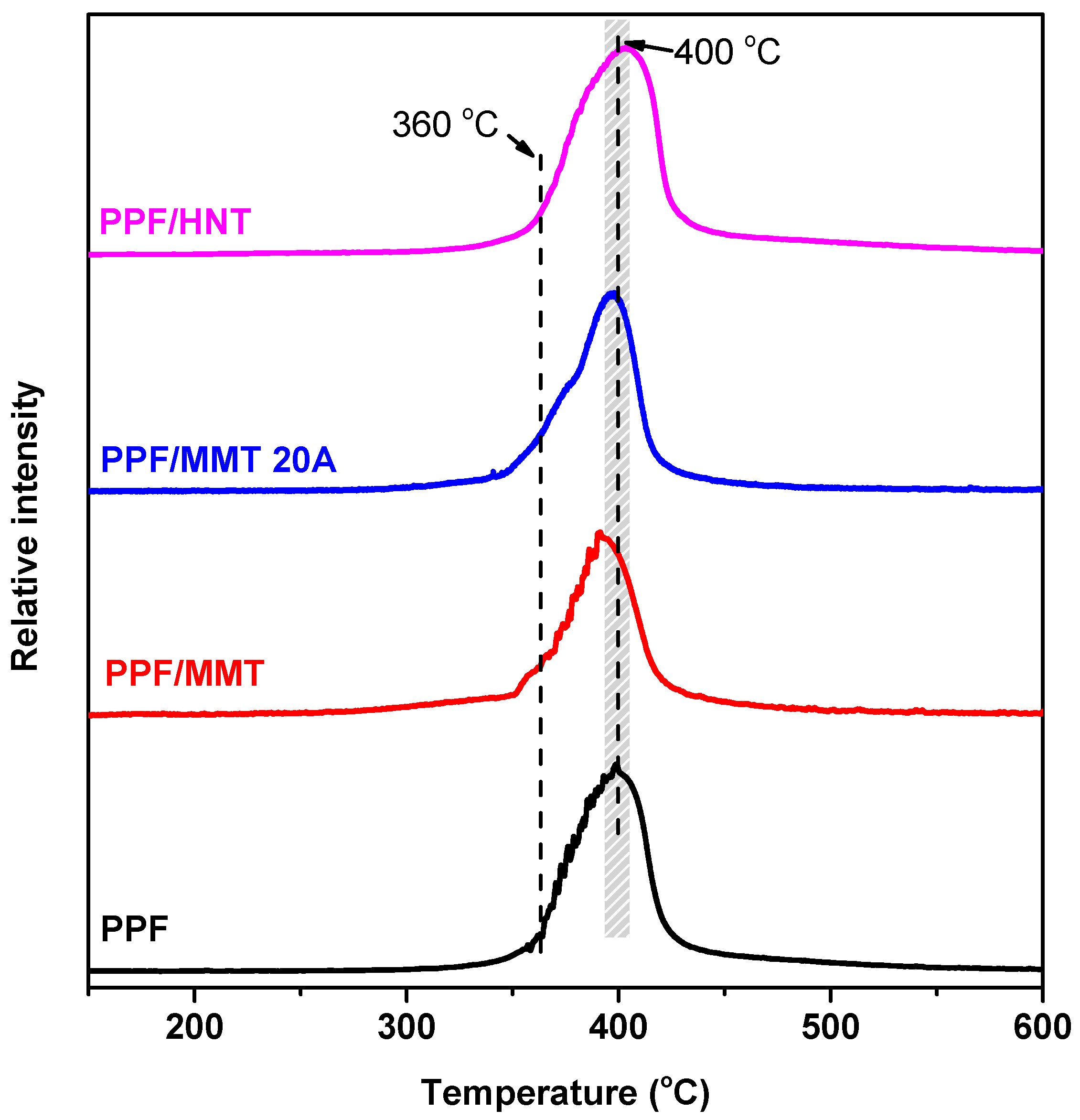
3.4. Thermal Degradation
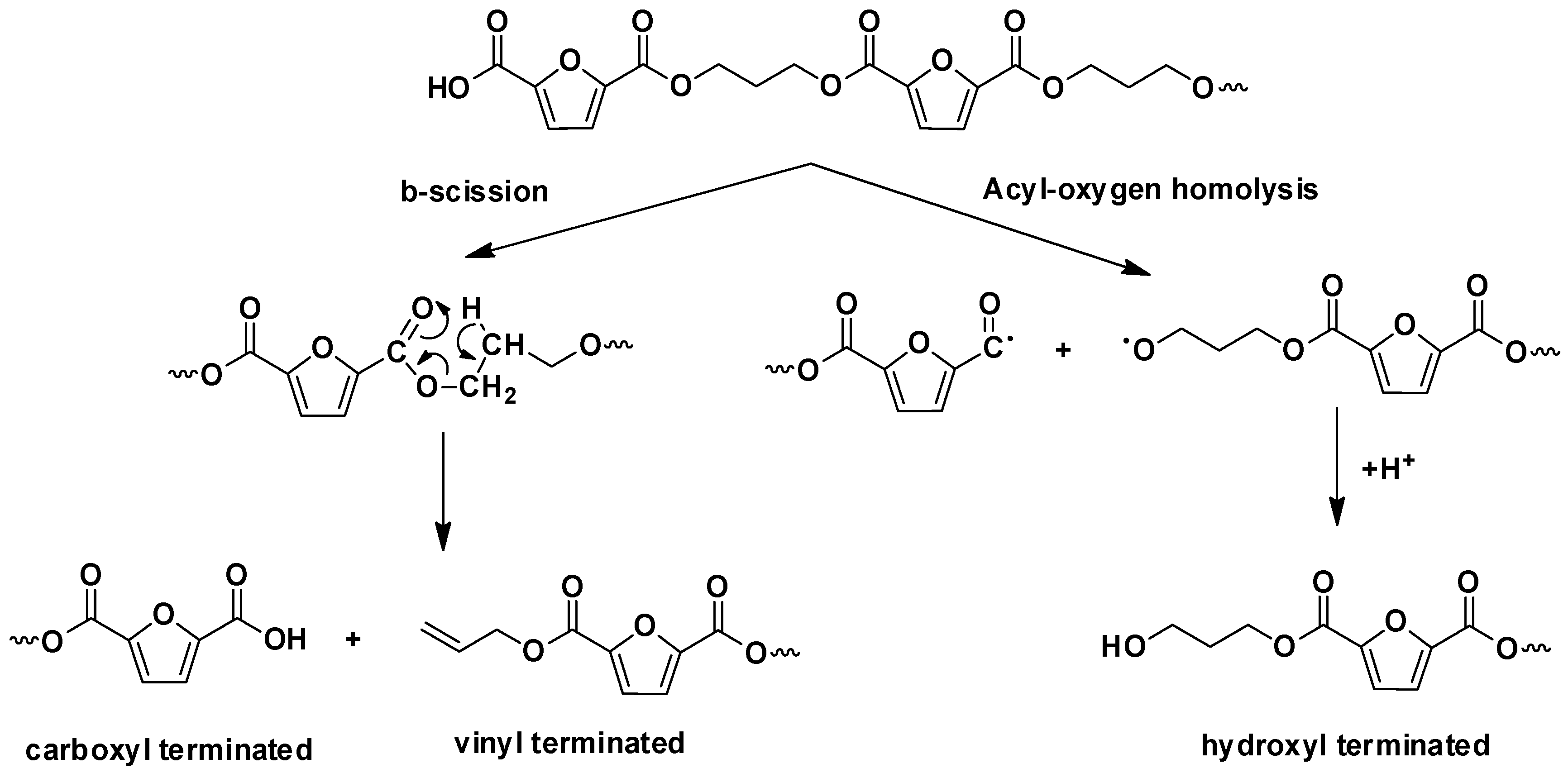
3.5. Thermal Degradation Mechanism Study by Py/GC-MS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sustainability and Circular Economy. Available online: http://ec.europa.eu/growth/industry/sustainability (accessed on 7 May 2018).
- What Is Biopreferred? Available online: https://www.biopreferred.gov/BioPreferred/faces/pages/AboutBioPreferred.xhtml (accessed on 7 May 2018).
- Bio-Based Products. Available online: http://ec.europa.eu/growth/sectors/biotechnology/bio-based-products_en (accessed on 7 May 2018).
- Terzopoulou, Z.; Karakatsianopoulou, E.; Kasmi, N.; Tsanaktsis, V.; Nikolaidis, N.; Kostoglou, M.; Papageorgiou, G.Z.; Lambropoulou, D.A.; Bikiaris, D.N. Effect of catalyst type on molecular weight increase and coloration of poly(ethylene furanoate) biobased polyester during melt polycondensation. Polym. Chem. 2017, 8. [Google Scholar] [CrossRef]
- Kasmi, N.; Papageorgiou, G.Z.; Achilias, D.S.; Bikiaris, D.N. Solid-State polymerization of poly(Ethylene Furanoate) biobased Polyester, II: An efficient and facile method to synthesize high molecular weight polyester appropriate for food packaging applications. Polymers 2018, 10, 471. [Google Scholar] [CrossRef]
- Kasmi, N.; Majdoub, M.; Papageorgiou, G.Z.; Achilias, D.S.; Bikiaris, D.N. Solid-state polymerization of poly(ethylene furanoate) biobased polyester, I: Effect of catalyst type on molecularweight increase. Polymers 2017, 9, 607. [Google Scholar] [CrossRef]
- Achilias, D.S.; Chondroyiannis, A.; Nerantzaki, M.; Adam, K.V.; Terzopoulou, Z.; Papageorgiou, G.Z.; Bikiaris, D.N. Solid State Polymerization of Poly(Ethylene Furanoate) and Its Nanocomposites with SiO2 and TiO2. Macromol. Mater. Eng. 2017, 302. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Papageorgiou, D.G.; Terzopoulou, Z.; Bikiaris, D.N. Production of bio-based 2,5-furan dicarboxylate polyesters: Recent progress and critical aspects in their synthesis and thermal properties. Eur. Polym. J. 2016, 83, 202–229. [Google Scholar] [CrossRef]
- Sousa, A.F.; Vilela, C.; Fonseca, A.C.; Matos, M.; Freire, C.S.R.; Gruter, G.-J.M.; Coelho, J.F.J.; Silvestre, A.J.D. Biobased polyesters and other polymers from 2,5-furandicarboxylic acid: A tribute to furan excellency. Polym. Chem. 2015, 6, 5961–5983. [Google Scholar] [CrossRef]
- Vannini, M.; Marchese, P.; Celli, A.; Lorenzetti, C. Fully biobased poly(propylene 2,5-furandicarboxylate) for packaging applications: Excellent barrier properties as a function of crystallinity. Green Chem. 2015, 17, 4162–4166. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Papageorgiou, D.G.; Tsanaktsis, V.; Bikiaris, D.N. Synthesis of the bio-based polyester poly(propylene 2,5-furan dicarboxylate). Comparison of thermal behavior and solid state structure with its terephthalate and naphthalate homologues. Polymer 2015, 62, 28–38. [Google Scholar] [CrossRef]
- Jiang, M.; Liu, Q.; Zhang, Q.; Ye, C.; Zhou, G. A series of furan-aromatic polyesters synthesized via direct esterification method based on renewable resources. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 1026–1036. [Google Scholar] [CrossRef]
- Haas, T.; Jaeger, B.; Weber, R.; Mitchell, S.F.; King, C.F. New diol processes: 1,3-propanediol and 1,4-butanediol. Appl. Catal. A Gen. 2005, 280, 83–88. [Google Scholar] [CrossRef]
- U.S.A.: World’s First Propanediol Production from Corn Sugar Opened. Available online: http://news.bio-based.eu/u-s-a-worlds-first-propanediol-production-from-corn-sugar-opened/ (accessed on 7 May 2018).
- DuPont Sorona. Available online: http://sorona.com/ (accessed on 7 May 2018).
- Cohen, G.M.; Nederberg, F.; Rajagopalan, B. Nucleated Crystallization of Poly(trimethylene-2,5-furandicarboxylate)(ptf) and Articles Made Therefrom. U.S. Patent US20160312001A1, 27 October 2016. [Google Scholar]
- Brun, Y.; Castagna, A.M.; Liao, K.-H.; Nederberg, F.; Forrester McCord, E.; Rasmussen, C.J. Transesterified Furan Based Polyesters and Articles Made Therefrom. U.S. Patent US20170058119A1, 2 March 2017. [Google Scholar]
- Nederberg, F.; Rajagopalan, B.; Uradnisheck, J. Polyesters and Articles Made Publication Classification Therefrom. PCT/US2013/034734, 30 March 2013. [Google Scholar]
- Nederberg, F.; Bell, R.; Torradas, J.M. Furan-Based Polymerc Hydrocarbon Fuel Barrier Structures. U.S. Patent US20160311208A1, 27 October 2016. [Google Scholar]
- DuPont Industrial Biosciences—Our Brightest Future Is Made of Biomaterials. Available online: http://www.pci-mag.com/contractors/recyclable-and-biodegradable-materials/dupont-industrial-biosciences/ (accessed on 7 May 2018).
- DuPont Wins 2017 European Company of the Year Award for Bio-Based Materials. Available online: http://www.bioplasticsmagazine.com/en/news/meldungen/20172006-Dupont-wins-FRost-and-Sullivan-Award-for-Biobased-Materials-2017.php (accessed on 7 May 2018).
- The Packaging Conference—Paul Fagan. Available online: https://www.thepackagingconference.com/speakers/paul-fagan.aspx (accessed on 7 May 2017).
- Paul, D.R.; Robeson, L.M. Polymer nanotechnology: Nanocomposites. Polymer 2008, 49, 3187–3204. [Google Scholar] [CrossRef]
- Chen, B. Polymer–clay nanocomposites: An overview with emphasis on interaction mechanisms. Br. Ceram. Trans. 2004, 103, 241–249. [Google Scholar] [CrossRef]
- Gul, S.; Kausar, A.; Muhammad, B.; Jabeen, S. Research progress on properties and applications of polymer/clay nanocomposite. Polym. Plast. Technol. Eng. 2016, 55, 684–703. [Google Scholar] [CrossRef]
- Nazir, M.S.; Haa, M.; Kassim, M. Nanoclay Reinforced Polymer Composites; Springer: Berlin, Germany, 2016; pp. 35–56. [Google Scholar] [CrossRef]
- Liu, M.; Jia, Z.; Jia, D.; Zhou, C. Recent advance in research on halloysite nanotubes-polymer nanocomposite. Prog. Polym. Sci. 2014, 39, 1498–1525. [Google Scholar] [CrossRef]
- Alexandre, M.; Dubois, P. Polymer-layered silicate nanocomposites: Preparation, properties and uses of a new class of materials. Mater. Sci. Eng. R Rep. 2000, 28, 1–63. [Google Scholar] [CrossRef]
- Fawaz, J.; Mittal, V. Synthesis of polymer nanocomposites: Review of various techniques. In Synthesis Techniques for Polymer Nanocomposites; Wiley-VCH Verlag GmbH & Co. KgaA: Weinheim, Germany, 2015; pp. 1–30. [Google Scholar]
- Martino, L.; Guigo, N.; van Berkel, J.G.; Sbirrazzuoli, N. Influence of organically modified montmorillonite and sepiolite clays on the physical properties of bio-based poly(ethylene 2,5-furandicarboxylate). Compos. Part B Eng. 2017, 110, 96–105. [Google Scholar] [CrossRef]
- Martino, L.; Niknam, V.; Guigo, N.; Gabriël van Berkel, J.; Sbirrazzuoli, N. Morphology and thermal properties of novel clay-based poly(ethylene 2,5-furandicarboxylate) (PEF) nanocomposites. RSC Adv. 2016, 6, 59800–59807. [Google Scholar] [CrossRef]
- Paszkiewicz, S.; Janowska, I.; Pawlikowska, D.; Szymczyk, A.; Irska, I.; Lisiecki, S.; Stanik, R.; Gude, M.; Piesowicz, E. New functional nanocomposites based on poly(Trimethylene 2,5-furanoate) and few layer graphene prepared by in situ polymerization. Express Polym. Lett. 2018, 12, 530–542. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Tsanaktsis, V.; Bikiaris, D.N. Synthesis of poly(ethylene furandicarboxylate) polyester using monomers derived from renewable resources: Thermal behavior comparison with PET and PEN. Phys. Chem. Chem. Phys. 2014, 16, 7946–7958. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, S. Viscosity–molecular weight relationships for poly(ethylene terephthalate) in hexafluoroisopropanol–pentafluorophenol using SEC–LALLS. J. Appl. Polym. Sci. 1984, 29, 4353–4361. [Google Scholar] [CrossRef]
- Karayannidis, G.P.; Kokkalas, D.E.; Bikiaris, D.N. Solid-state polycondensation of poly(ethylene terephthalate) recycled from postconsumer soft-drink bottles. II. J. Appl. Polym. Sci. 1995, 56, 405–410. [Google Scholar] [CrossRef]
- Lorenzo, A.T.; Arnal, M.L.; Albuerne, J.; Müller, A.J. DSC isothermal polymer crystallization kinetics measurements and the use of the Avrami equation to fit the data: Guidelines to avoid common problems. Polym. Test. 2007, 26, 222–231. [Google Scholar] [CrossRef]
- Gomes, M.; Gandini, A.; Silvestre, A.J.D.; Reis, B. Synthesis and characterization of poly(2,5-furan dicarboxylate) s based on a variety of diols. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 3759–3768. [Google Scholar] [CrossRef]
- Vassiliou, A.A.; Chrissafis, K.; Bikiaris, D.N. In situ prepared PET nanocomposites: Effect of organically modified montmorillonite and fumed silica nanoparticles on PET physical properties and thermal degradation kinetics. Thermochim. Acta 2010, 500, 21–29. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Karandrea, E.; Giliopoulos, D.; Papageorgiou, D.G.; Ladavos, A.; Katerinopoulou, A.; Achilias, D.S.; Triantafyllidis, K.S.; Bikiaris, D.N. Effect of clay structure and type of organomodifier on the thermal properties of poly(ethylene terephthalate) based nanocomposites. Thermochim. Acta 2014, 576, 84–96. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of Phase Change. I General Theory. J. Chem. Phys. 1939, 7, 1103–1112. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of Phase Change. II Transformation-Time Relations for Random Distribution of Nuclei. J. Chem. Phys. 1940, 8, 212–224. [Google Scholar] [CrossRef]
- Minakov, A.A.; Mordvintsev, D.A.; Schick, C. Melting and reorganization of poly(ethylene terephthalate) on fast heating (1000 K/s). Polymer 2004, 45, 3755–3763. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Bikiaris, D.N. Crystallization and melting behavior of three biodegradable poly(alkylene succinates). A comparative study. Polymer 2005, 46, 12081–12092. [Google Scholar] [CrossRef]
- Genovese, L.; Lotti, N.; Siracusa, V.; Munari, A. Poly(neopentyl glycol furanoate): A member of the furan-based polyester family with smart barrier performances for sustainable food packaging applications. Materials 2017, 10, 1028. [Google Scholar] [CrossRef] [PubMed]
- Terzopoulou, Z.; Tsanaktsis, V.; Nerantzaki, M.; Achilias, D.S.; Vaimakis, T.; Papageorgiou, G.Z.; Bikiaris, D.N. Thermal degradation of biobased polyesters: Kinetics and decomposition mechanism of polyesters from 2,5-furandicarboxylic acid and long-chain aliphatic diols. J. Anal. Appl. Pyrolysis 2016, 117, 162–175. [Google Scholar] [CrossRef]
- Tsanaktsis, V.; Terzopoulou, Z.; Exarhopoulos, S.; Bikiaris, D.N.; Achilias, D.S.; Papageorgiou, D.G.; Papageorgiou, G.Z. Sustainable, eco-friendly polyesters synthesized from renewable resources: Preparation and thermal characteristics of poly(dimethyl-propylene furanoate). Polym. Chem. 2015, 6. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Karakatsianopoulou, E.; Kasmi, N.; Majdoub, M.; Papageorgiou, G.Z.; Bikiaris, D.N. Effect of catalyst type on recyclability and decomposition mechanism of poly(ethylene furanoate) biobased polyester. J. Anal. Appl. Pyrolysis 2017, 126, 357–370. [Google Scholar] [CrossRef]
- Konstantopoulou, M.; Terzopoulou, Z.; Nerantzaki, M.; Tsagkalias, J.; Achilias, D.S.; Bikiaris, D.N.; Exarhopoulos, S.; Papageorgiou, D.G.; Papageorgiou, G.Z. Poly(ethylene furanoate-co-ethylene terephthalate) biobased copolymers: Synthesis, thermal properties and cocrystallization behavior. Eur. Polym. J. 2017, 89, 349–366. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Kasmi, N.; Tsanaktsis, V.; Doulakas, N.; Bikiaris, D.N.; Achilias, D.S.; Papageorgiou, G.Z. Synthesis and characterization of bio-based polyesters: Poly(2-methyl-1,3-propylene-2,5-furanoate), Poly(isosorbide-2,5-furanoate), Poly(1,4-cyclohexanedimethylene-2,5-furanoate). Materials 2017, 10, 801. [Google Scholar] [CrossRef] [PubMed]
- Terzopoulou, Z.; Tsanaktsis, V.; Nerantzaki, M.; Papageorgiou, G.Z.; Bikiaris, D.N. Decomposition mechanism of polyesters based on 2,5-furandicarboxylic acid and aliphatic diols with medium and long chain methylene groups. Polym. Degrad. Stab. 2016, 132, 127–136. [Google Scholar] [CrossRef]
- Tsanaktsis, V.; Vouvoudi, E.; Papageorgiou, G.Z.; Papageorgiou, D.G.; Chrissafis, K.; Bikiaris, D.N. Thermal degradation kinetics and decomposition mechanism of polyesters based on 2,5-furandicarboxylic acid and low molecular weight aliphatic diols. J. Anal. Appl. Pyrolysis 2015, 112, 369–378. [Google Scholar] [CrossRef]












| Temperature | PPF | PPF/MMT 20A | |||
| n | K(min−n) | n | K(min−n) | ||
| 130 | 3.7 | 1 × 10−4 | 130 | 2.6 | 6.3 × 10−4 |
| 135 | 3.6 | 4 × 10−5 | 135 | 2.7 | 3.1 × 10−4 |
| 140 | 3.8 | 1.2 × 10−5 | 140 | 2.7 | 2 × 10−4 |
| 145 | 3.7 | 5 × 10−6 | 145 | 2.5 | 1.2 × 10−4 |
| 150 | 3.5 | 2 × 10−6 | 150 | 2.6 | 4 × 10−5 |
| 160 | 3.7 | 2 × 10−7 | 160 | 2.4 | 2. × 10−5 |
| Temperature | PPF/MMT | PPF/HNT | |||
| n | K(min−n) | n | K(min−n) | ||
| 130 | 2.5 | 7 × 10−3 | 130 | 2.8 | 3 × 10−4 |
| 135 | 2.7 | 1 × 10−4 | 135 | 2.6 | 5 × 10−5 |
| 140 | 2.6 | 5.5 × 10−5 | 140 | 2.5 | 1.4 × 10−5 |
| 145 | 2.4 | 9 × 10−6 | 145 | 2.3 | 3.1 × 10−6 |
| 150 | 2.6 | 5.2 × 10−6 | 150 | 2.6 | 1.1 × 10−6 |
| 160 | 2.7 | 6.1 × 10−7 | 160 | 2.7 | 1 × 10−7 |
| Sample | Td,5% (°C) | Td,10% (°C) | Td,max (°C) |
|---|---|---|---|
| PPF | 361 | 369 | 393 |
| PPF/HNT | 362 | 370 | 391 |
| PPF/MMT | 359 | 368 | 395 |
| PPF/MMT 20A | 358 | 366 | 386 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadopoulos, L.; Terzopoulou, Z.; Bikiaris, D.N.; Patsiaoura, D.; Chrissafis, K.; Papageorgiou, D.G.; Papageorgiou, G.Z. Synthesis and Characterization of In-Situ-Prepared Nanocomposites Based on Poly(Propylene 2,5-Furan Dicarboxylate) and Aluminosilicate Clays. Polymers 2018, 10, 937. https://doi.org/10.3390/polym10090937
Papadopoulos L, Terzopoulou Z, Bikiaris DN, Patsiaoura D, Chrissafis K, Papageorgiou DG, Papageorgiou GZ. Synthesis and Characterization of In-Situ-Prepared Nanocomposites Based on Poly(Propylene 2,5-Furan Dicarboxylate) and Aluminosilicate Clays. Polymers. 2018; 10(9):937. https://doi.org/10.3390/polym10090937
Chicago/Turabian StylePapadopoulos, Lazaros, Zoi Terzopoulou, Dimitrios N. Bikiaris, Dimitra Patsiaoura, Kostantinos Chrissafis, Dimitrios G. Papageorgiou, and George Z. Papageorgiou. 2018. "Synthesis and Characterization of In-Situ-Prepared Nanocomposites Based on Poly(Propylene 2,5-Furan Dicarboxylate) and Aluminosilicate Clays" Polymers 10, no. 9: 937. https://doi.org/10.3390/polym10090937