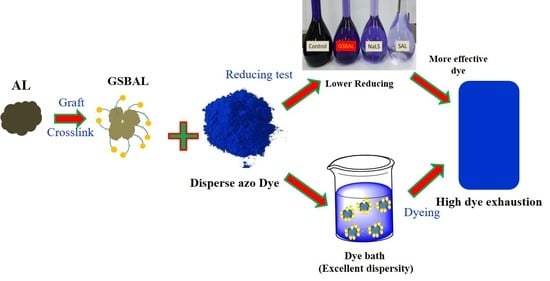
Preparation of a Low Reducing Effect Sulfonated Alkali Lignin and Application as Dye Dispersant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Sulfomethylated Alkali Lignin (SAL)
2.3. Grafting Sulfonated and Blocking Condensed Alkali Lignin (GSBAL)
2.4. FTIR Analysis
2.5. HSQC Analysis
2.6. Molecular Weight Distribution Analysis
2.7. Sulfonic Group Content Measurement
2.8. Phenolic Hydroxyl Content Measurement
2.9. Preparation of Dye Suspension
2.10. Dye Exhaustion Measurement
2.11. Scanning Electron Microscope (SEM) Images Measurement
2.12. Reducing Rate of Dispersant on Azo Dye
3. Results and Discussion
3.1. FTIR Analysis
3.2. 1H–13C Correlation NMR Spectrum (HSQC) of AL and GSBAL
3.3. Molecular Weight and the Functional Group
3.4. Dispersion Performance
3.5. Reducing Effect of Dispersant on Azo Dye
3.6. Dye Exhaustion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, X.; Hardin, I.R.; Hwang, H.-M. Biodegradation of a model azo disperse dye by the white rot fungus Pleurotus ostreatus. Int. Biodeter. Biodegr. 2006, 57, 1–6. [Google Scholar] [CrossRef]
- Koh, J. Dyeing with Disperse Dyes, In Textile Dyeing; IntechOpen: Rijeka, Croatia, 2011; pp. 32–33. [Google Scholar]
- Huynh, L.; Beattie, D.A.; Fornasiero, D.; Ralston, J. Effect of polyphosphate and naphthalene sulfonate formaldehyde condensate on the rheological properties of dewatered tailings and cemented paste backfill. Miner. Eng. 2006, 19, 28–36. [Google Scholar] [CrossRef]
- Dilling, P. Color Reduction Process for Non-sulfonated Lignin. U.S. Patent 4486346A, 4 December 1984. [Google Scholar]
- Dilling, P. Lignosulfonate dispersants and the role of the reduction of azo dyes. Text. Chem. Col. 1986, 8, 17–24. [Google Scholar]
- Liu, L.; Li, F.B.; Feng, C.H.; Li, X.Z. Microbial fuel cell with an azo-dye-feeding cathode. Appl. Microbiol. Biot. 2009, 85, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.Q.; Yu, J.; Yang, D.J.; Wang, J.Y.; Mo, W.J.; Qian, Y. Whitening sulfonated alkali lignin via H2O2/UV radiation and its application as dye dispersant. ACS Sustain. Chem. Eng. 2017, 6, 1055–1060. [Google Scholar] [CrossRef]
- Dilling, P.; Samaranayake, G.S. Mixtures of Amine Modified Lignin with Sulfonated Lignin for Disperse Dye. U.S. Patent 5989299A, 23 November 1999. [Google Scholar]
- Yang, D.J.; Qiu, X.Q.; Pang, Y.X.; Zhou, M.S. Physicochemical properties of calcium lignosulfonate with different molecular weights as dispersant in aqueous suspension. J. Dispers. Sci. Technol. 2008, 29, 1296–1303. [Google Scholar] [CrossRef]
- Lin, S.Y. Process for Reduction of Lignin Color. U.S. Patent 4184845A, 22 January 1980. [Google Scholar]
- Qian, Y.; Zhong, X.; Li, Y.; Qiu, X.Q. Fabrication of uniform lignin colloidal spheres for developing natural broad-spectrum sunscreens with high sun protection factor. Ind. Crops Prod. 2017, 101, 54–60. [Google Scholar] [CrossRef]
- Pu, Y.; Ragauskas, A.J. Structural analysis of acetylated hardwood lignins and their photoyellowing properties. Can. J. Chem. 2005, 83, 2132–2139. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, B.; Zhou, W.; Liu, X.; Chen, F. High-value utilization of eucalyptus kraft lignin: Preparation and characterization as efficient dye dispersant. Int. J. Biol. Macromol. 2018, 109, 1232–1238. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.L.; Yang, D.J.; Guo, W.Y.; Qiu, X.Q. Investigation of grafted sulfonated alkali lignin polymer as dispersant in coal-water slurry. J. Ind. Eng. Chem. 2015, 27, 192–200. [Google Scholar] [CrossRef]
- Yang, D.J.; Qiu, X.Q.; Zhou, M.S.; Lou, H.M. Properties of sodium lignosulfonate as dispersant of coal water slurry. Energ. Convers. Manag. 2007, 48, 2433–2438. [Google Scholar] [CrossRef]
- Pang, Y.X.; Qiu, X.Q.; Yang, D.J.; Lou, H.M. Influence of oxidation, hydroxymethylation and sulfomethylation on the physicochemical properties of calcium lignosulfonate. Colloids Surf. A 2008, 312, 154–159. [Google Scholar] [CrossRef]
- Park, K.M.; Yoon, I.; Lee, S.S.; Choi, G.; Lee, J.S. X-ray crystal structure of CI Disperse Blue 79. Dyes Pigments 2002, 54, 155–161. [Google Scholar] [CrossRef]
- Geng, X.F.; Hu, X.Q.; Xia, J.J.; Jia, X.C. Synthesis and surface activities of a novel di-hydroxyl-sulfate-betaine-type zwitterionic gemini surfactants. Appl. Surf. Sci. 2013, 271, 284–290. [Google Scholar] [CrossRef]
- Lou, H.M.; Lai, H.R.; Wang, M.X.; Pang, Y.X.; Yang, D.J.; Qiu, X.Q.; Wang, B.; Zhang, H.B. Preparation of lignin-based superplasticizer by graft sulfonation and investigation of the dispersive performance and mechanism in a cementitious system. Ind. Eng. Chem. Res. 2013, 52, 16101–16109. [Google Scholar] [CrossRef]
- Sousa, F.D.; Reimann, A.; Jansson, M.B.; Nilberbrant, N. Estimating the amount of phenolic hydroxyl groups in lignins. In Proceedings of the 11th ISWPC, Nice, France, 11–14 June 2001; pp. 649–653. [Google Scholar]
- Zhou, H.; Yang, D.; Qiu, X.; Wu, X.; Li, Y. A novel and efficient polymerization of lignosulfonates by horseradish peroxidase/H2O2 incubation. Appl. Microbiol. Biot. 2013, 97, 10309–10320. [Google Scholar] [CrossRef] [PubMed]
- Tejado, A.; Pena, C.; Labidi, J.; Echeverria, J.; Mondragon, I. Physico-chemical characterization of lignins from different sources for use in phenol–formaldehyde resin synthesis. Bioresour. Technol. 2007, 98, 1655–1663. [Google Scholar] [CrossRef] [PubMed]
- Faix, O. Fourier transform infrared spectroscopy. In Methods in Lignin Chemistry; Lin, S.Y., Dence, C.W., Eds.; Springer: Berlin, Germany, 1992; pp. 83–109. [Google Scholar]
- Song, Y.; Wang, Z.; Yan, N.; Zhang, R.; Li, J. Demethylation of wheat straw alkali lignin for application in phenol formaldehyde adhesives. Polymers 2016, 8, 209. [Google Scholar] [CrossRef]
- Jahan, M.S.; Chowdhury, D.; Islam, M.K.; Moeiz, S. Characterization of lignin isolated from some nonwood available in Bangladesh. Bioresour. Technol. 2007, 98, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Bu, L.; Xing, Y.; Yu, H.; Gao, Y.; Jiang, J. Comparative study of sulfite pretreatments for robust enzymatic saccharification of corn cob residue. Biotechnol. Biofuels 2012, 5, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hage, R.E.; Brosse, N.; Chrusciel, L.; Sanchez, C.; Sannigrahi, P.; Ragauskas, A. Characterization of milled wood lignin and ethanol organosolv lignin from miscanthus. Polym. Degrad. Stabil. 2009, 94, 1632–1639. [Google Scholar] [CrossRef]
- .Gan, L.; Zhou, M.; Yang, D.; Qiu, X. Preparation and Evaluation of Carboxymethylated Lignin as Dispersant for Aqueous Graphite Suspension Using Turbiscan Lab Analyzer. J. Dispers. Sci. Technol. 2013, 34, 644–650. [Google Scholar] [CrossRef]
- Hu, T. Characterization of Lignocellulosic Materials; Blackwell: Oxford, UK, 2009; pp. 68–71. [Google Scholar]
- Lutnaes, B.F.; Myrvold, B.O.; Lauten, R.A.; Endeshaw, M.M. 1H and 13C NMR data of benzylsulfonic acids-model compounds for lignosulfonate. Magn. Reson. Chem. 2008, 46, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Sakkayawong, N.; Thiravetyan, P.; Nakbanpote, W. Adsorption mechanism of synthetic reactive dye wastewater by chitosan. J. Colloid Interf. Sci. 2005, 286, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gellerstedt, G. Quantitative 2D HSQC NMR determination of polymer structures by selecting suitable internal standard references. Magn. Reson. Chem. 2007, 45, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Willerich, I.; Li, Y.; Grohn, F. Influencing Particle Size and Stability of Ionic Dendrimer Dye Assemblies. J. Phys. Chem. B 2010, 114, 15466–15476. [Google Scholar] [CrossRef] [PubMed]
- Kissa, E. Partitioning and stability of aqueous dispersions. Effect of electrolytes on the stability of aqueous dye dispersions. Langmuir 1990, 6, 1217–1221. [Google Scholar] [CrossRef]
- Ujhelyiova, A.; Bolhova, E.; Oravkinova, J.; Tiňo, R.; Marcinčin, A. Kinetics of dyeing process of blend polypropylene/polyester fibres with disperse dye. Dyes Pigments 2007, 72, 212–216. [Google Scholar] [CrossRef]




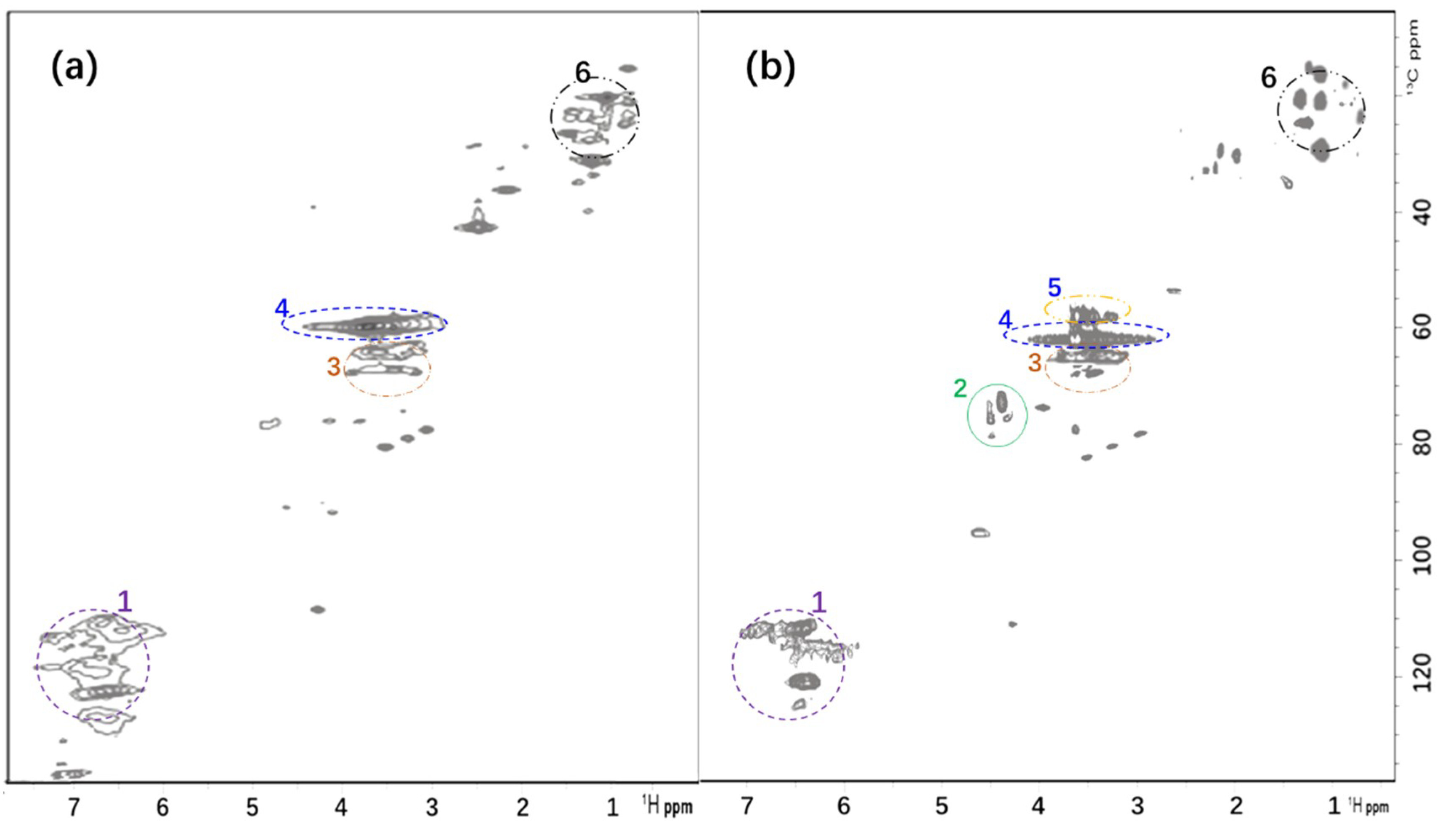




| Marked Number | δH/δc ppm | Assignment |
|---|---|---|
| 1 | 6.2–7.5/102–125 | Aromatic 1H–13C [29] |
| 2 | 4.4–4.9/68–80 | 1H–13C correlation in sulfonated chain (–CH2CH(OH)CH2SO3Na) [29,30] |
| 3 | 3.4–3.7/62–72 | Aliphatic oxygenated 1H–13C [31] |
| 4 | 3.0–4.4/56–62 | Methoxyl [32] |
| 5 | 3.0–3.6/53–57 | Methylene (part of sulfonated chain) [33] |
| 6 | 0.5–1.5/15–30 | Aliphatic non-oxygenated 1H–13C [31,32] |
| Dispersants | Mw (Da) | Mn (Da) | Mw/Mn | Sulfonic (mmol·g−1) | Phenolic Hydroxyl (mmol·g−1) |
|---|---|---|---|---|---|
| AL | 4920 | 1900 | 2.59 | — | 2.27 |
| NaLS | 10,250 | 4780 | 2.14 | 1.36 | 1.88 |
| SAL | 6140 | 2200 | 2.79 | 1.14 | 2.16 |
| GSBAL | 11,350 | 5880 | 1.93 | 2.21 | 0.42 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, Y.; Lin, X.; Lu, Y.; Wu, S.; Yang, D.; Qiu, X.; Fang, Y.; Wang, T. Preparation of a Low Reducing Effect Sulfonated Alkali Lignin and Application as Dye Dispersant. Polymers 2018, 10, 982. https://doi.org/10.3390/polym10090982
Qin Y, Lin X, Lu Y, Wu S, Yang D, Qiu X, Fang Y, Wang T. Preparation of a Low Reducing Effect Sulfonated Alkali Lignin and Application as Dye Dispersant. Polymers. 2018; 10(9):982. https://doi.org/10.3390/polym10090982
Chicago/Turabian StyleQin, Yanlin, Xuliang Lin, Yaoqin Lu, Siyuan Wu, Dongjie Yang, Xueqing Qiu, Yanxiong Fang, and Tiejun Wang. 2018. "Preparation of a Low Reducing Effect Sulfonated Alkali Lignin and Application as Dye Dispersant" Polymers 10, no. 9: 982. https://doi.org/10.3390/polym10090982
APA StyleQin, Y., Lin, X., Lu, Y., Wu, S., Yang, D., Qiu, X., Fang, Y., & Wang, T. (2018). Preparation of a Low Reducing Effect Sulfonated Alkali Lignin and Application as Dye Dispersant. Polymers, 10(9), 982. https://doi.org/10.3390/polym10090982