3.3.1. LOI and UL 94 Tests
LOI and UL94 tests were performed, and the corresponding results are collected in
Table 4. Indeed, in order to compare the RPUF specimens, total burning time—the time in which the five specimens from the UL94 tests were burning until being total extinguished—was herein recorded and presented. The LOI of neat RPUF (PU) was 19.2%, indicating that it was a very flammable material under atmospheric conditions (
ca. 21% O
2). LOI values and UL94 rating increased along with the increasing amount of EG into the foam. A V-0 rating in the vertical burning test was only achieved above 8 wt % onwards on the EG1-RPUF sample, which provided a LOI value of 27.8%. The sample containing 10 wt % of EG1 increased by a 50% the initial value of the PU to 29.8, whereas UL94 burning total time decreased (20.9 s) and maintained the V-0 rating. When EG1 was replaced by EG particles with a higher rate of expansion; such as EG2 (350 cm
3/g), the effects on the LOI values were positive. LOI increased from 27.8% to 30.0% in EG2–8 and higher LOI values were achieved using 10 wt % of EG-2, showing a value of 31.8%. UL94 rating of the EG2–8 and EG2–10 samples was indeed V-0. In spite of the increasing the total time for UL94 test, the performance of EG2 was almost similar to EG1 on the UL94 test. So, the rate of expansion of EG, had no big influence on the UL94 test, maintaining the same rating. Moreover, EG3, with higher particle size and same rate of expansion as EG2, obtained similar LOI performance. The samples’ LOI values were clearly improved with the increasing rate of expansion, which might be explained by the formation and densification of an isolation layer that became larger with increasing volume of the EG particles and formed a more intumescent char [
23,
25]. On the contrary, decrease in particle number (caused by the higher particle size for the same loading) had no important effect on the formation of a compact isolation layer during the fire process, increasing slowly the LOI values as it was reported by other authors [
23,
25]. Interestingly, the UL94 values obtained by EG3-RPUFs were outstanding. In fact, a V-1 rating was achieved with a loading as low as 6 wt % of EG3, whereas for 8 wt % onwards, the UL94 rating was V-0. This behavior may be explained considering the carbonaceous char layer under the polyurethane surface, which protects the polymer from the flame on these tests. Therefore, an increasing amount of EG added into the foam should contribute to an increased barrier effect due to EG expansion, giving eventually a good fire performance [
17,
18,
19,
20]. For this reason, EG3—the EG particles with higher size—should be able to create a more compact isolation layer, thus improving UL94 performance [
24,
46]. In summary, the role of particle size and rate of expansion of EG, as intrinsic characteristics of the material, seemed to be crucial for the flame retardancy performance of EG-filled RPUFs. On one hand, the expansion rate was important for a variable O
2 % test as LOI, in which a higher concentration of O
2 could easily start a fire process. On the other hand, the particle size of EG3 was determined on the vertical burning test under ambient conditions in order to prevent fire spreading in a more efficient way [
24].
3.3.2. Cone Calorimeter Test
The cone calorimeter test has been extensively used to investigate the fire hazard of polyurethane matrix foams when subjected to heat flow, since this test correlates well with large-scale fire tests and simulates a real fire scenario [
47]. This experiment was based on the oxygen-combustion principle test, and was performed at a heat flux of 50 kW/m
2, thus giving a quantitative analysis, since it provides different characteristic parameters such as pHRR (Peak Heat Release Rate), TSP (Total Smoke Production), TSR (Total Smoke Release), THR (Total Heat Released), and mass loss rate (Mass %). All these data for the RPUFs are summarized in
Figure 5 (at 10 wt % loading of the EGs) and
Table 5. Each RPUF loaded with different EG types was discussed individually.
The pHRR and TSP curves for the PU foams loaded with EG1 are shown in
Figure S2, whereas their THR and mass loss curves are displayed in
Figure S3. pHRR is the most important parameter for fire safety evaluation, since it indicates the burning behavior of materials that form residues after the burning process [
48]. It is noteworthy that the samples rapidly raised their maximum value (pHRR) after ignition. The reason behind this phenomena was their porous structure, which facilitated a large surface contact area between material and air, currently being more exposed to heat [
49]. For instance, neat RPUFs burned fiercely once ignited and exhibited a peak value of 220 kW/m
2. Two peaks were detected on these samples. The first peak may be attributed to the development of an intumescent protective char layer, and the second peak should be mainly due to the degradation of this layer to heat exposure [
50]. Thermal feedback may also contribute to this second peak, when the pyrolysis front approaches the insulated back surface of the sample [
25,
48]. The maximum values of these second peaks decreased as the amount of EG increased, since pyrolysis at the samples’ bottom parts was then blocked [
24,
25]. Therefore, the addition of EG1 reduced pHRR values by 32%, 39%, and 49% for the samples containing 6, 8, and 10 wt % of EG, respectively, with the lowest peak for the EG1-10 samples being 111 kW/m
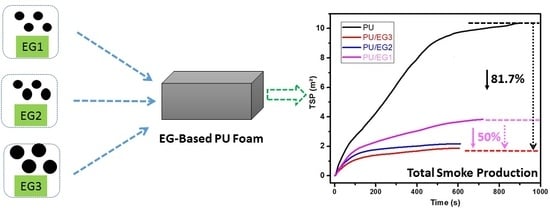
2. TSP is a critical parameter, since smoke is a dangerous agent on a fire scenario, and mainly due to the toxicity of CO. Hence, reducing the TSP or TSR evidenced a good flame retardant effect. TSP of neat PU showed a value of 10.4 m
2, and the addition of EG continuously reduced the TSP reference value: they were reduced by 25%, 63% and 81% in the samples containing 6, 8 and 10 wt % of EG respectively, with the lowest value for the EG1-10 sample being 2.0 m
2.
THR curves for RPUFs loaded with EG1 are shown in
Figure S3. THR of the reference sample (PU) gave a value of 68.7 MJ/m
2. The introduction of EG continuously reduced this value by 18%, 34%, and 27% for samples containing 6, 8, and 10 wt %, respectively of EG1, with the lowest value for the EG1-8 sample being 45.3 MJ/m
2. The mass loss rate is another important parameter in order to evaluate the flame retardant properties of a polymer. Commonly, neat polymers made from petroleum derivatives are very flammable, therefore flame retardant treatments are mandatory, and this parameter must be improved. In our case, mass loss of the neat PU sample showed a value of 87 wt %. In this way, most polymers were transformed into volatiles during the process. Significantly, the addition of EG reduced this reference value by 17%, 28%, and 33% for samples containing 6, 8, and 10 wt % of EG, respectively, the lowest mass loss for the EG1-10 sample being 54 wt %. For our EG-filled RPUFs, increasing quantities due to the EG expansion led to the formation of a carbon char when exposed to a heat source, thus improving the polymer flame retardancy. As concluded previously by other scientists, the intumescent char formed by the EG expansion contributed flame suppression, and limited heat and mass transfers from the polymer to the heat source, thereby preventing further decomposition and limiting RPUFs’ weight loss [
2,
24,
37,
50].
pHRR and TSP curves for RPUFs loaded with EG2 are shown in
Figure S4. THR and mass loss are plotted in
Figure S5. As well as EG1 samples, EG-filled RPUF samples reached their maximum peak very rapidly; less than 20 s. Thus the time of EG2-10 increased by 5 s compared to that of EG1. Alike, the addition of EG reduced the pHRR value of neat PU continuously by a 25%, 42%, and 48% in the samples containing 6, 8, and 10 wt % of EG2, respectively, with the lowest peak for the sample with EG2-10 being 113 kW/m
2. In this way, the addition of EG2 reduced continuously the reference TSP values (PU) by a 52%, 79%, and 79% in the samples containing 6, 8, and 10 wt % of EG2, respectively. Then, 2.1 m
2 was shown as the lowest value for the sample with EG2-10.
THR and mass loss curves for RPUF containing EG2 are shown in
Figure S5. The introduction of EG2 also reduced the THR value continuously by a 29%, 43%, and 40% in the samples containing 6, 8, and 10 wt % of EG2, respectively, with the lowest result for the sample EG2-8 being 38.9 MJ/m
2. The addition of EG2 also reduced the reference value of mass loss up to a 34% reduction on EG2-10. So far, 54 wt % was the lowest amount of mass loss during the experiment for the sample EG2-10.
pHRR and TSP for the RPUFs loaded with EG3 are shown in
Figure S6. THR and mass loss are displayed in
Figure S7. As described above for previous samples, PU containing EG3 again reached their pHRR before 15 s, in the same range as other EGs. In this case, the addition of EG also reduced the reference pHRR value continuously by a 43%, 51%, and 54% for the samples containing 6, 8, and 10 wt % of EG3, respectively, with the lowest peak for the sample with EG3-10 being 101 kW/m
2. Thus, the addition of EG3 also continuously reduced the TSP reference value by a 67%, 82%, and 84% for samples containing 6, 8, and 10 wt % of EG respectively. In this case, an impressive 1.7 m
2 was the lowest value for the EG3-10 sample.
THR and mass loss curves for RPUFs containing EG3 are shown in
Figure S7. Again, the introduction of EG3 reduced the reference value by 18%, 47%, and 47% for the samples containing 6, 8, and 10 wt % of EG3, respectively, with the lowest result for a sample with higher EG wt % as EG3-10 being 36.3 MJ/m
2. Therefore, the addition of EG3 also reduced the reference value of mass loss by 18%, 39%, and 37% for the samples containing 6, 8, and 10 wt % of EG3, respectively. The lowest amount of mass loss during the cone calorimeter test for the sample with 8 wt % EG3 was 49 wt %.
In order to clarify which intrinsic characteristics of EG were decisive for its performance as flame retardant, an analysis including the impact of particle size and rate of expansion on the flame retardant performance was carried out. Thus, the effect of rate of expansion could be studied by comparing EG1 and EG2, bearing in mind that these two types of EG had the same particle size at different rates of expansion (250 and 350 cm
3/g, respectively). The pHRR at the maximum difference point was 6% lower for EG2, which had a higher rate of expansion. Afterwards, THR of samples with a 10 wt % of EG was 18% lower for the foams with higher expansion rates, although it was a 15% lower considering the average values. Owing to the rate of expansion of EG2, THR was lower. One possible explanation could be that the heat could not be transferred so easyily trough a higher volume material after the expansion mechanism of EG. In this case, a higher amount of PU remained unaltered in the condensed phase, which was not available for pyrolysis, which eventually reduced THR [
25]. On the other side, EG2 reduced TSP and TSR at higher rates than EG1, with a higher reduction of 43% lower than EG1 when an 8 wt % EG-2 was used. On average, the amount of smoke was 24% lower for EG with higher expansion. Interestingly the above difference between EGs decreased with an increase in EG loading. At last, the mass lost during the cone calorimeter test was slightly lower for EG2, although no big differences were found. All in all, these results support that EG2 was a better flame retardant than EG1. The above mentioned higher performance could be explained considering the fact that a higher expansion of EG promoted the development of a thicker worm structure, which can block more efficiently the heat and mass transfer during the pyrolysis, leading to lower values of pHRR, THR, and smoke production [
25,
37]. It can be concluded that the higher rate of expansion of EG2, has a very positive effect regarding the THR, mostly the TSP and TSR, where the differences were highlighted at lower loadings of EG, with a higher effectivity at higher rates of EG expansion.
On the other hand, to study the effect of particle size on flame retardancy, the behavior of EG3 and EG2 was compared next, taking into consideration that particle size increased from 300 to 500 µm, whereas the rate of expansion remained unaltered (350 cm
3/g). Commonly, bigger particle sizes and expansion rates lead to higher flame retardant performance [
51,
52,
53,
54]. Consequently, the pHRR of EG3 samples was 24%, 15%, and 11% lower than that of EG2 samples containing 6, 8, and 10 wt % of EG3, respectively. In other words, particles with higher size were able to reduce more efficiently the pHRR. Considering the average values, the decrease of pHRR for EG2 was 17% lower than that of EG3. Even so, when the amount of EG increased, this difference was continuously reduced. In a similar trend, EG3 also reduced the smoke production (TSP) and the smoke released (TSR) at higher rates than EG2. In fact, a 30%, 13%, and 20% respective reduction with respect to EG2 was obtained for samples containing 6, 8, and 10 wt % of EG3. This result indicated that particles of higher sizes formed a more stable char barrier, which blocked the smoke production, as described by others [
23,
24,
37]. On the other hand, THR of the sample with EG3 was higher for those samples containing 8 wt % of EG or less. Even though particles of higher sizes favored char barrier formation, bigger cracks could occur at the surface, thus reducing the effect of heat blocking and increasing THR as consequence [
37]. Moreover, the thermal conductivity increased with the average cell size; as reported on the morphology analysis above; and with the breaking of the cell walls [
23]. Finally, when comparing the mass loss rate of both samples, those containing EG3 improved the mass loss rate rather than those with EG2. This was due to the increasing number of particles forming a char barrier, and thus forming a more compact isolation layer [
37]. For this reason, the heat flux delivered to the pyrolysis front of the sample was also reduced [
25]. On the contrary, at 6 wt % of EG3, a higher amount of gases could be generated, as the small number of particles could not form a sufficient barrier to hinder heat penetration [
37]. Therefore, the defects in structure also became larger, resulting in a less stable structure, and producing less amounts of residual char [
24]. When the loading increased, the char became more compact and the initial PU structure remained almost unaltered, reducing the mass loss rate along with the aforementioned parameters. Therefore, the combination of higher particle sizes and higher rates of expansion had a beneficial effect on the formation of a compact residual char, which was the main characteristic of the mechanism of EG as flame retardant [
14]. In summary, the higher the particle size, the bigger impact on the results of the cone calorimeter test, such as pHRR and THR, mostly TSP and TSR. At the same time, a higher rate of expansion was a necessary characteristic of this flame retardant to obtain an outstanding performance.