Investigation of Chitosan Nanoparticles Loaded with Protocatechuic Acid (PCA) for the Resistance of Pyricularia oryzae Fungus against Rice Blast
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Chitosan Nanoparticles
2.3. Preparation of Chitosan Nanoparticles Loading PCA
2.4. Characterizations
2.5. Agar Diffusion Test
3. Results and Discussion
3.1. The Content of PCA in CS@PCA
3.2. Zeta Potentials
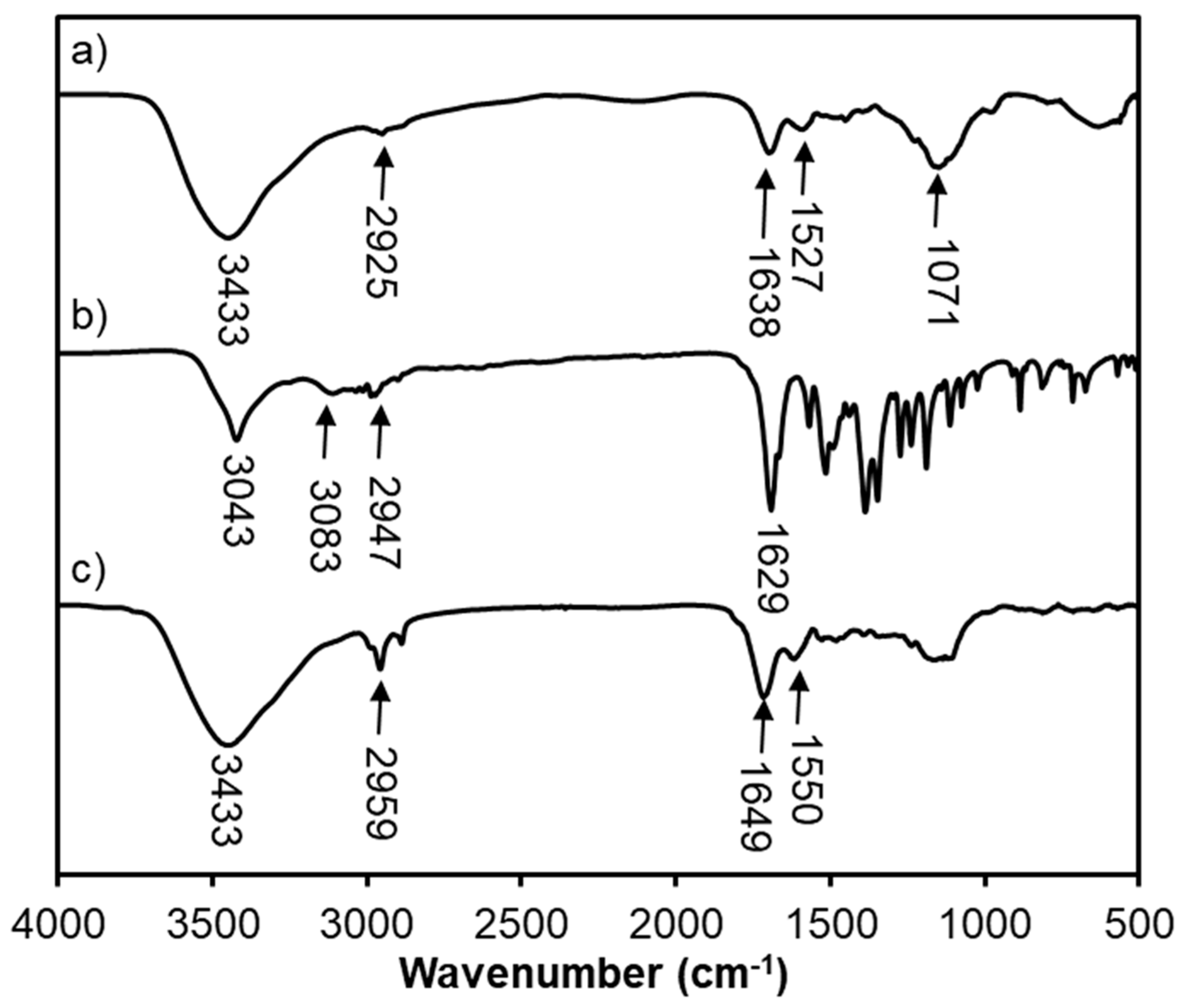
3.3. FTIR Analysis
3.4. TEM Analysis
3.5. Antifungal Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rossman, A.Y.; Howard, R.J.; Valent, B. Pyricularia grisea, the Correct Name for the Rice Blast Disease Fungus. Mycologia 1990, 82, 509. [Google Scholar] [CrossRef]
- General Statistics Office of Vietnam. Rice Production in 2016 and Trends in 2017 in Vietnam; General Statistics Office of Vietnam: Hanoi, Vietnam, 2017.
- Rai, M.; Ingle, A. Role of nanotechnology in agriculture with special reference to management of insect pests. Appl. Microbiol. Biotechnol. 2012, 94, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Ngoc, U.T.P.; Nguyen, D.H. Synergistic antifungal effect of fungicide and chitosan-silver nanoparticles on Neoscytalidium dimidiatum. Green Process. Synth. 2018, 7, 132–138. [Google Scholar] [CrossRef]
- Stojković, D.S.; Živković, J.; Soković, M.; Glamočlija, J.; Ferreira, I.C.F.R.; Janković, T.; Maksimović, Z. Antibacterial activity of Veronica montana L. extract and of protocatechuic acid incorporated in a food system. Food Chem. Toxicol. 2013, 55, 209–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, M.; Chao, C. Anti-Campylobacter, anti-aerobic, and anti-oxidative effects of roselle calyx extract and protocatechuic acid in ground beef. Int. J. Food Microbiol. 2008, 127, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.-F.; Wang, G.-Y.; Shi, Y.-P. Molecularly imprinted polymer microspheres for solid-phase extraction of protocatechuic acid in Rhizoma homalomenae. J. Sep. Sci. 2011, 34, 2602–2610. [Google Scholar] [CrossRef] [PubMed]
- Semaming, Y.; Pannengpetch, P.; Chattipakorn, S.C.; Chattipakorn, N. Pharmacological Properties of Protocatechuic Acid and Its Potential Roles as Complementary Medicine. J. Evid.-Based Complement. Altern. Med. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.-Y.; Yin, M.-C. Antibacterial Effects of Roselle Calyx Extracts and Protocatechuic Acid in Ground Beef and Apple Juice. Foodborne Pathog. Dis. 2008, 6, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Ajiboye, T.O.; Habibu, R.S.; Saidu, K.; Haliru, F.Z.; Ajiboye, H.O.; Aliyu, N.O.; Ibitoye, O.B.; Uwazie, J.N.; Muritala, H.F.; Bello, S.A.; et al. Involvement of oxidative stress in protocatechuic acid-mediated bacterial lethality. MicrobiologyOpen. 2017, 6, e00472. [Google Scholar] [CrossRef]
- Friedman, M.; Henika, P.R.; Mandrell, R.E. Antibacterial Activities of Phenolic Benzaldehydes and Benzoic Acids against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J. Food Prot. 2003, 66, 1811–1821. [Google Scholar] [CrossRef]
- Tanaka, T.; Tanaka, T.; Tanaka, M. Potential Cancer Chemopreventive Activity of Protocatechuic Acid. J. Exp. Clin. Med. 2011, 3, 27–33. [Google Scholar] [CrossRef]
- Yamabe, N.; Park, J.Y.; Lee, S.; Cho, E.-J.; Lee, S.; Kang, K.S.; Hwang, G.S.; Kim, S.-N.; Kim, H.Y.; Shibamoto, T. Protective effects of protocatechuic acid against cisplatin-induced renal damage in rats. J. Funct. Foods 2015, 19, 20–27. [Google Scholar] [CrossRef] [Green Version]
- Kakkar, S.; Bais, S. A Review on Protocatechuic Acid and Its Pharmacological Potential. ISRN Pharmacol. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Usman, M.S.; Hussein, M.Z.; Kura, A.U.; Fakurazi, S.; Masarudin, M.J.; Saad, F.F.A. Synthesis and characterization of protocatechuic acid-loaded gadolinium-layered double hydroxide and gold nanocomposite for theranostic application. Appl. Nanosci. 2018, 5, 973–986. [Google Scholar] [CrossRef]
- Oh, J.-M.; Choi, S.-J.; Lee, G.-E.; Han, S.-H.; Choy, J.-H. Inorganic Drug-Delivery Nanovehicle Conjugated with Cancer-Cell-Specific Ligand. Adv. Funct. Mater. 2009, 19, 1617–1624. [Google Scholar] [CrossRef]
- Hadwiger, L.A. Multiple effects of chitosan on plant systems: Solid science or hype. Plant Sci. 2013, 208, 42–49. [Google Scholar] [CrossRef]
- El Hadrami, A.; Adam, L.R.; El Hadrami, I.; Daayf, F. Chitosan in plant protection. Mar. Drugs 2010, 8, 968–987. [Google Scholar]
- Aziz, A.; Trotel-Aziz, P.; Conreux, A.; Jeandet, D.L.P.; Couderchet, M. Chitosan induces phytoalexin synthesis, chitinase and β-1,3-glucanase activities, and resistance of grapevine to fungal pathogens. In Macromolecules and Secondary Metabolites of Grapevine and Wine; 2007; pp. 83–88. Available online: https://www.researchgate.net/publication/234094409_Chitosan_induces_phytoalexin_synthesis_chitinase_and_b-13-glucanase_activities_and_resistance_of_grapevine_to_fungal_pathogens (accessed on 21 January 2019).
- Riva, R.; Ragelle, H.; des Rieux, A.; Duhem, N.; Jérôme, C.; Préat, V. Chitosan and Chitosan Derivatives in Drug Delivery and Tissue Engineering. In Chitosan for Biomaterials II; Jayakumar, R., Prabaharan, M., Muzzarelli, R.A.A., Eds.; Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2011; pp. 19–44. ISBN 9783642240614. [Google Scholar]
- Mohandas, A.; Deepthi, S.; Biswas, R.; Jayakumar, R. Chitosan based metallic nanocomposite scaffolds as antimicrobial wound dressings. Bioact. Mater. 2018, 3, 267–277. [Google Scholar] [CrossRef]
- Nguyen, T.B.T.; Dang, L.H.; Nguyen, T.T.T.; Tran, D.L.; Nguyen, D.H.; Nguyen, V.T.; Nguyen, C.K.; Nguyen, T.H.; Le, V.T.; Tran, N.Q. Green processing of thermosensitive nanocurcumin-encapsulated chitosan hydrogel towards biomedical application. Green Process. Synth. 2016, 5. [Google Scholar] [CrossRef] [Green Version]
- Hiep, N.T.; Hai, N.D.; Toi, V.V. Fabrication of Core-Shell PLGA-Chitosan Microparticles Using Electrospinning: Effects of Polymer Concentration. Int. J. Polym. Sci. 2017, 2017, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Hoang, D.Q.; Tran, T.V.; Tran, N.Q.; Nguyen, C.K.; Nguyen, T.H.; Truong, M.D.; Tran, D.L.; Thu, L.V.; Nguyen, D.H. Functionalization of Fe3O4 nanoparticles with biodegradable chitosan-grafted-mPEG for paclitaxel delivery. Green Process. Synth. 2016, 5. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Tran, N.Q.; Nguyen, C.K. Tetronic-grafted chitosan hydrogel as an injectable and biocompatible scaffold for biomedical applications. J. Biomater. Sci. Polym. Ed. 2013, 24, 1636–1648. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.D.; Van Dang, P.; Le, A.Q.; Nguyen, T.K.L.; Pham, D.H.; Van Nguyen, N.; Nguyen, Q.H. Effect of oligochitosan and oligo-β-glucan supplementation on growth, innate immunity, and disease resistance of striped catfish (Pangasianodon hypophthalmus): Oligosaccharide Immunostimulants. Biotechnol. Appl. Biochem. 2017, 64, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Phuong, N.T.; Anh Ho, V.; Hai Nguyen, D.; Khoa, N.C.; Quyen, T.N.; Lee, Y.; Park, K.D. Enzyme-mediated fabrication of an oxidized chitosan hydrogel as a tissue sealant. J. Bioact. Compat. Polym. 2015, 30, 412–423. [Google Scholar] [CrossRef]
- Akter Mukta, J.; Rahman, M.; As Sabir, A.; Gupta, D.R.; Surovy, M.Z.; Rahman, M.; Islam, M.T. Chitosan and plant probiotics application enhance growth and yield of strawberry. Biocatal. Agric. Biotechnol. 2017, 11, 9–18. [Google Scholar] [CrossRef]
- Wanichpongpan, P.; Suriyachan, K.; Chandrkrachang, S. Effects of Chitosan on the growth of Gerbera flower plant (Gerbera jamesonii). In Chitin and Chitosan in Life Science; Uragami, T., Kurita, K., Fukamizo, T., Eds.; Kodansha Scientific: Yamaguchi, Japan, 2001; pp. 198–201. ISBN 4-906464-43-0. [Google Scholar]
- Desai, K.G. Chitosan Nanoparticles Prepared by Ionotropic Gelation: An Overview of Recent Advances. Crit. Rev. TM Ther. Drug Carr. Syst. 2016, 33, 107–158. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Meng, C.; Yan, Y.; Shan, Y.; Kan, J.; Jin, C. Protocatechuic acid grafted onto chitosan: Characterization and antioxidant activity. Int. J. Biol. Macromol. 2016, 89, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Paomephan, P.; Assavanig, A.; Chaturongakul, S.; Cady, N.C.; Bergkvist, M.; Niamsiri, N. Insight into the antibacterial property of chitosan nanoparticles against Escherichia coli and Salmonella Typhimurium and their application as vegetable wash disinfectant. Food Control 2018, 86, 294–301. [Google Scholar] [CrossRef]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J. Controll. Release 2004, 100, 5–28. [Google Scholar] [CrossRef]
- Sathiyabama, M.; Manikandan, A. Chitosan nanoparticle induced defense responses in fingermillet plants against blast disease caused by Pyricularia grisea (Cke.) Sacc. Carbohydr. Polym. 2016, 154, 241–246. [Google Scholar] [CrossRef]
- Katas, H.; Mohamad, A.; Zin, N.M. Physicochemical Effects of Chitosan-Tripolyphosphate Nanoparticles on Antibacterial Activity against Gram-positive and Gram-negative Bacteria. J. Med. Sci. 2011, 11, 192–197. [Google Scholar] [CrossRef]
- Manikandan, A.; Sathiyabama, M. Preparation of Chitosan nanoparticles and its effect on detached rice leaves infected with Pyricularia grisea. Int. J. Biol. Macromol. 2016, 84, 58–61. [Google Scholar] [CrossRef] [PubMed]






| Sample | Optical Density |
|---|---|
| CSNPs | 0.036 |
| PCA (1) | 0.386 |
| PCA (2) | 0.388 |
| PCA (3) | 0.386 |
| Concentration of PCA (ppm) | PCA (mm) | CSNPs (mm) | CS@PCA (mm) | ||
|---|---|---|---|---|---|
| 24 h | 48 h | 96 h | |||
| 500 | 6 ± 0.33 | 9 ± 0.56 | 8 ± 0.4 | 10 ± 0.3 | 6 ± 0.5 |
| 1000 | 8 ± 0.36 | 8 ± 0.56 | 10 ± 0.3 | 12 ± 0.3 | 9 ± 0.3 |
| 2500 | 12 ± 0.4 | 6 ± 0.48 | 12 ± 0.5 | 14 ± 0.7 | 12 ± 0.4 |
| 5000 | 14 ± 0.56 | 5 ± 0.6 | 14 ± 0.3 | 15 ± 0.4 | 16 ± 0.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pham, T.T.; Nguyen, T.H.; Thi, T.V.; Nguyen, T.-T.; Le, T.D.; Vo, D.M.H.; Nguyen, D.H.; Nguyen, C.K.; Nguyen, D.C.; Nguyen, T.T.; et al. Investigation of Chitosan Nanoparticles Loaded with Protocatechuic Acid (PCA) for the Resistance of Pyricularia oryzae Fungus against Rice Blast. Polymers 2019, 11, 177. https://doi.org/10.3390/polym11010177
Pham TT, Nguyen TH, Thi TV, Nguyen T-T, Le TD, Vo DMH, Nguyen DH, Nguyen CK, Nguyen DC, Nguyen TT, et al. Investigation of Chitosan Nanoparticles Loaded with Protocatechuic Acid (PCA) for the Resistance of Pyricularia oryzae Fungus against Rice Blast. Polymers. 2019; 11(1):177. https://doi.org/10.3390/polym11010177
Chicago/Turabian StylePham, The Trinh, Thi Hiep Nguyen, Thuan Vo Thi, Thanh-Truc Nguyen, Tien Dung Le, Do Minh Hoang Vo, Dai Hai Nguyen, Cuu Khoa Nguyen, Duy Chinh Nguyen, Trong Tuan Nguyen, and et al. 2019. "Investigation of Chitosan Nanoparticles Loaded with Protocatechuic Acid (PCA) for the Resistance of Pyricularia oryzae Fungus against Rice Blast" Polymers 11, no. 1: 177. https://doi.org/10.3390/polym11010177





