Color Tuning by Oxide Addition in PEDOT:PSS-Based Electrochromic Devices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Electrochromic Ink Formulation
2.2. Thin Film Deposition and Display Processing
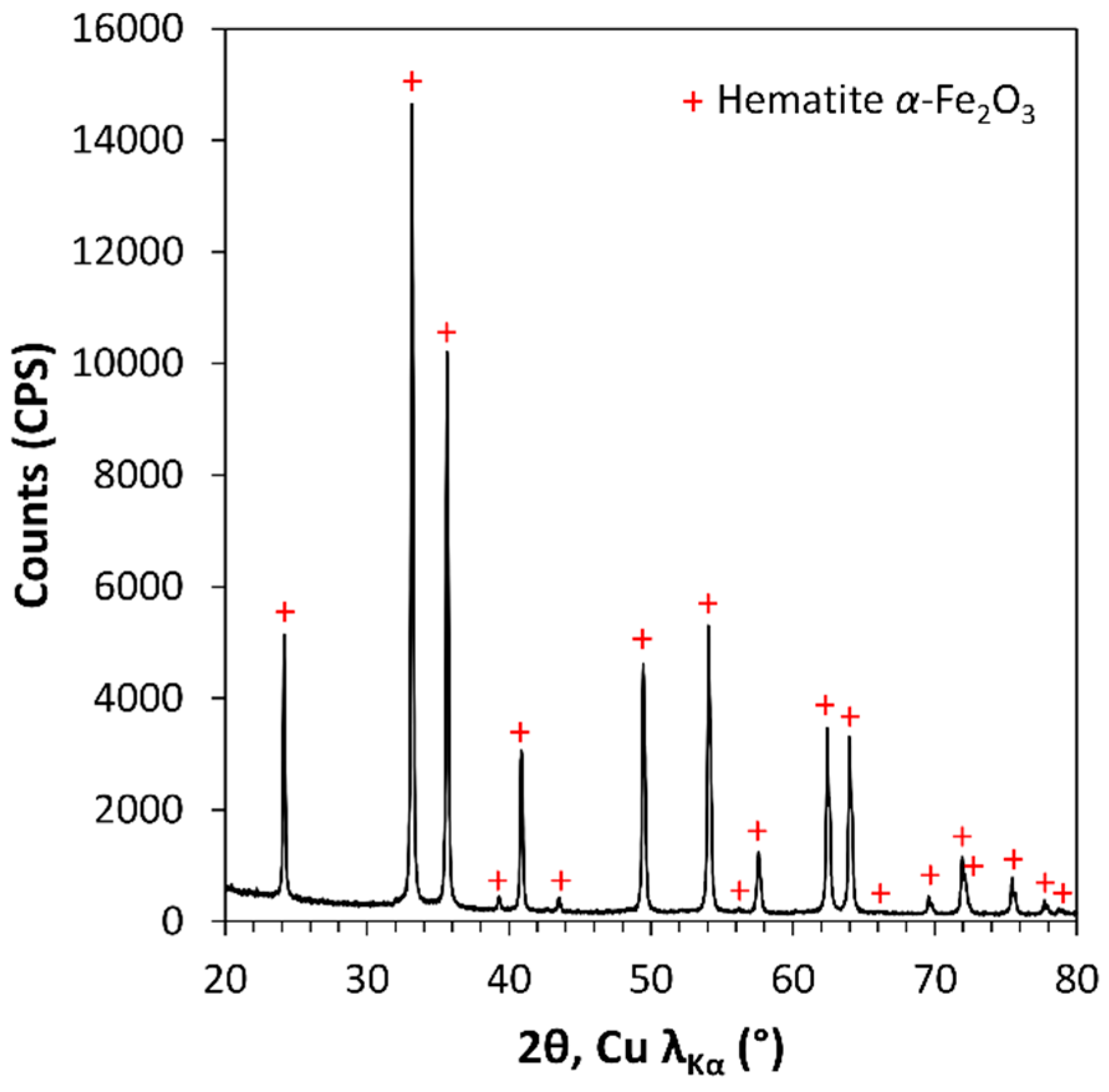
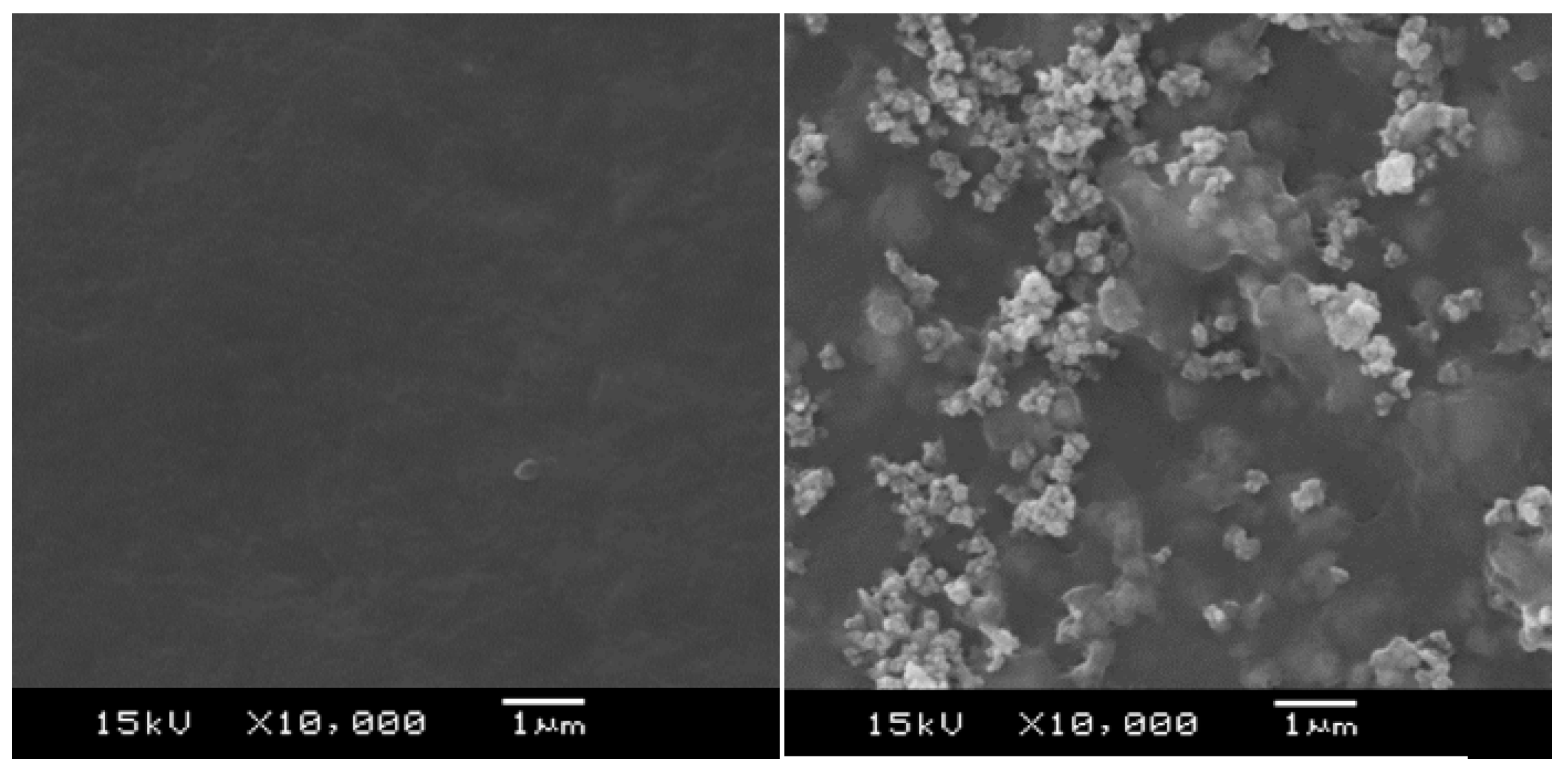
2.3. Structure, Morphology, and Thickness Measurements
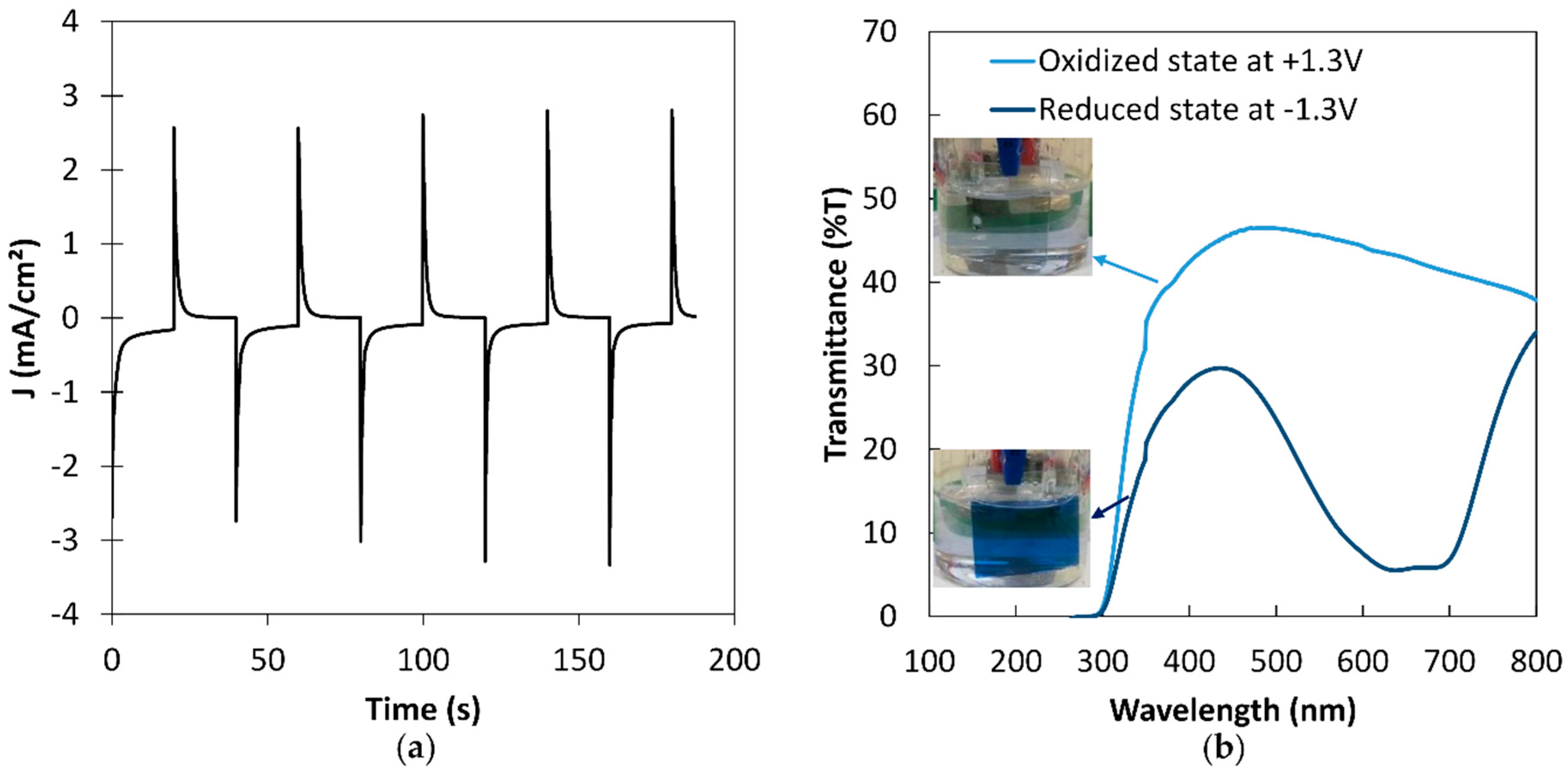
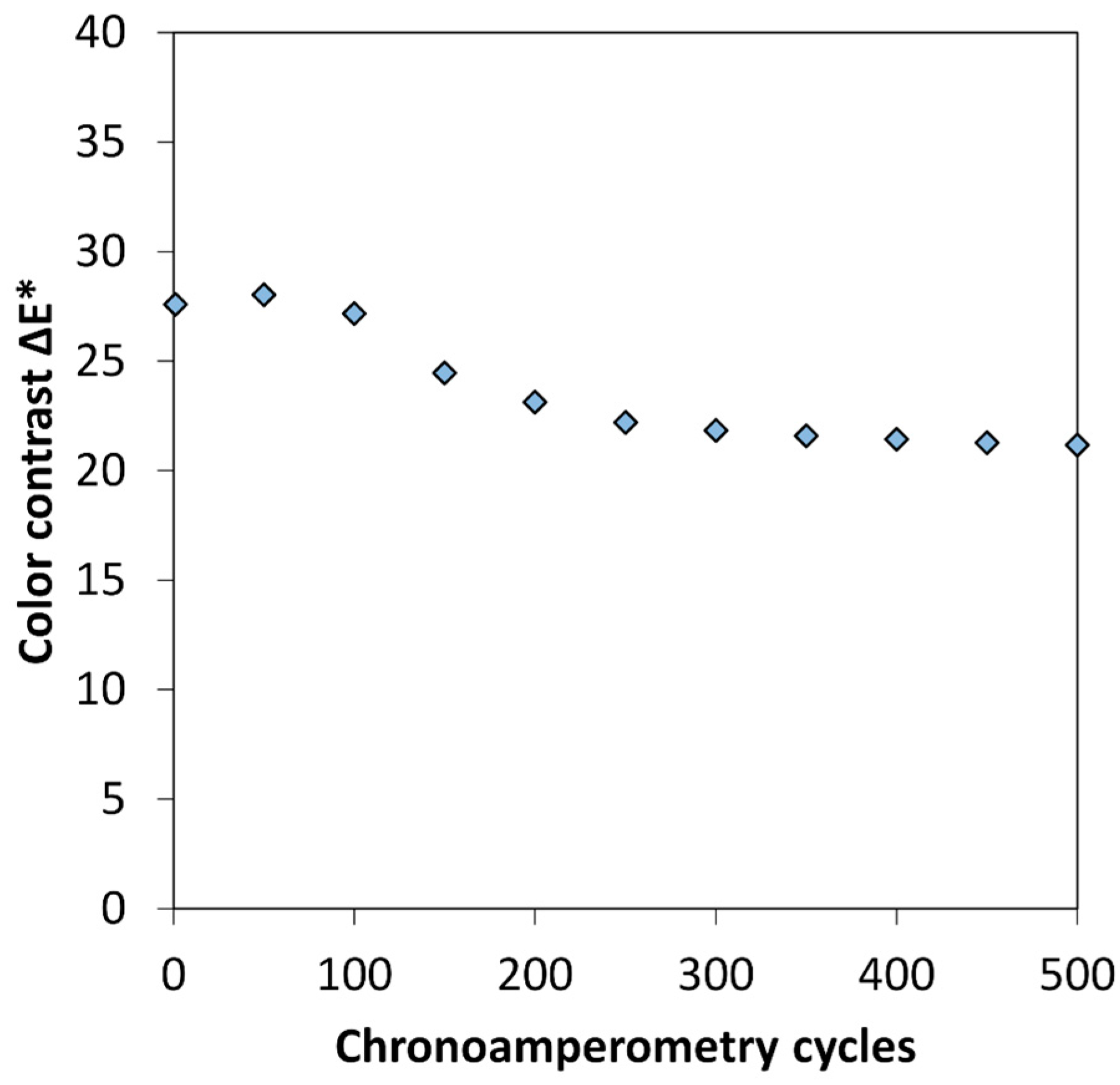
2.4. Electrochromic Measurements
3. Results and Discussion
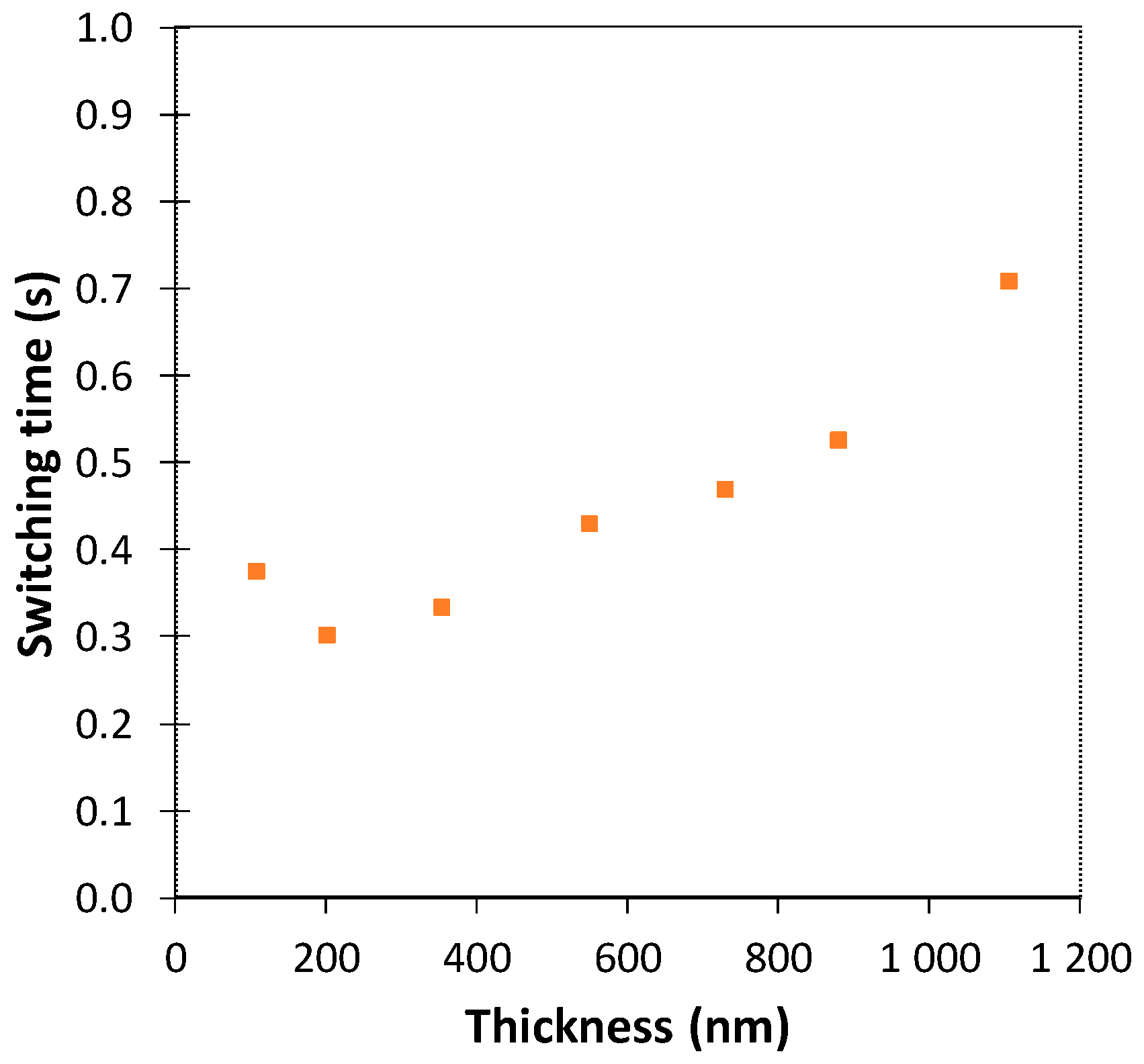
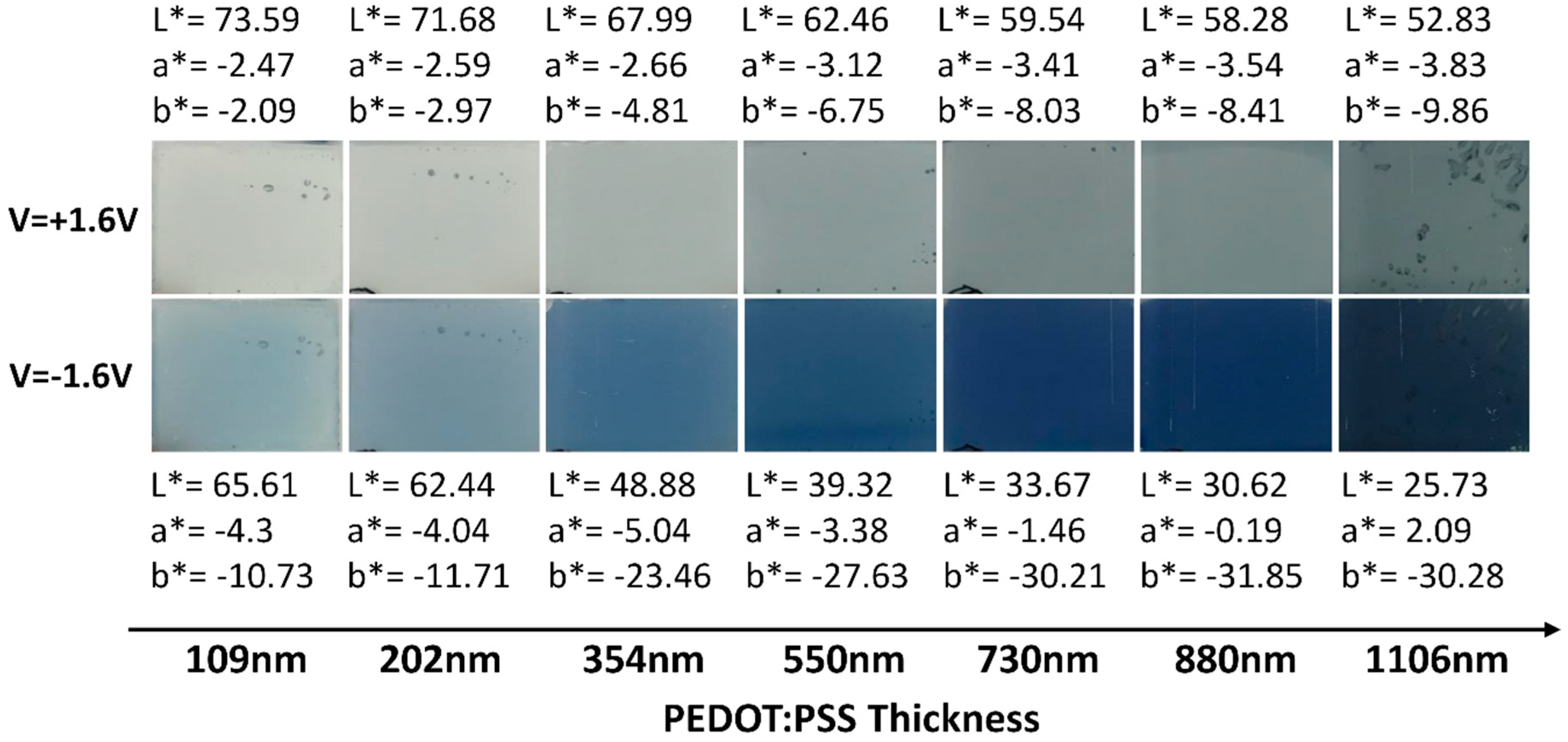
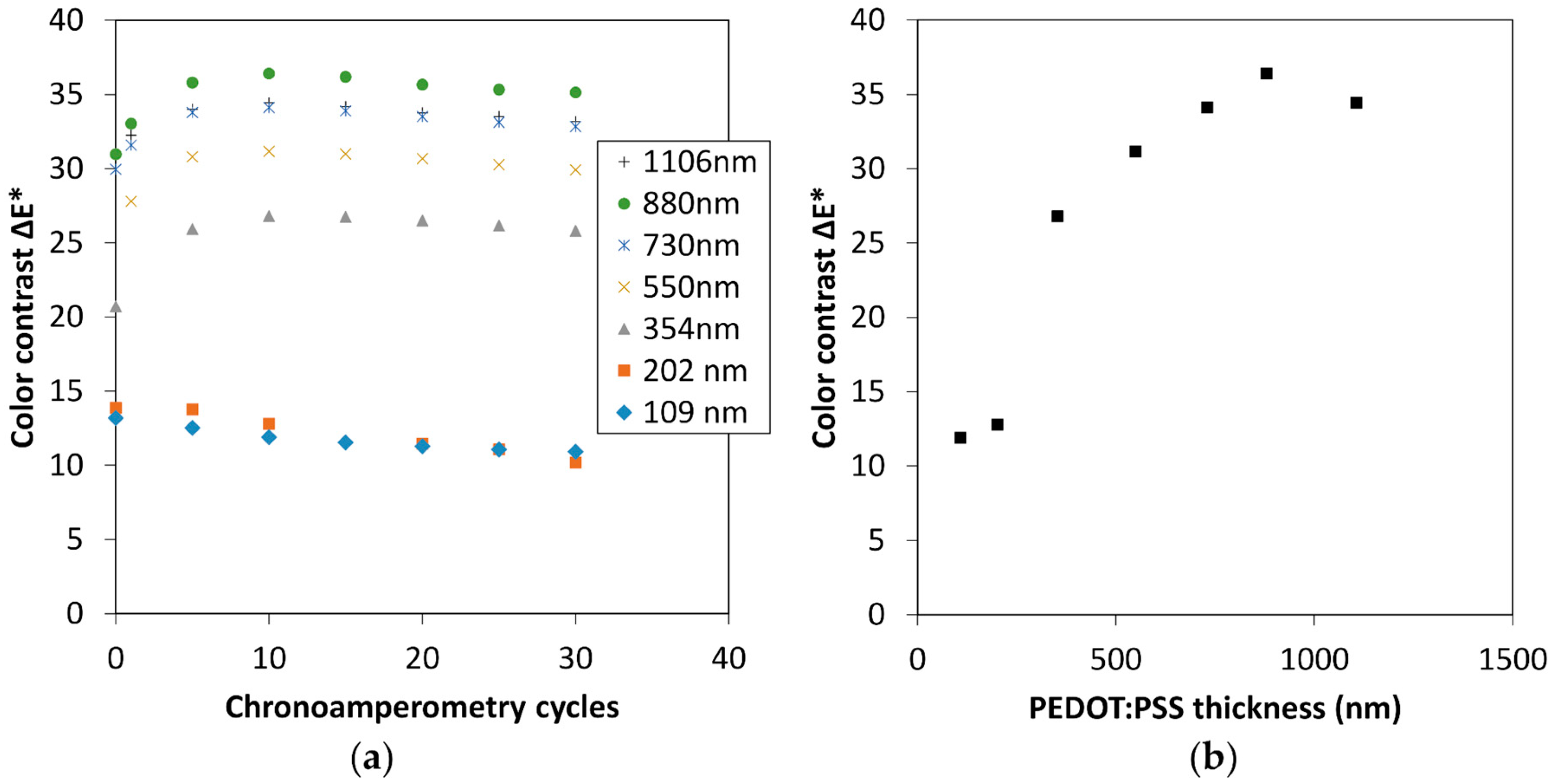
3.1. Optimization of the PEDOT:PSS Thickness
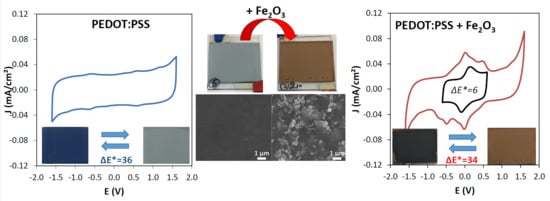
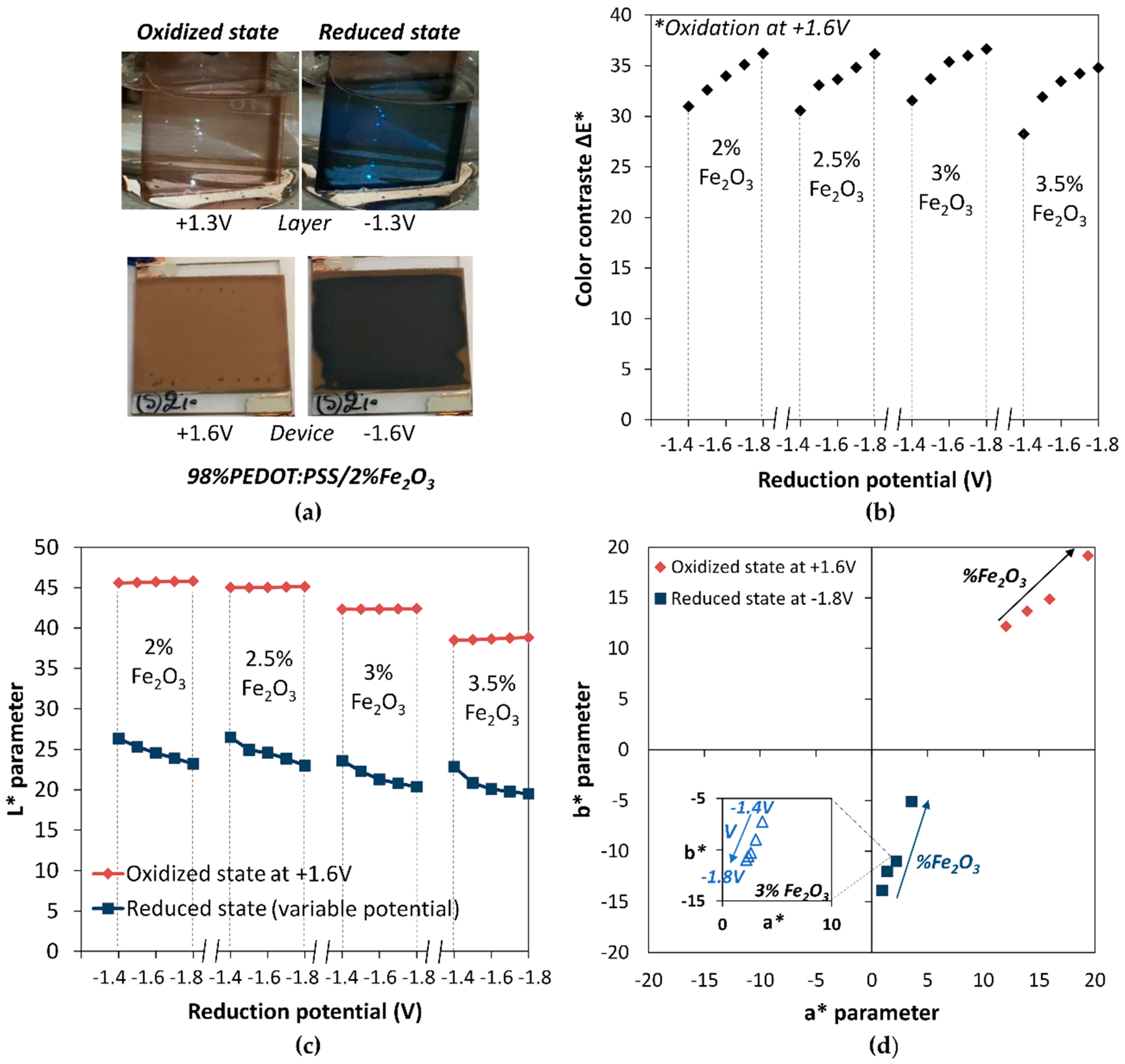
3.2. Tuning of the PEDOT:PSS Color by Fe2O3 Pigment Addition
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
References
- Rauh, R.D.; Wang, F.; Reynolds, J.R.; Meeker, D.L. High coloration efficiency electrochromics and their application to multi-color devices. Electrochim. Acta 2001, 46, 2023–2029. [Google Scholar] [CrossRef]
- Granqvist, C.G. Oxide electrochromics: An introduction to devices and materials. Sol. Energy Mater. Sol. Cells 2012, 99, 1–13. [Google Scholar] [CrossRef]
- Granqvist, C.G.; Arvizu, M.A.; Qu, H.-Y.; Wen, R.-T.; Niklasson, G.A. Advances in electrochromic device technology: Multiple roads towards superior durability. Surf. Coat. Technol. 2019, 357, 619–625. [Google Scholar] [CrossRef]
- Invernale, M.A.; Ding, Y.; Sotzing, G.A. All-Organic Electrochromic Spandex. ACS Appl. Mater. Interfaces 2010, 2, 296–300. [Google Scholar] [CrossRef]
- Österholm, A.M.; Shen, D.E.; Kerszulis, J.A.; Bulloch, R.H.; Kuepfert, M.; Dyer, A.L.; Reynolds, J.R. Four Shades of Brown: Tuning of Electrochromic Polymer Blends Toward High-Contrast Eyewear. ACS Appl. Mater. Interfaces 2015, 7, 1413–1421. [Google Scholar] [CrossRef]
- Cai, G.; Eh, A.L.-S.; Ji, L.; Lee, P.S. Recent Advances in Electrochromic Smart Fenestration. Adv. Sustain. Syst. 2017, 1, 1700074. [Google Scholar] [CrossRef]
- Mjejri, I.; Manceriu, L.M.; Gaudon, M.; Rougier, A.; Sediri, F. Nano-vanadium pentoxide films for electrochromic displays. Solid State Ion. 2016, 292, 8–14. [Google Scholar] [CrossRef]
- Danine, A.; Cojocaru, L.; Faure, C.; Olivier, C.; Toupance, T.; Campet, G.; Rougier, A. Room Temperature UV treated WO3 thin films for electrochromic devices on paper substrate. Electrochim. Acta 2014, 129, 113–119. [Google Scholar] [CrossRef]
- Da Rocha, M.; He, Y.; Diao, X.; Rougier, A. Influence of cycling temperature on the electrochromic properties of WO 3//NiO devices built with various thicknesses. Sol. Energy Mater. Sol. Cells 2018, 177, 57–65. [Google Scholar] [CrossRef]
- Huang, L.-M.; Peng, C.-Y.; Hu, C.-W.; Lu, H.-C.; Chen, C.-H.; Yang, D.-J.; Kuo, C.-C.; Ho, K.-C. Spectroelectrochemical and adhesion properties of chemically synthesized ion conducting poly (vinyl butyral) in Prussian blue and poly (3,4-ethylenedioxythiophene) laminated electrochromic glazing. Sol. Energy Mater. Sol. Cells 2017, 171, 258–266. [Google Scholar] [CrossRef]
- Mortimer, R.J.; Dyer, A.L.; Reynolds, J.R. Electrochromic organic and polymeric materials for display applications. Displays 2006, 27, 2–18. [Google Scholar] [CrossRef] [Green Version]
- Maier, A.; Cheng, K.; Savych, J.; Tieke, B. Double-Electrochromic Coordination Polymer Network Films. ACS Appl. Mater. Interfaces 2011, 3, 2710–2718. [Google Scholar] [CrossRef] [PubMed]
- Österholm, A.M.; Shen, D.E.; Gottfried, D.S.; Reynolds, J.R. Full Color Control and High-Resolution Patterning from Inkjet Printable Cyan/Magenta/Yellow Colored-to-Colorless Electrochromic Polymer Inks. Adv. Mater. Technol. 2016, 1, 1600063. [Google Scholar] [CrossRef]
- Li, S.; Liu, G.; Ju, X.; Zhang, Y.; Zhao, J. Synthesis, Characterization and Application of Four Novel Electrochromic Materials Employing Nitrotriphenylamine Unit as the Acceptor and Different Thiophene Derivatives as the Donor. Polymers 2017, 9, 173. [Google Scholar] [CrossRef]
- He, J.; Mukherjee, S.; Zhu, X.; You, L.; Boudouris, B.W.; Mei, J. Highly Transparent Crosslinkable Radical Copolymer Thin Film as the Ion Storage Layer in Organic Electrochromic Devices. ACS Appl. Mater. Interfaces 2018, 10, 18956–18963. [Google Scholar] [CrossRef]
- Kuo, C.-W.; Wu, B.-W.; Chang, J.-K.; Chang, J.-C.; Lee, L.-T.; Wu, T.-Y.; Ho, T.-H. Electrochromic Devices Based on Poly(2,6-di(9H-carbazol-9-yl)pyridine)-Type Polymer Films and PEDOT-PSS. Polymers 2018, 10, 604. [Google Scholar] [CrossRef]
- Padilla, J.; Österholm, A.M.; Dyer, A.L.; Reynolds, J.R. Process controlled performance for soluble electrochromic polymers. Sol. Energy Mater. Sol. Cells 2015, 140, 54–60. [Google Scholar] [CrossRef]
- Andersson, P.; Forchheimer, R.; Tehrani, P.; Berggren, M. Printable All-Organic Electrochromic Active-Matrix Displays. Adv. Funct. Mater. 2007, 17, 3074–3082. [Google Scholar] [CrossRef]
- Shim, G.H.; Han, M.G.; Sharp-Norton, J.C.; Creager, S.E.; Foulger, S.H. Inkjet-printed electrochromic devices utilizing polyaniline–silica and poly(3,4-ethylenedioxythiophene)–silica colloidal composite particles. J. Mater. Chem. 2008, 18, 594–601. [Google Scholar] [CrossRef]
- Costa, C.; Pinheiro, C.; Henriques, I.; Laia, C.A.T. Inkjet Printing of Sol–Gel Synthesized Hydrated Tungsten Oxide Nanoparticles for Flexible Electrochromic Devices. ACS Appl. Mater. Interfaces 2012, 4, 1330–1340. [Google Scholar] [CrossRef]
- Shi, P.; Amb, C.M.; Dyer, A.L.; Reynolds, J.R. Fast Switching Water Processable Electrochromic Polymers. ACS Appl. Mater. Interfaces 2012, 4, 6512–6521. [Google Scholar] [CrossRef]
- Layani, M.; Darmawan, P.; Foo, W.L.; Liu, L.; Kamyshny, A.; Mandler, D.; Magdassi, S.; Lee, P.S. Nanostructured electrochromic films by inkjet printing on large area and flexible transparent silver electrodes. Nanoscale 2014, 6, 4572. [Google Scholar] [CrossRef] [PubMed]
- De Keersmaecker, M.; Lang, A.W.; Österholm, A.M.; Reynolds, J.R. All Polymer Solution Processed Electrochromic Devices: A Future without Indium Tin Oxide? ACS Appl. Mater. Interfaces 2018, 10, 31568–31579. [Google Scholar] [CrossRef]
- Sze, P.-W.; Lee, K.-W.; Huang, P.-C.; Chou, D.-W.; Kao, B.-S.; Huang, C.-J. The Investigation of High Quality PEDOT:PSS Film by Multilayer-Processing and Acid Treatment. Energies 2017, 10, 716. [Google Scholar] [CrossRef]
- Kawahara, J.; Ersman, P.A.; Engquist, I.; Berggren, M. Improving the color switch contrast in PEDOT:PSS-based electrochromic displays. Org. Electron. 2012, 13, 469–474. [Google Scholar] [CrossRef] [Green Version]
- Burke, L.D.; Lyons, M.E.G. The formation and stability of hydrous oxide films on iron under potential cycling conditions in aqueous solution at high pH. J. Electroanal. Chem. Interfacial Electrochem. 1986, 198, 347–368. [Google Scholar] [CrossRef]
- Gutiérrez, C.; Beden, B. UV-Visible differential reflectance spectroscopy of the electrochromic oxide layer on iron in 0.1 M NaOH. J. Electroanal. Chem. Interfacial Electrochem. 1990, 293, 253–259. [Google Scholar] [CrossRef]
- Campet, G.; Wen, S.J.; Han, S.D.; Shastry, M.C.R.; Portier, J.; Guizard, C.; Cot, L.; Xu, Y.; Salardenne, J. Reversible electrochemical insertion of lithium in fine-grained polycrystalline thin films of mixed-valency metal oxides: Application to Li_Fe203 thin film electrodes prepared by Film the sol-gel process. Mater. Sci. Eng. B 1993, 18, 201–208. [Google Scholar] [CrossRef]
- Orel, B.; Maček, M.; Švegl, F.; Kalcher, K. Electrochromism of iron oxide films prepared via the sol-gel route by the dip-coating technique. Thin Solids 1994, 246, 131–142. [Google Scholar] [CrossRef]
- Maruyama, T. Electrochromic Properties of Iron Oxide Thin Films Prepared by Chemical Vapor Deposition. J. Electrochem. Soc. 1996, 143, 1675. [Google Scholar] [CrossRef]
- Aegerter, M.A.; Avellaneda, C.O.; Pawlicka, A.; Atik, M. Electrochromism in materials prepared by the sol-gel process. J. Sol-Gel. Sci. Technol. 1997, 8, 689–696. [Google Scholar] [CrossRef]
- Garcia-Lobato, M.A.; Martinez, A.I.; Zarate, R.A.; Castro-Roman, M. New Insight into the Electrochromic Properties of Iron Oxides. Appl. Phys. Express 2010, 3, 115801. [Google Scholar] [CrossRef]
- Garcia-Lobato, M.A.; Martinez, A.I.; Perry, D.L.; Castro-Roman, M.; Zarate, R.A.; Escobar-Alarcon, L. Elucidation of the electrochromic mechanism of nanostructured iron oxides films. Sol. Energy Mater. Sol. Cells 2011, 95, 751–758. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levasseur, D.; Mjejri, I.; Rolland, T.; Rougier, A. Color Tuning by Oxide Addition in PEDOT:PSS-Based Electrochromic Devices. Polymers 2019, 11, 179. https://doi.org/10.3390/polym11010179
Levasseur D, Mjejri I, Rolland T, Rougier A. Color Tuning by Oxide Addition in PEDOT:PSS-Based Electrochromic Devices. Polymers. 2019; 11(1):179. https://doi.org/10.3390/polym11010179
Chicago/Turabian StyleLevasseur, Delphin, Issam Mjejri, Thomas Rolland, and Aline Rougier. 2019. "Color Tuning by Oxide Addition in PEDOT:PSS-Based Electrochromic Devices" Polymers 11, no. 1: 179. https://doi.org/10.3390/polym11010179