Effects of Tung Oil-Based Polyols on the Thermal Stability, Flame Retardancy, and Mechanical Properties of Rigid Polyurethane Foam
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of GTO and EGTO
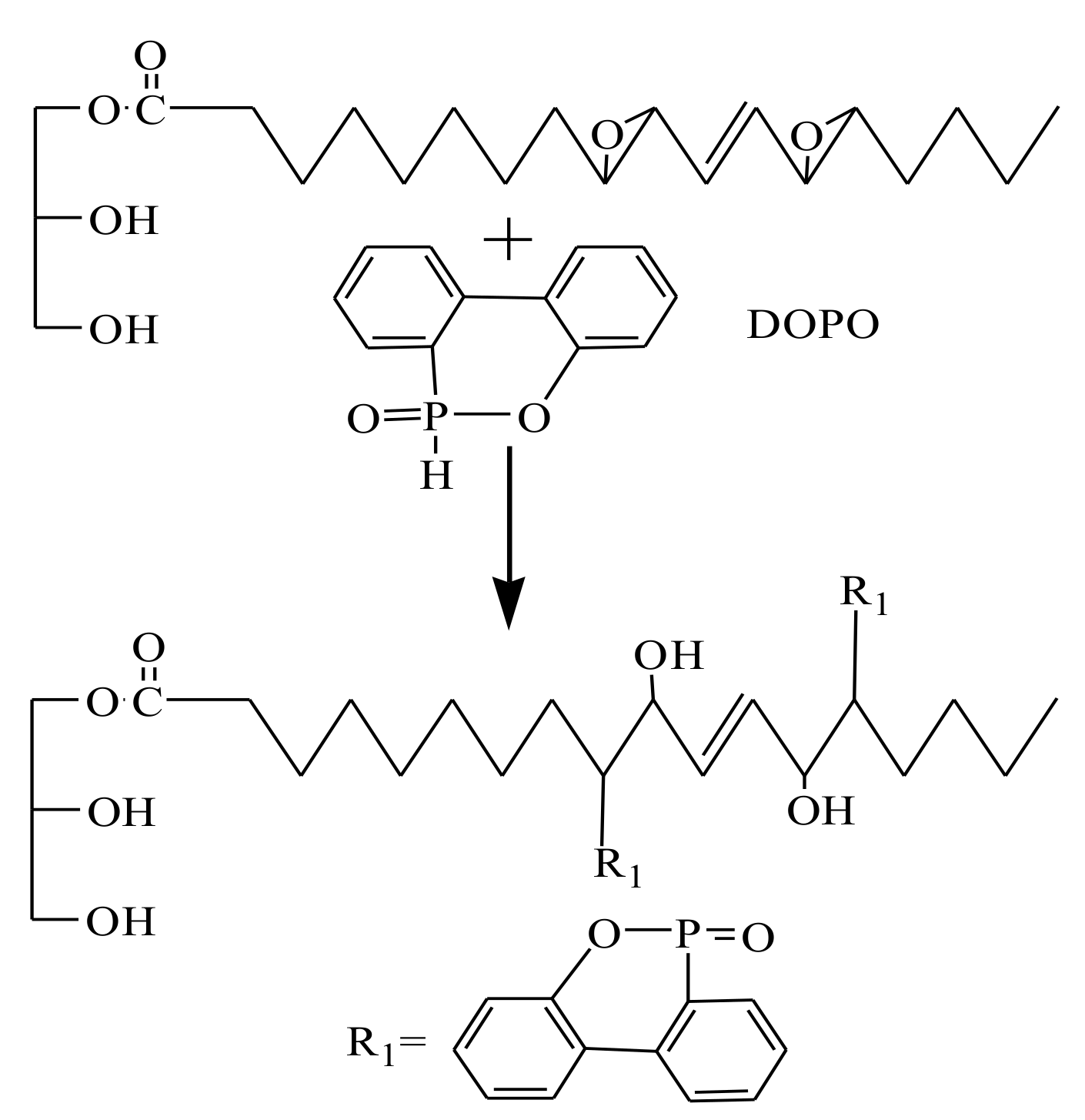
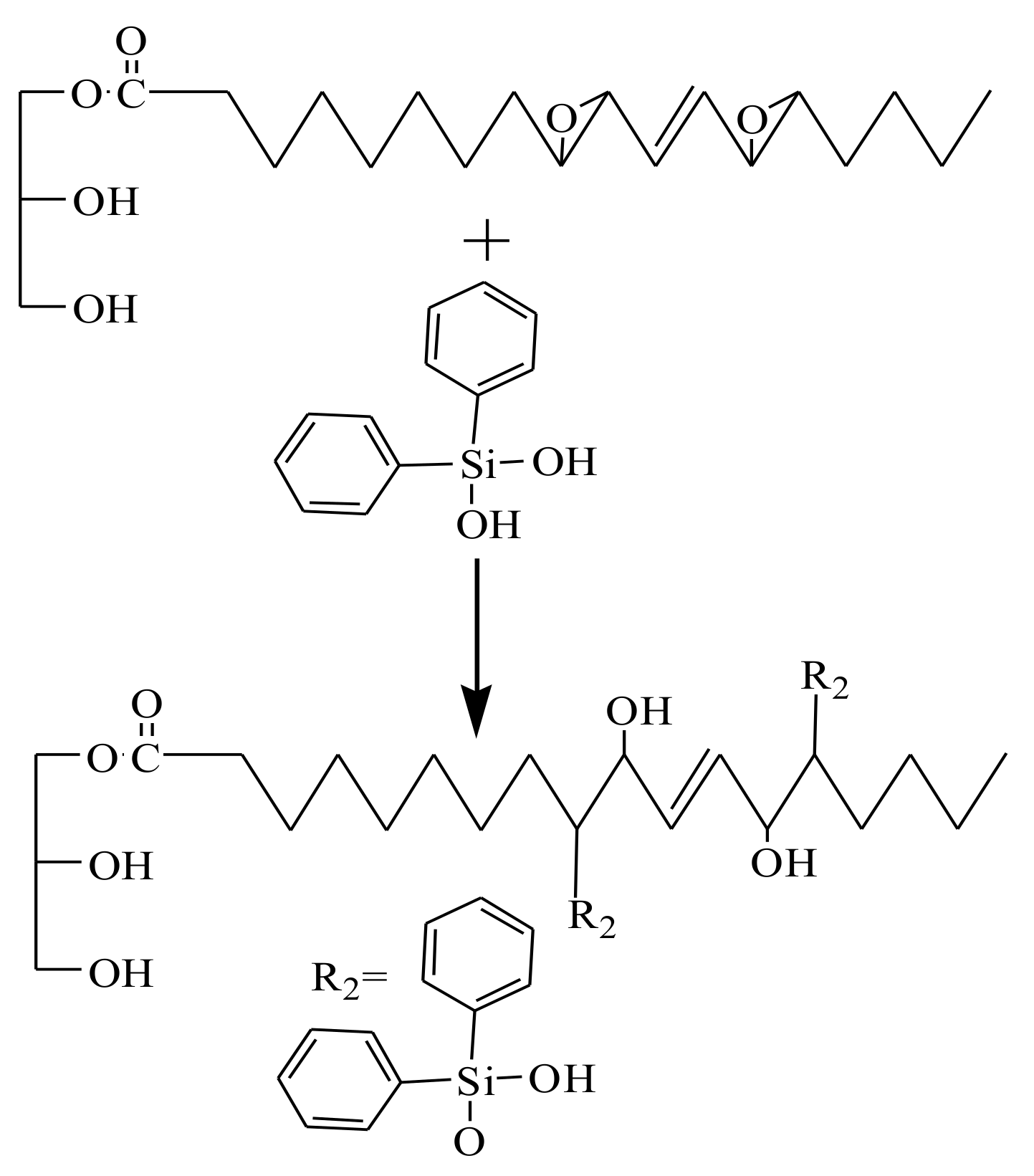
2.3. Synthesis of PTOP and PTOSi
2.4. Preparation of RPUF
2.5. Characterization
2.5.1. Physical Parameter Analysis
2.5.2. Gel Permeation Chromatography (GPC)
2.5.3. Fourier Transform Infrared (FTIR)
2.5.4. Nuclear Magnetic Resonance (NMR)
2.5.5. Scanning Electron Microscope (SEM)
2.5.6. Thermogravimetric Analysis (TGA)
2.5.7. Differential Scanning Calorimeter (DSC)
2.5.8. Limit Oxygen Index (LOI) Test
2.5.9. Cone Calorimeter Test (CTT)
2.5.10. Compressive Strength Test
2.5.11. Thermal Conductivity Test
2.5.12. Density Test
3. Results
3.1. Synthesis of PTOP
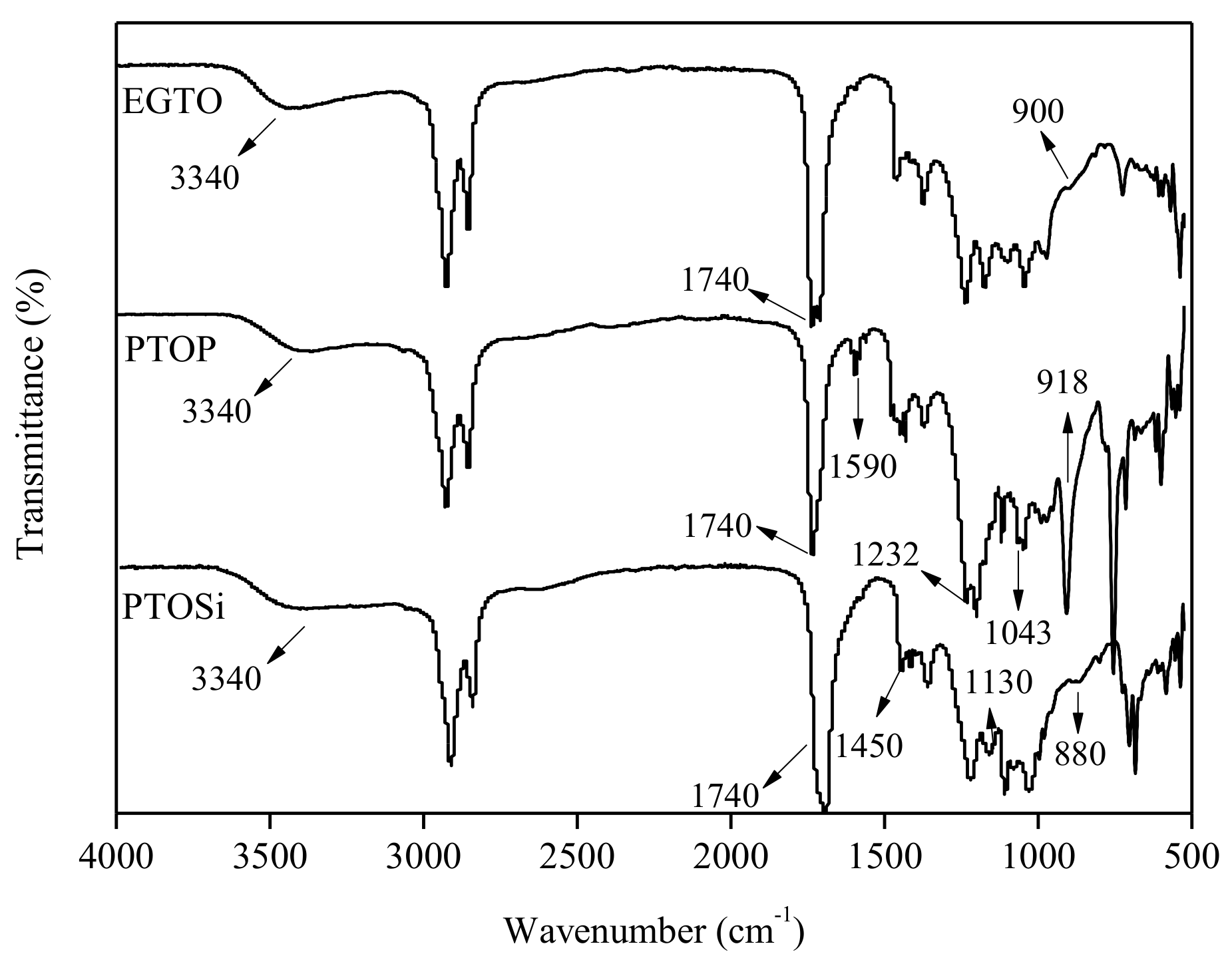
3.2. FTIR of EGTO, PTOP, and PTOSi
3.3. 1H NMR and 31P NMR
3.4. SEM Micrographs of RPUF
3.5. Thermal Property of PTOP, PTOSi, and RPUF
3.5.1. Thermal stability of PTOP, PTOSi, and RPUF
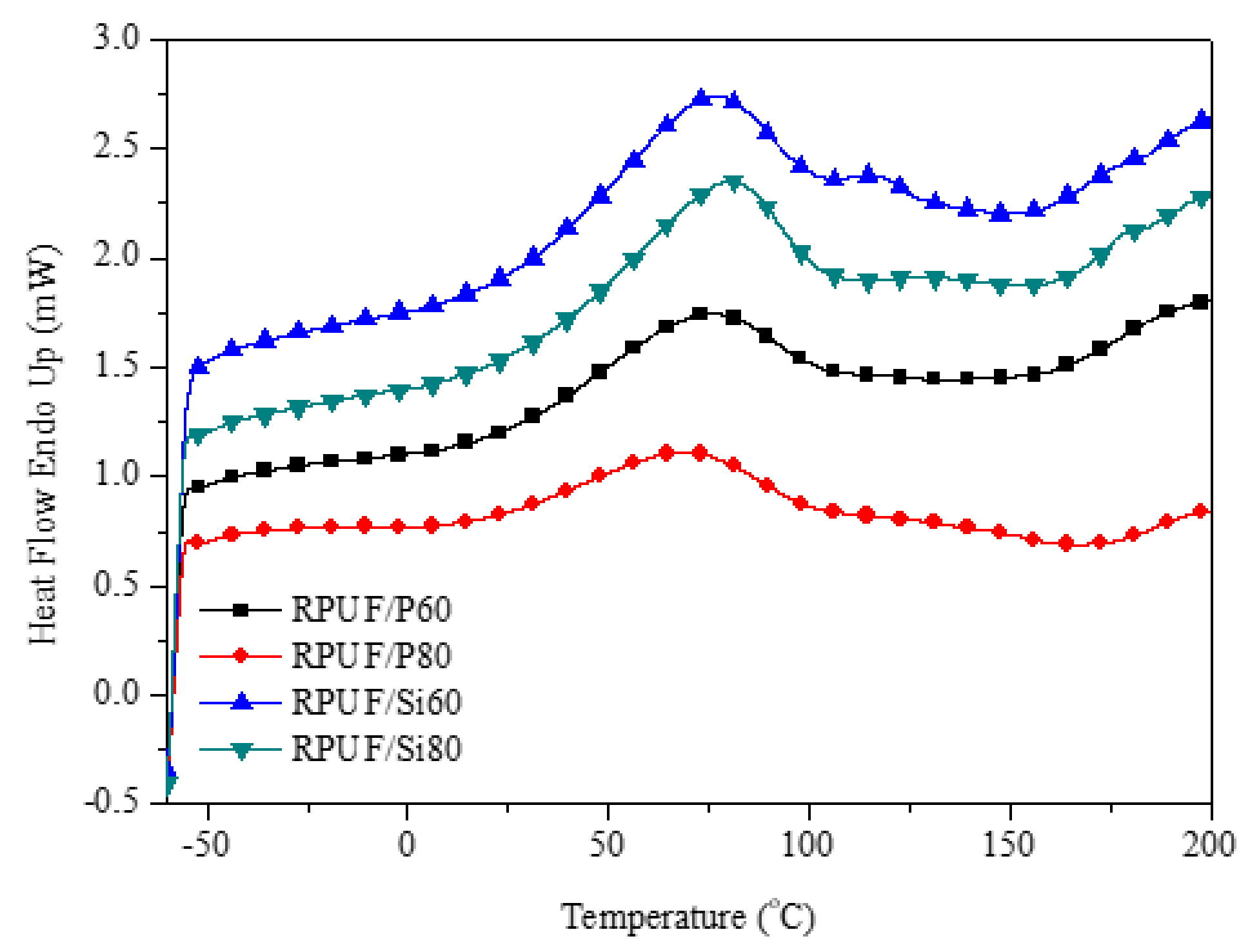
3.5.2. DSC of RPUF
3.6. Flame Retardancy
3.6.1. Limiting Oxygen Index (LOI) Test of RPUF
3.6.2. Fire Behaviors of RPUF
3.6.3. SEM and EDAX of RPUF Residue
3.7. Mechanical Property of RPUF
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sylwia, C.; Massimo, F.B.; Jan, K.; Anna, S.; Marcin, M.; Krzysztof, S. Linseed oil as a natural modifier of rigid polyurethane foams. Ind. Crops Prod. 2018, 115, 40–51. [Google Scholar]
- Lligadas, G.; Ronda, J.C.; Galia, M.; Cadiz, V. Renewable polymeric materials from vegetable oils: A perspective. Mater. Today 2013, 16, 337–343. [Google Scholar] [CrossRef]
- Athanasia, A.S.; David, A.C.E.; Celine, C.; Darren, J.M.; Pratheep, K.A. A systematic study substituting polyether polyol with palm kernel oil based polyester polyol in rigid polyurethane foam. Ind. Crops Prod. 2015, 66, 16–26. [Google Scholar]
- Zhou, X.; Sain, M.M.; Oksman, K. Semi-rigid biopolyurethane foams based on palm-oil polyol and reinforced with cellulose nanocrystals. Compos. Part A Appl. Sci. Manuf. 2015, 83, 56–62. [Google Scholar] [CrossRef]
- Zieleniewska, M.; Leszczynski, M.K.; Kuranska, M.; Prociak, A.; Szczepkowski, L.; Krzyzowska, M.; Ryszkowska, J. Preparation and characterisation of rigid polyurethane foams using a rapeseed oil-based polyol. Ind. Crops Prod. 2015, 74, 887–897. [Google Scholar] [CrossRef]
- Shirke, A.G.; Dholakiya, B.Z.; Kuperkar, K. Modification of tung oil-based polyurethane foam by anhydrides and inorganic content through esterification process. J. Appl. Polym. Sci. 2017, 135, 45786. [Google Scholar] [CrossRef]
- Wang, C.; Wu, Y.C.; Li, Y.C.; Shao, Q.; Yan, X.G.; Han, C.; Wang, Z.; Liu, Z.; Guo, Z.H. Flame-retardant rigid polyurethane foam with a phosphorus-nitrogen single intumescent flame retardant. Polym. Adv. Technol. 2018, 29, 668–676. [Google Scholar] [CrossRef]
- Shi, X.X.; Jiang, S.H.; Zhu, J.Y.; Li, G.H.; Peng, X.F. Establishment of a highly efficient flame-retardant system for rigid polyurethane foams based on biphase flame-retardant actions. RSC Adv. 2018, 8, 9985–9995. [Google Scholar] [CrossRef]
- Shi, S.Y.; Neisius, M.; Mispreuve, H.; Naescher, R.; Gaan, S. Flame retardancy and thermal decomposition of flexible polyurethane foams: Structural influence of organophosphorus compounds. Polym. Degrad. Stab. 2012, 97, 2428–2440. [Google Scholar]
- Thirumal, M.; Khastgir, D.; Nando, G.B.; Naik, Y.P.; Singha, N.K. Halogen-free flame retardant PUF: Effect of melamine compounds on mechanical, thermal and flame retardant properties. Polym. Degrad. Stab. 2010, 95, 1138–1145. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Webster, D.C. Thermal stability and flame retardancy of polyurethanes. Prog. Polym. Sci. 2009, 34, 1068–1133. [Google Scholar] [CrossRef]
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.M.; Dubosi, P. New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mater. Sci. Eng. R Rep. 2009, 63, 100–125. [Google Scholar] [CrossRef]
- Perret, B.; Schartel, B.; Stob, K.; Ciesielski, M.; Diederichs, J.; Doring, M.; Kramer, J.; Altstadt, V. Novel DOPO-based flame retardants in high-performance carbon fibre epoxy composites for aviation. Eur. Polym. 2011, 47, 1081–1089. [Google Scholar] [CrossRef]
- Li, J.L.; Li, Z.W.; Wang, H.G.; Wu, Z.J.; Wang, Z.; Li, S.C. Liquid oxygen compatibility and cryogenic mechanical properties of a novel phosphorous/silicon containing epoxy-based hybrid. RSC Adv. 2016, 6, 91012–91023. [Google Scholar] [CrossRef]
- Gaan, S.; Liang, S.Y.; Mispreuve, H.; Perler, H.; Naescher, R. Flame retardant flexible polyurethane foams from novel DOPO-phosphonamidate additives. Polym. Degrad. Stab. 2015, 113, 180–188. [Google Scholar] [CrossRef]
- Granzow, A. Flame retardation by phosphorus compounds. Acc. Chem. Res. 1978, 11, 177–183. [Google Scholar] [CrossRef]
- Zhang, M.; Luo, Z.Y.; Zhang, J.W.; Chen, S.G.; Zhou, Y.H. Effects of a novel phosphorus-nitrogen flame retardant on rosin-based rigid polyurethane foams. Polym. Degrad. Stab. 2015, 120, 427–434. [Google Scholar] [CrossRef]
- Liu, Y.L.; He, J.Y.; Yang, R.J. The thermal properties and flame retardancy of 9, 10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (10)-Mg/Polyisocyanate -polyurethane foam composites. Bull. Chem. Soc. Jpn. 2016, 89, 779–785. [Google Scholar] [CrossRef]
- Shi, Y.C.; Wang, G.J. The novel silicon-containing epoxy/PEPA phosphate flame retardant for transparent intumescent fire resistant coating. Appl. Surf. Sci. 2016, 385, 453–463. [Google Scholar] [CrossRef]
- Wang, J.H.; Ji, C.T.; Yan, Y.T.; Zhao, D.; Shi, L.Y. Mechanical and ceramifiable properties of silicone rubber filled with different inorganic fillers. Polym. Degrad. Stab. 2015, 121, 149–156. [Google Scholar] [CrossRef]
- Bian, X.C.; Tang, J.H.; Li, Z.M. Flame retardancy of whisker silicon oxide/rigid polyurethane foam composites with expandable graphite. J. Appl. Polym. Sci. 2010, 110, 3871–3879. [Google Scholar] [CrossRef]
- Zhou, H.F.; Wang, H.; Tian, X.Y.; Zheng, K.; Cheng, Q.T. Effect of 3-Aminopropyltriethoxysilane on polycarbonate based waterborne polyurethane transparent coatings. Prog. Org. Coat. 2014, 77, 1073–1078. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.C.; Liu, Q.; Cai, X.F. Synergistic effects between silicon-containing flame retardant and DOPO on flame retardancy of epoxy resins. J. Therm. Anal. Calorim. 2016, 123, 1343–1350. [Google Scholar] [CrossRef]
- Li, M.L.; Zhong, Y.; Wang, Z.; Fischer, A.; Ranft, F.; Drummer, D.; Wu, W. Flame retarding mechanism of polyamide 6 with phosphorus-nitrogen flame retardant and DOPO derivatives. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Zhou, W.; Jia, P.Y.; Zhou, Y.H.; Zhang, M. Preparation and characterization of tung oil-based flame retardant polyols. Chin. J. Chem. Eng. 2018. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Xu, G.L.; Liang, Y.; Yang, J.; Hu, J. Preparation of flame retardant epoxy resins containing DOPO group. Thermochim. Acta 2016, 643, 33–40. [Google Scholar] [CrossRef]
- Xiong, Y.Q.; Zhang, X.Y.; Liu, J.; Li, M.M.; Guo, F.; Xia, X.N.; Xu, W.J. Synthesis of novel phosphorus-containing epoxy hardeners and thermal stability and flame-retardant properties of cured products. J. Appl. Polym. Sci. 2012, 125, 1219–1225. [Google Scholar] [CrossRef]
- Han, M.S.; Kim, Y.H.; Han, S.J.; Choi, S.J.; Kim, S.B.; Kim, W.N. Effects of a silane coupling agent on the exfoliation of organoclay layers in polyurethane/organoclay nanocomposite foams. J. Appl. Polym. Sci. 2008, 110, 376–386. [Google Scholar] [CrossRef]
- Chen, M.J.; Chen, C.R.; Tan, Y.; Huang, J.Q.; Wang, X.L.; Chen, L. Inherently flame-retardant flexible polyurethane foam with low content of phosphorus-containing cross-linking agent. Ind. Eng. Chem. Res. 2014, 53, 1160–1171. [Google Scholar] [CrossRef]
- Wu, K.; Song, L.; Hu, Y.; Lu, H.D.; Kandola, B.K.; Kandare, E. Synthesis and characterization of a functional polyhedral oligomeric silsesquioxane and its flame retardancy in epoxy resin. Prog. Org. Coat. 2009, 65, 490–497. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.C.; Li, X.M.; Yang, R.J. Pyrolysis and fire behavior of epoxy resin composites based on a phosphorus-containing polyhedral oligomeric silsesquioxane (DOPO-POSS). Polym. Degrad. Stab. 2011, 96, 1821–1832. [Google Scholar] [CrossRef]
- Hamdani, S.; Longuet, C.; Perrin, D.; Lopez-cuesta, J.M.; Ganachaud, F. Flame retardancy of silicone-based materials. Polym. Degrad. Stab. 2009, 94, 465–495. [Google Scholar] [CrossRef]
- Wu, X.; Wang, L.; Wu, C.; Yu, J.; Xie, L.; Wang, G.; Jiang, P. Influence of char residues on flammability of EVA/EG, EVA/NG and EVA/GO composites. Polym. Degrad. Stab. 2012, 97, 54–63. [Google Scholar] [CrossRef]
- Zhao, B.; Chen, L.; Long, J.W.; Chen, H.B.; Wang, Y.Z. Aluminum hypophosphite versus alkyl-substituted phosphinate in polyamide 6: Flame retardance, thermal degradation, and pyrolysis behavior. Ind. Eng. Chem. Res. 2013, 52, 2875–2886. [Google Scholar] [CrossRef]









| Sample | DOPO (mol) | EGTO/Epoxy Group (g/mol) | DPSD (mol) | Conversion Rate (%) | Viscosity (Pa·s) | P (%) | Si (%) | Hydroxyl Value (mg KOH/g) |
|---|---|---|---|---|---|---|---|---|
| PTOP | 0.072 | 100/0.1875 | 0 | 95.85 | 2.19 | 1.85 | 0 | 339.96 |
| PTOSi | 0 | 100/0.1875 | 0.084 | 95.48 | 2.42 | 0 | 1.95 | 317.93 |
| Sample | Content Weight (g) | |||||||
|---|---|---|---|---|---|---|---|---|
| PPG4110 | PTOP | PTOSi | AK8804 | DBTDL | Water | HFC-365mfc | PAPI | |
| Neat RPUF | 100 | 0 | 0 | 2.4 | 1.2 | 1.2 | 20 | 120 |
| RPUF/P60 | 40 | 60 | 0 | 2.4 | 1.2 | 1.2 | 20 | 120 |
| RPUF/P80 | 20 | 80 | 0 | 2.4 | 1.2 | 1.2 | 20 | 120 |
| RPUF/Si60 | 40 | 0 | 60 | 4.0 | 0.28 | 0.17 | 20 | 100 |
| RPUF/Si80 | 20 | 0 | 80 | 4.0 | 0.11 | 0.11 | 20 | 100 |
| Sample | N2 Atmosphere | The Maximum Decomposition Rate (%/min) | Residue Rate (wt %) | |
|---|---|---|---|---|
| Tonset (°C) | Tmax (°C) | |||
| PTOP | 272.40 | 468.10 | 11.31 | 4.13 |
| PTOSi | 228.20 | 440.10 | 6.18 | 8.58 |
| Sample | Tonset (°C) | Tmax (°C) | Residue Rate (wt %) | ||
|---|---|---|---|---|---|
| Step I | Step II | Step III | |||
| Neat RPUF | 313.10 | 354.30 | 478.10 | / | 17.54 |
| RPUF/P60 | 282.60 | 330.90 | 408.70 | 461.40 | 20.48 |
| RPUF/P80 | 261.80 | 319.60 | 402.30 | 460.60 | 19.20 |
| RPUF/Si60 | 291.40 | 335.10 | 463.70 | / | 18.03 |
| RPUF/Si80 | 288.50 | 338.60 | 464.50 | / | 17.00 |
| Sample | Neat RPUF | RPUF/P60 | RPUF/P80 | RPUF/Si60 | RPUF/Si80 |
|---|---|---|---|---|---|
| LOI value (%) | 19.0 | 21.3 | 24.0 | 21.0 | 23.4 |
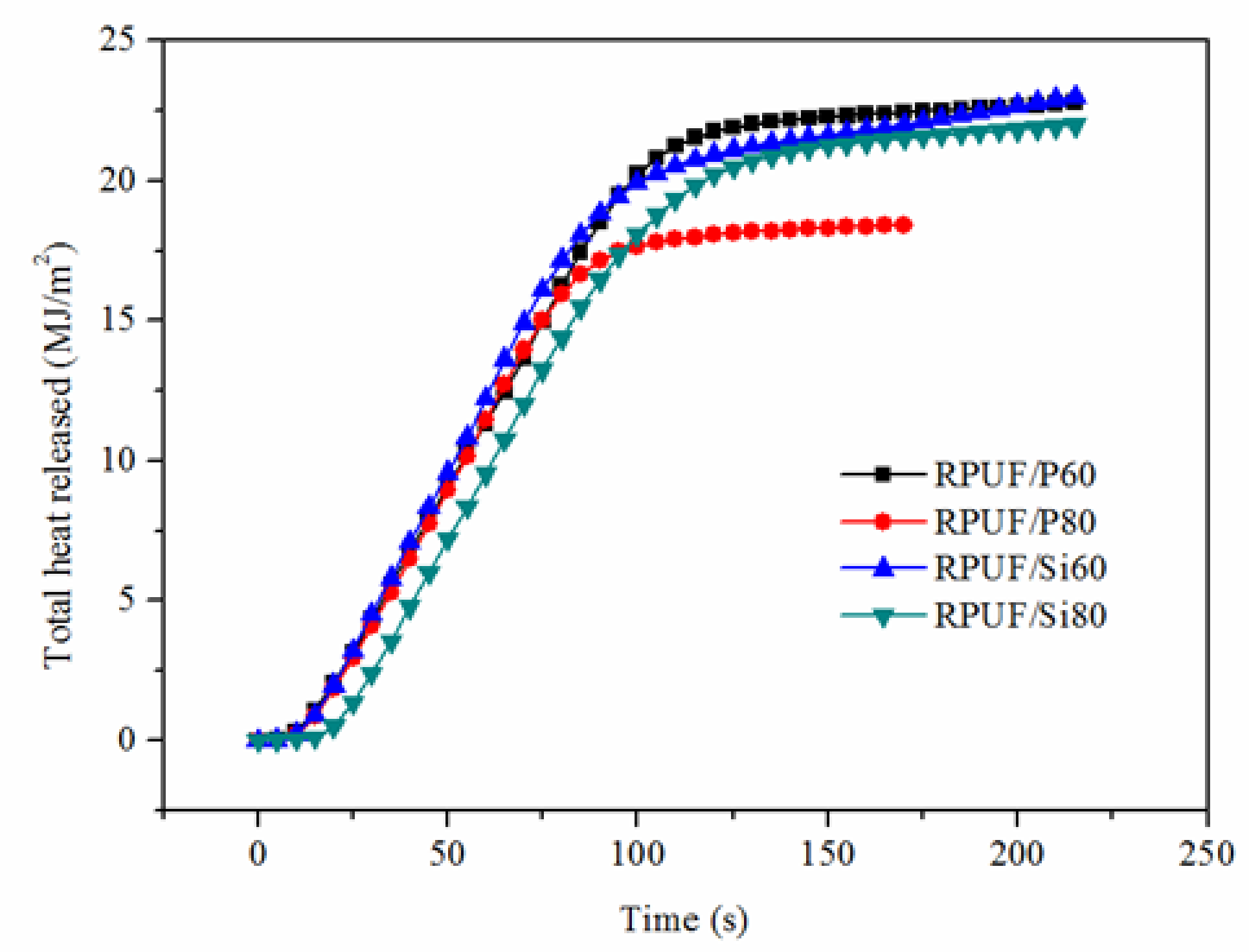
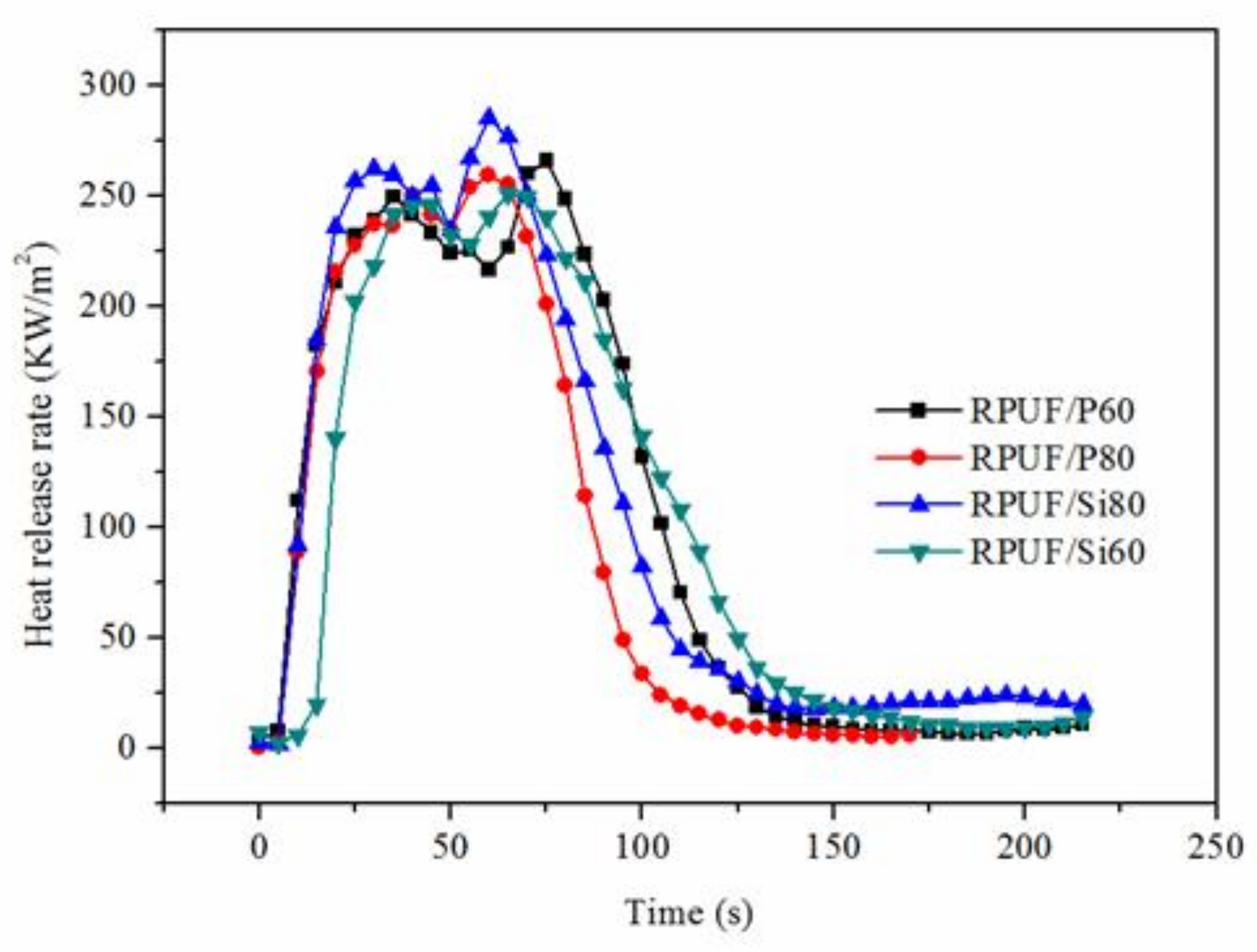
| Sample | TII (s) | PHRR (kW·m−2) | Time to PHRR (s) | THR (MJ·m−2) |
|---|---|---|---|---|
| RPUF/P60 | 2 | 266.03 | 35, 75 | 22.78 |
| RPUF/P80 | 3 | 259.38 | 40, 60 | 18.40 |
| RPUF/Si60 | 2 | 285.36 | 30, 60 | 22.80 |
| RPUF/Si80 | 3 | 255.20 | 40, 65 | 21.99 |
| Sample | C (wt %) | O (wt %) | P (wt %) | Si (wt %) | Others (wt %) |
|---|---|---|---|---|---|
| RPUF/P60 | 79.67 | 17.72 | 1.83 | 0 | 0.78 |
| RPUF/P80 | 83.38 | 14.35 | 0.83 | 0 | 0.84 |
| RPUF/Si60 | 77.37 | 20.61 | 0 | 1.13 | 0.89 |
| RPUF/Si80 | 79.61 | 17.08 | 0 | 2.31 | 1.01 |
| Sample | ρ (kg·m−3) | σ (MPa) | σsp (N.m.kg−1) | K (W·mk−1) |
|---|---|---|---|---|
| RPUF/P60 | 97.08 ± 0.44 | 0.82 ± 0.02 | 8446 | 0.039 |
| RPUF/P80 | 89.48 ± 0.80 | 0.75 ± 0.03 | 8381 | 0.038 |
| RPUF/Si60 | 57.09 ± 0.38 | 0.18 ± 0.02 | 3152 | 0.051 |
| RPUF/Si80 | 52.94 ± 0.32 | 0.25 ± 0.02 | 4722 | 0.044 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, W.; Bo, C.; Jia, P.; Zhou, Y.; Zhang, M. Effects of Tung Oil-Based Polyols on the Thermal Stability, Flame Retardancy, and Mechanical Properties of Rigid Polyurethane Foam. Polymers 2019, 11, 45. https://doi.org/10.3390/polym11010045
Zhou W, Bo C, Jia P, Zhou Y, Zhang M. Effects of Tung Oil-Based Polyols on the Thermal Stability, Flame Retardancy, and Mechanical Properties of Rigid Polyurethane Foam. Polymers. 2019; 11(1):45. https://doi.org/10.3390/polym11010045
Chicago/Turabian StyleZhou, Wei, Caiying Bo, Puyou Jia, Yonghong Zhou, and Meng Zhang. 2019. "Effects of Tung Oil-Based Polyols on the Thermal Stability, Flame Retardancy, and Mechanical Properties of Rigid Polyurethane Foam" Polymers 11, no. 1: 45. https://doi.org/10.3390/polym11010045





