Facile and Green Preparation of Pectin/Cellulose Composite Films with Enhanced Antibacterial and Antioxidant Behaviors
Abstract
:1. Introduction
2. Materials and Methods
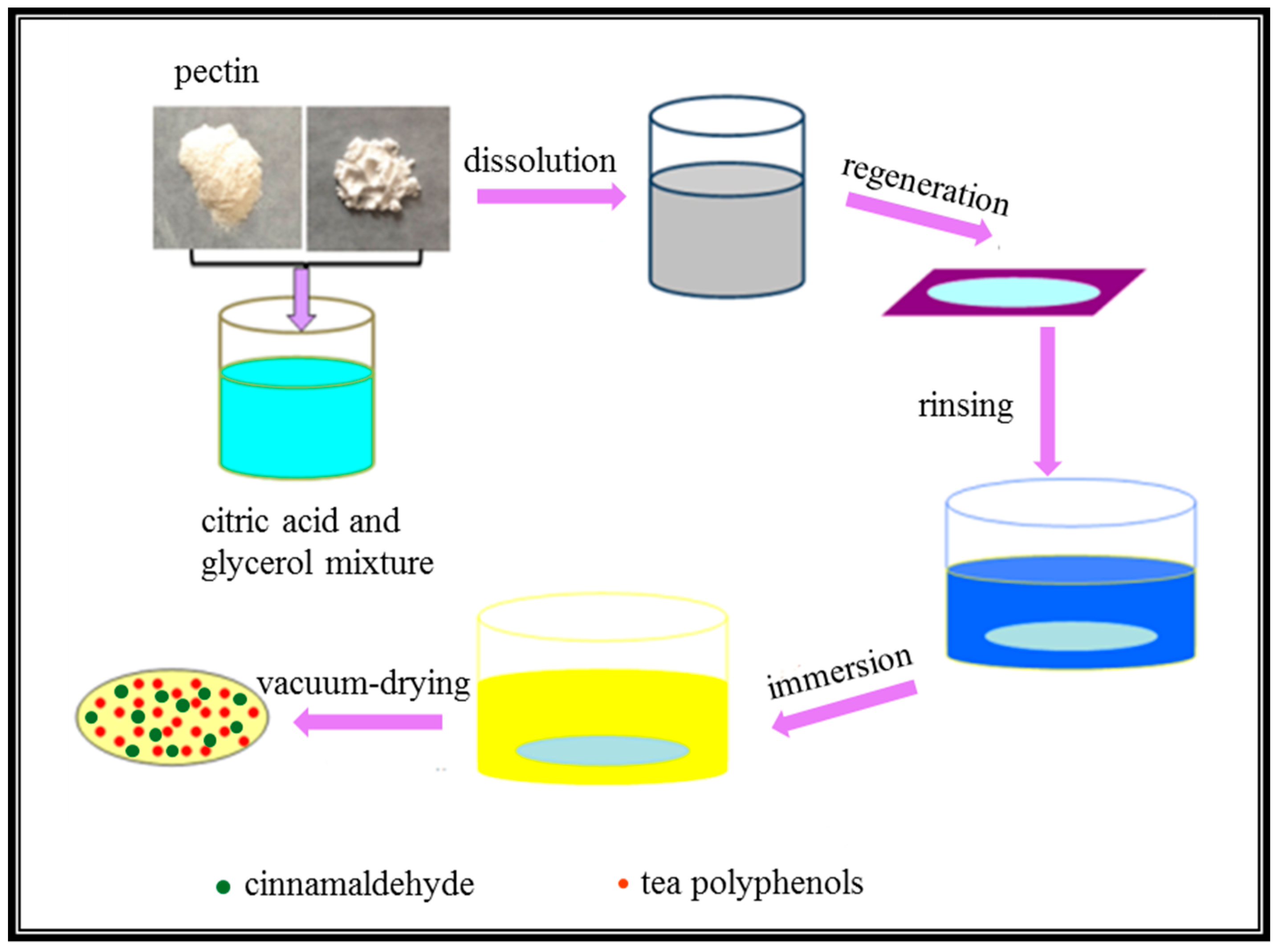
2.1. Preparation of PC Composite Films
2.2. Characterization
2.3. Antibacterial and Antifungal Activities
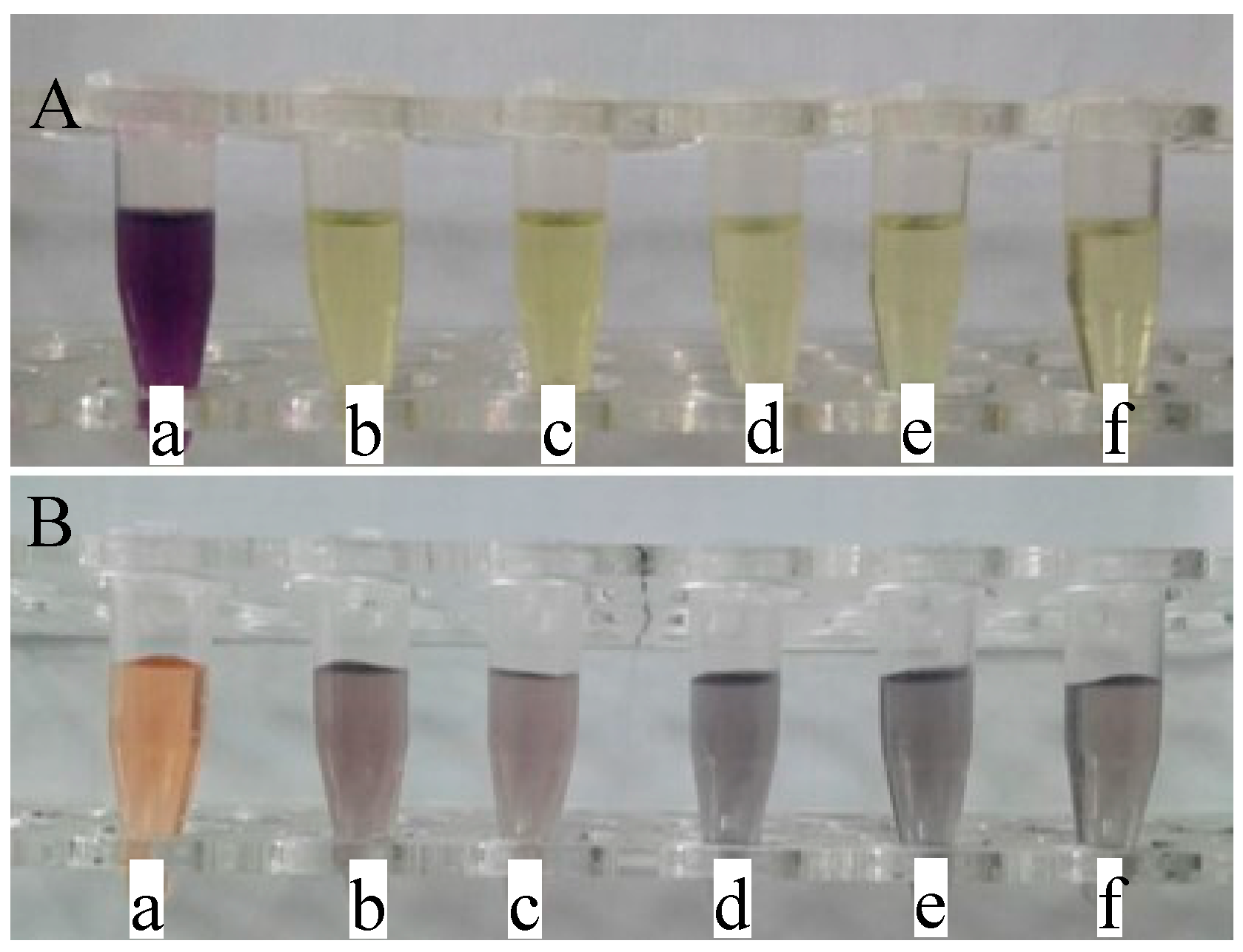
2.4. Antioxidant Activities
2.5. Mechanical Properties
3. Results
3.1. SEM Morphology
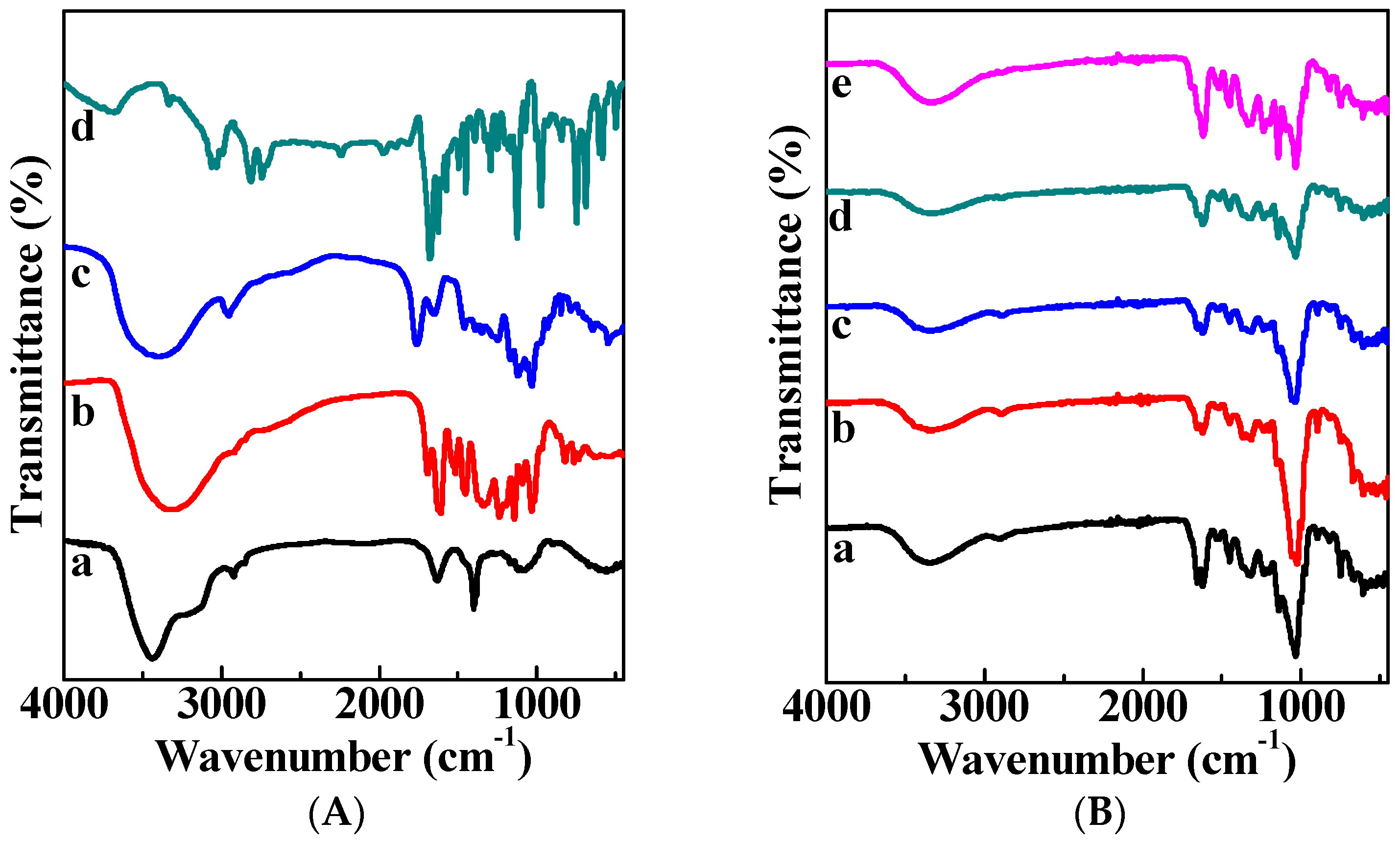
3.2. FTIR Characterization
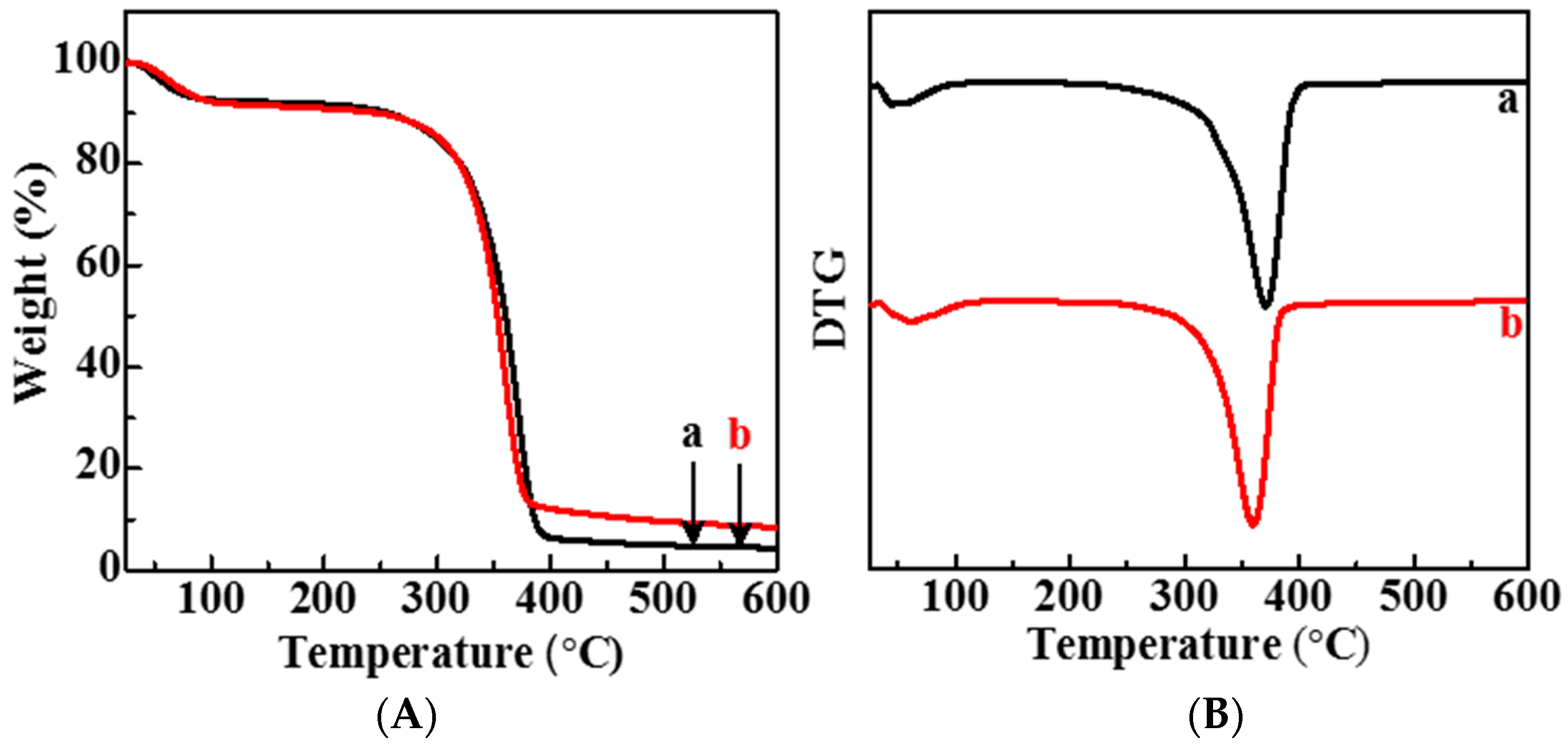
3.3. Thermogravimetric Analysis
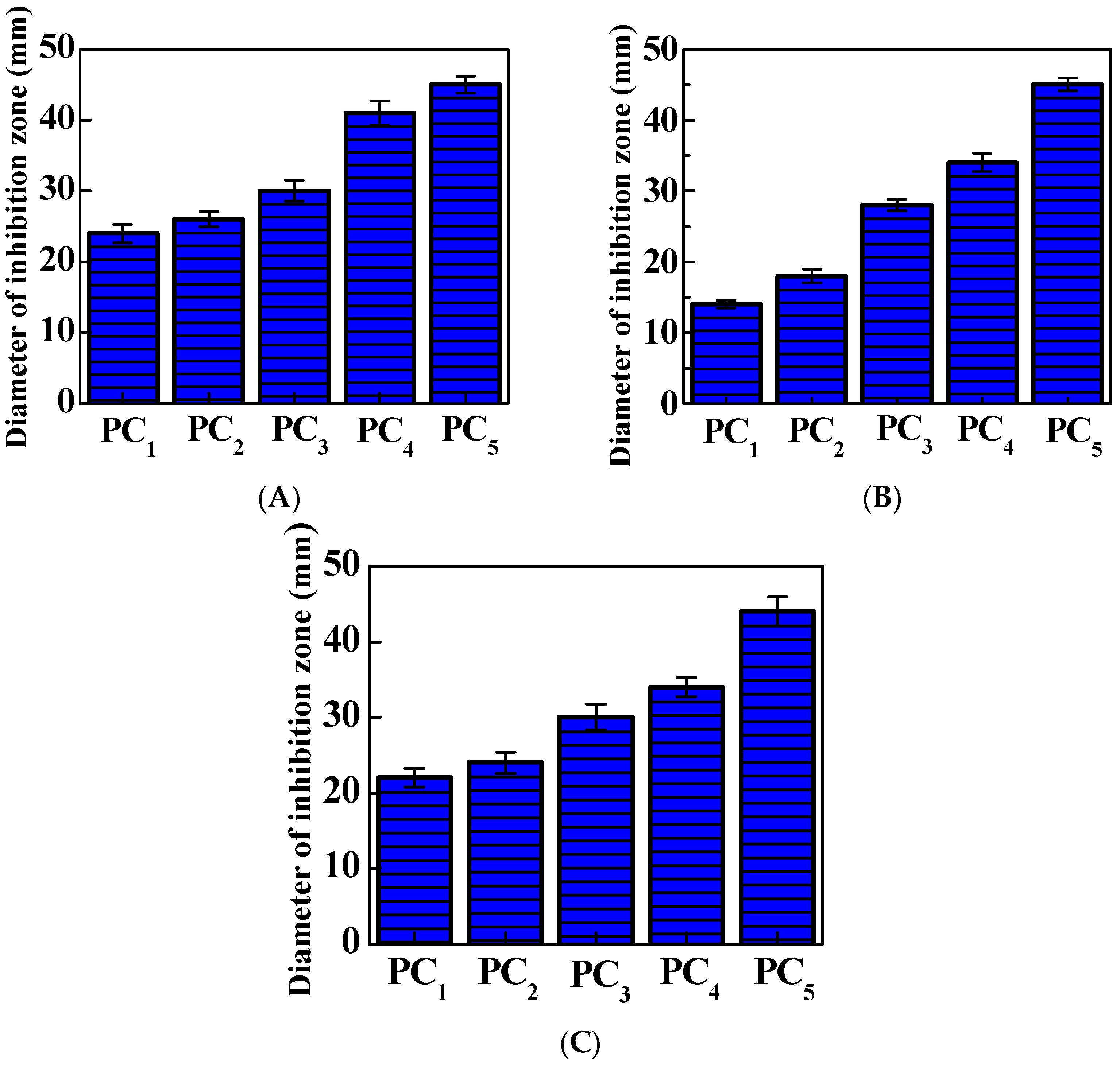
3.4. Bacterial Inhibition Zones
3.5. Antioxidant Properties
3.6. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yu, Z.; Li, B.; Chu, J.; Zhang, P. Silica in situ enhanced PVA/chitosan biodegradable films for food packages. Carbohydr. Polym. 2018, 184, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Huang, X.; Li, Y.C.; Xiao, H.; Wang, X. Novel chitosan films with laponite immobilized Ag nanoparticles for active food packaging. Carbohydr. Polym. 2018, 199, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Gregorova, A.; Saha, N.; Kitano, T.; Saha, P. Hydrothermal effect and mechanical stress properties of carboxymethylcellulose based hydrogel food packaging. Carbohydr. Polym. 2015, 117, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Tanomrod, N.; Rawdkuen, S.; Rhim, J.W. Preparation of pectin/silver nanoparticles composite films with UV-light barrier and properties. Int. J. Biol. Macromol. 2016, 92, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Farahnaky, A.; Sharifi, S.; Imani, B.; Dorodmand, M.M.; Majzoobi, M. Physicochemical and mechanical properties of pectin-carbon nanotubes films produced by chemical bonding. Food Packag. Shelf Life 2018, 16, 8–14. [Google Scholar] [CrossRef]
- Pandele, A.M.; Andronescu, C.; Vasile, E.; Radu, I.C.; Stanescu, P.; Iovu, H. Non-covalent functionalization of GO for improved mechanical performances of pectin composite films. Compos. Part A Appl. Sci. Manuf. 2017, 103, 188–195. [Google Scholar] [CrossRef]
- Bermudez-Oria, A.; Rodriguez-Gutierrez, G.; Vioque, B.; Rubio-Senent, F.; Fernandez-Bolanos, J. Physical and functional properties of pectin-fish gelatin films containing the olive phenols hydroxytyrosol and 3,4-dihydroxyphenylglycol. Carbohydr. Polym. 2017, 178, 368–377. [Google Scholar] [CrossRef]
- Rezvanian, M.; Ahmad, N.; Mohd Amin, M.C.; Ng, S.F. Optimization, characterization, and in vitro assessment of alginate-pectin ionic cross-linked hydrogel film for wound dressing applications. Int. J. Biol. Macromol. 2017, 97, 131–140. [Google Scholar] [CrossRef]
- Chiarappa, G.; De’Nobili, M.D.; Rojas, A.M.; Abrami, M.; Lapasin, R.; Grassi, G.; Ferreira, J.A.; Gudiño, E.; de Oliveira, P.; Grassi, M. Mathematical modeling of L-(+)-ascorbic acid delivery from pectin films (packaging) to agar hydrogels (food). J. Food Eng. 2018, 234, 73–81. [Google Scholar] [CrossRef]
- Hennink, W.E.; van Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 223–236. [Google Scholar] [CrossRef]
- Tummalapalli, M.; Berthet, M.; Verrier, B.; Deopura, B.L.; Alam, M.S.; Gupta, B. Composite wound dressings of pectin and gelatin with aloe vera and curcumin as bioactive agents. Int. J. Biol. Macromol. 2016, 82, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Nesic, A.; Onjia, A.; Davidovic, S.; Dimitrijevic, S.; Errico, M.E.; Santagata, G.; Malinconico, M. Design of pectin-sodium alginate based films for potential healthcare application: Study of chemico-physical interactions between the components of films and assessment of their antimicrobial activity. Carbohydr. Polym. 2017, 157, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Marquez, G.R.; Di Pierro, P.; Mariniello, L.; Esposito, M.; Giosafatto, C.V.L.; Porta, R. Fresh-cut fruit and vegetable coatings by transglutaminase-crosslinked whey protein/pectin edible films. LWT-Food Sci. Technol. 2017, 75, 124–130. [Google Scholar] [CrossRef]
- Seslija, S.; Nesic, A.; Ruzic, J.; Krusic, M.K.; Velickovic, S.; Avolio, R.; Santagata, G.; Malinconico, M. Edible blend films of pectin and poly(ethylene glycol): Preparation and physico-chemical evaluation. Food Hydrocoll. 2018, 77, 494–501. [Google Scholar] [CrossRef]
- Ye, S.; He, S.; Su, C.; Jiang, L.; Wen, Y.Y.; Zhu, Z.J.; Shao, W. Morphological, Release and Antibacterial Performances of Amoxicillin-Loaded Cellulose Aerogels. Molecules 2018, 23, 2082. [Google Scholar] [CrossRef]
- Lee, J.H.; Hwang, C.E.; Son, K.S.; Cho, K.M. Comparisons of nutritional constituents in soybeans during solid state fermentation times and screening for their glucosidase enzymes and antioxidant properties. Food Chem. 2019, 272, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Yu, L.; Zhu, P.; Zhou, X.; Liu, H.; Yang, Y.; Zhou, J.; Gao, C.; Bao, X.; Chen, P. Development and preparation of active starch films carrying tea polyphenol. Carbohydr. Polym. 2018, 196, 162–167. [Google Scholar] [CrossRef]
- Muhoza, B.; Xia, S.; Cai, J.; Zhang, X.; Duhoranimana, E.; Su, J. Gelatin and pectin complex coacervates as carriers for cinnamaldehyde: Effect of pectin esterification degree on coacervate formation, and enhanced thermal stability. Food Hydrocoll. 2019, 87, 712–722. [Google Scholar] [CrossRef]
- Zhang, D.-Y.; Wan, Y.; Hao, J.-Y.; Hu, R.-Z.; Chen, C.; Yao, X.-H.; Zhao, W.-G.; Liu, Z.-Y.; Li, L. Evaluation of the alkaloid, polyphenols, and antioxidant contents of various mulberry cultivars from different planting areas in eastern China. Ind. Crops Prod. 2018, 122, 298–307. [Google Scholar] [CrossRef]
- Jia, Y.; He, Y.; Lu, F. The structure-antioxidant activity relationship of dehydrodiferulates. Food Chem. 2018, 269, 480–485. [Google Scholar] [CrossRef]
- Luo, Q.L.; Tang, Z.H.; Zhang, X.F.; Zhong, Y.H.; Yao, S.Z.; Wang, L.S.; Lin, C.W.; Luo, X. Chemical properties and antioxidant activity of a water-soluble polysaccharide from Dendrobium officinale. Int. J. Biol. Macromol. 2016, 89, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Li, B.; Zhang, K.; Chu, X.; Hou, H. Physical properties and antioxidant activity of gelatin-sodium alginate edible films with tea polyphenols. Int. J. Biol. Macromol. 2018, 118, 1377–1383. [Google Scholar] [CrossRef] [PubMed]




| Cinnamaldehyde (%) | TP Concentration (mg/mL) | |
|---|---|---|
| PC1 | 1 | 0.2 |
| PC2 | 2 | 0.4 |
| PC3 | 3 | 0.6 |
| PC4 | 4 | 0.8 |
| PC5 | 5 | 1 |
| Tensile Strength (MPa) | Elongation at Break (%) | Thickness (μm) | |
|---|---|---|---|
| PC film | 0.31 ± 0.03 | 28.1 ± 1.9 | 504 ± 6 |
| PC5 film | 5.18 ± 0.10 | 7.4 ± 0.4 | 345 ± 9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, S.; Zhu, Z.; Wen, Y.; Su, C.; Jiang, L.; He, S.; Shao, W. Facile and Green Preparation of Pectin/Cellulose Composite Films with Enhanced Antibacterial and Antioxidant Behaviors. Polymers 2019, 11, 57. https://doi.org/10.3390/polym11010057
Ye S, Zhu Z, Wen Y, Su C, Jiang L, He S, Shao W. Facile and Green Preparation of Pectin/Cellulose Composite Films with Enhanced Antibacterial and Antioxidant Behaviors. Polymers. 2019; 11(1):57. https://doi.org/10.3390/polym11010057
Chicago/Turabian StyleYe, Shan, Zhongjie Zhu, Yanyi Wen, Chen Su, Lei Jiang, Shu He, and Wei Shao. 2019. "Facile and Green Preparation of Pectin/Cellulose Composite Films with Enhanced Antibacterial and Antioxidant Behaviors" Polymers 11, no. 1: 57. https://doi.org/10.3390/polym11010057





