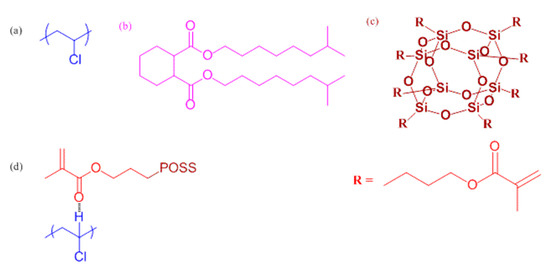
Using Methacryl-Polyhedral Oligomeric Silsesquioxane as the Thermal Stabilizer and Plasticizer in Poly(vinyl chloride) Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. PVC/DINCH, PVC/MA-POSS, and PVC/DINCH/MA-POSS Blend Films
2.3. Characterization
3. Results
3.1. Thermal Analysis of PVC/DINCH Blends
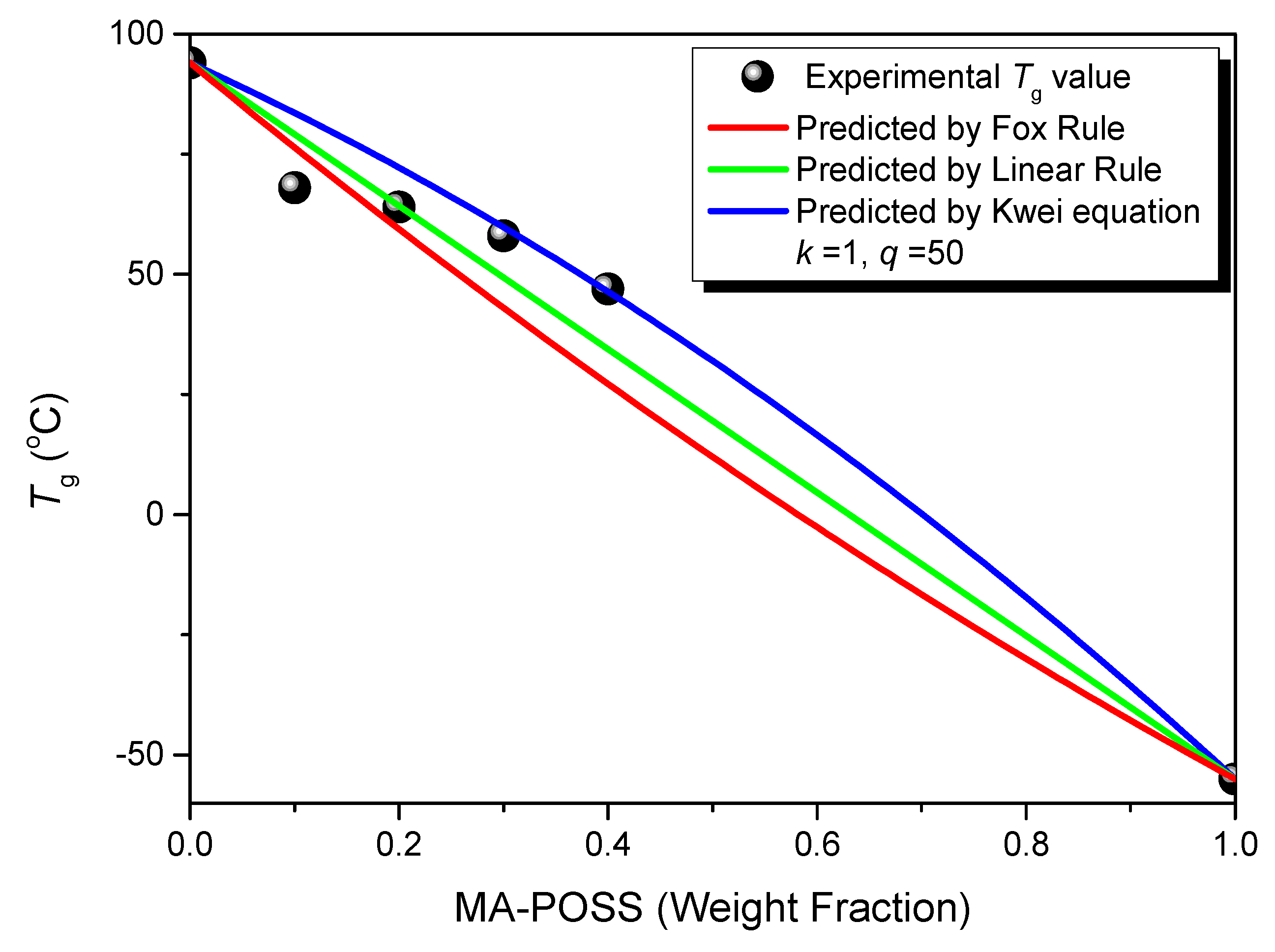
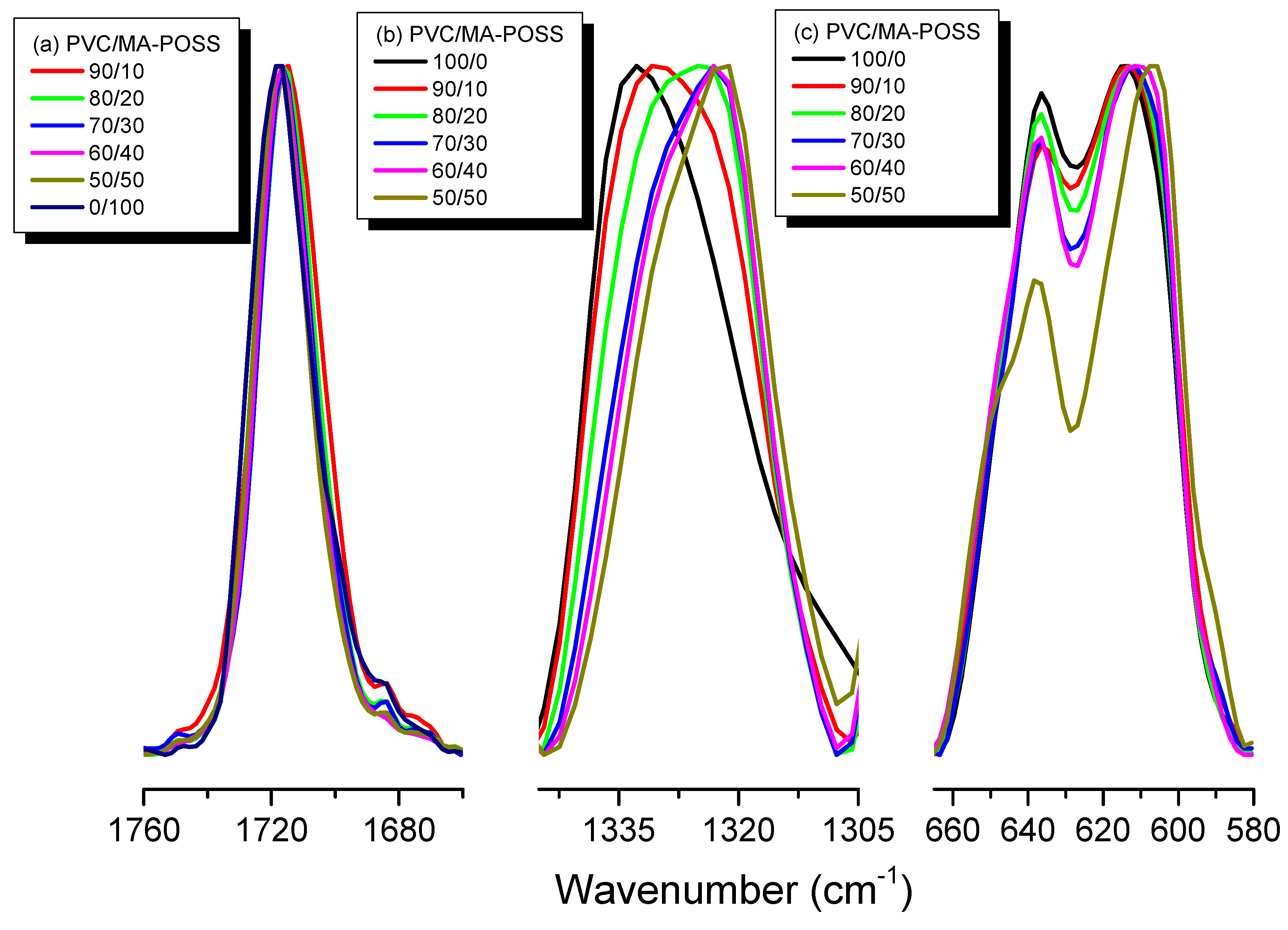
3.2. Thermal Properties and FTIR Spectra of PVC/MA-POSS Blends
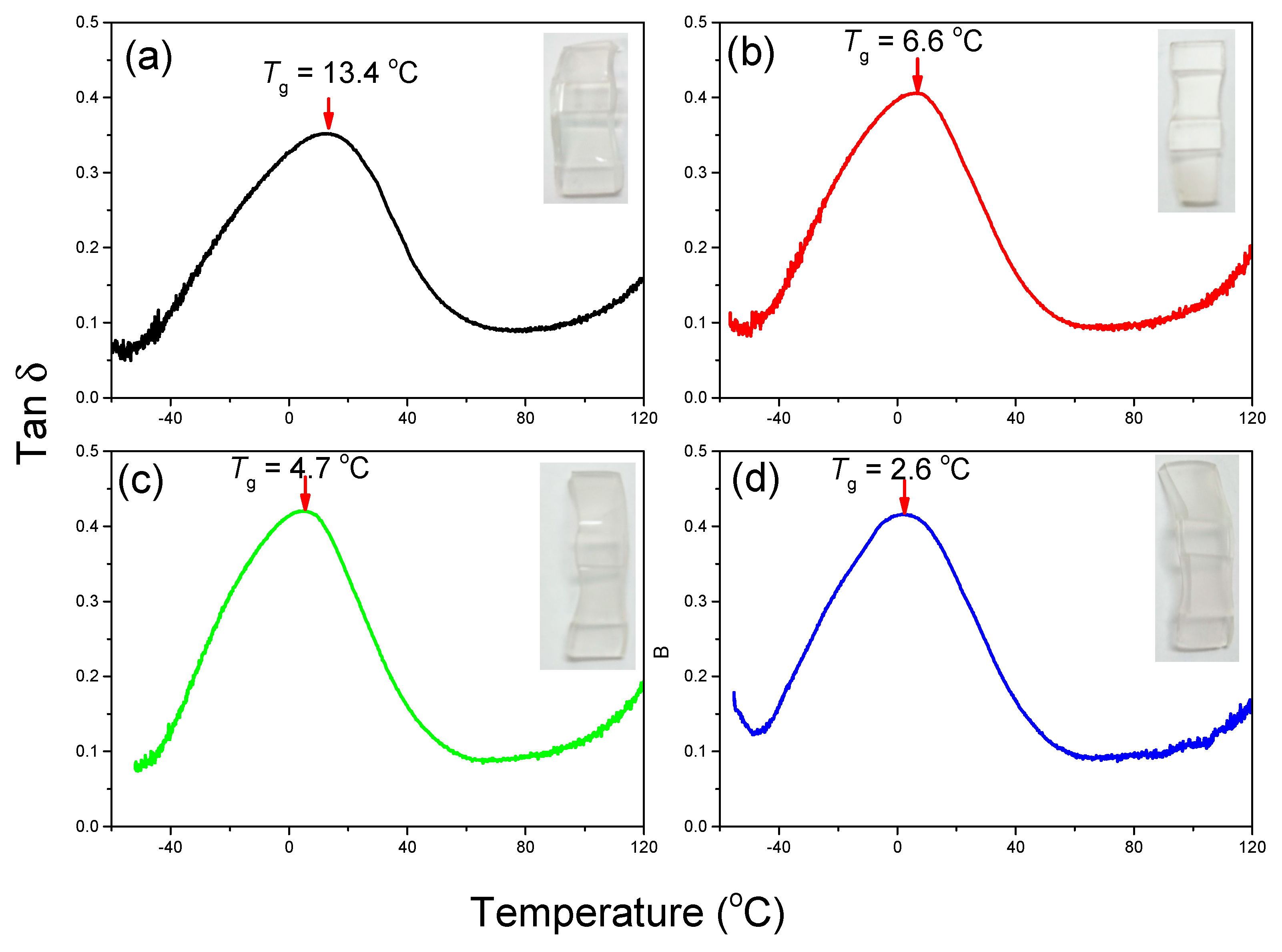
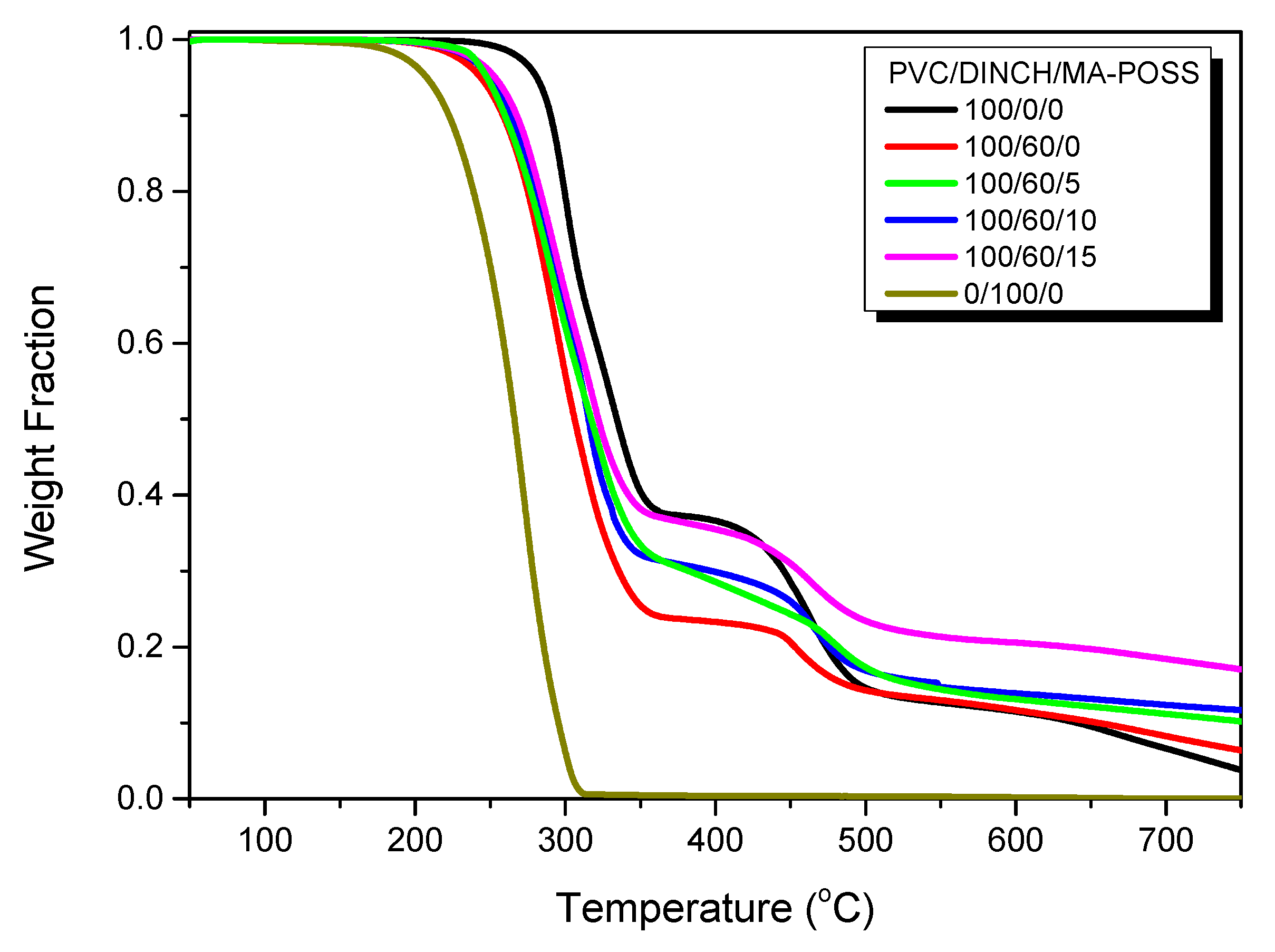
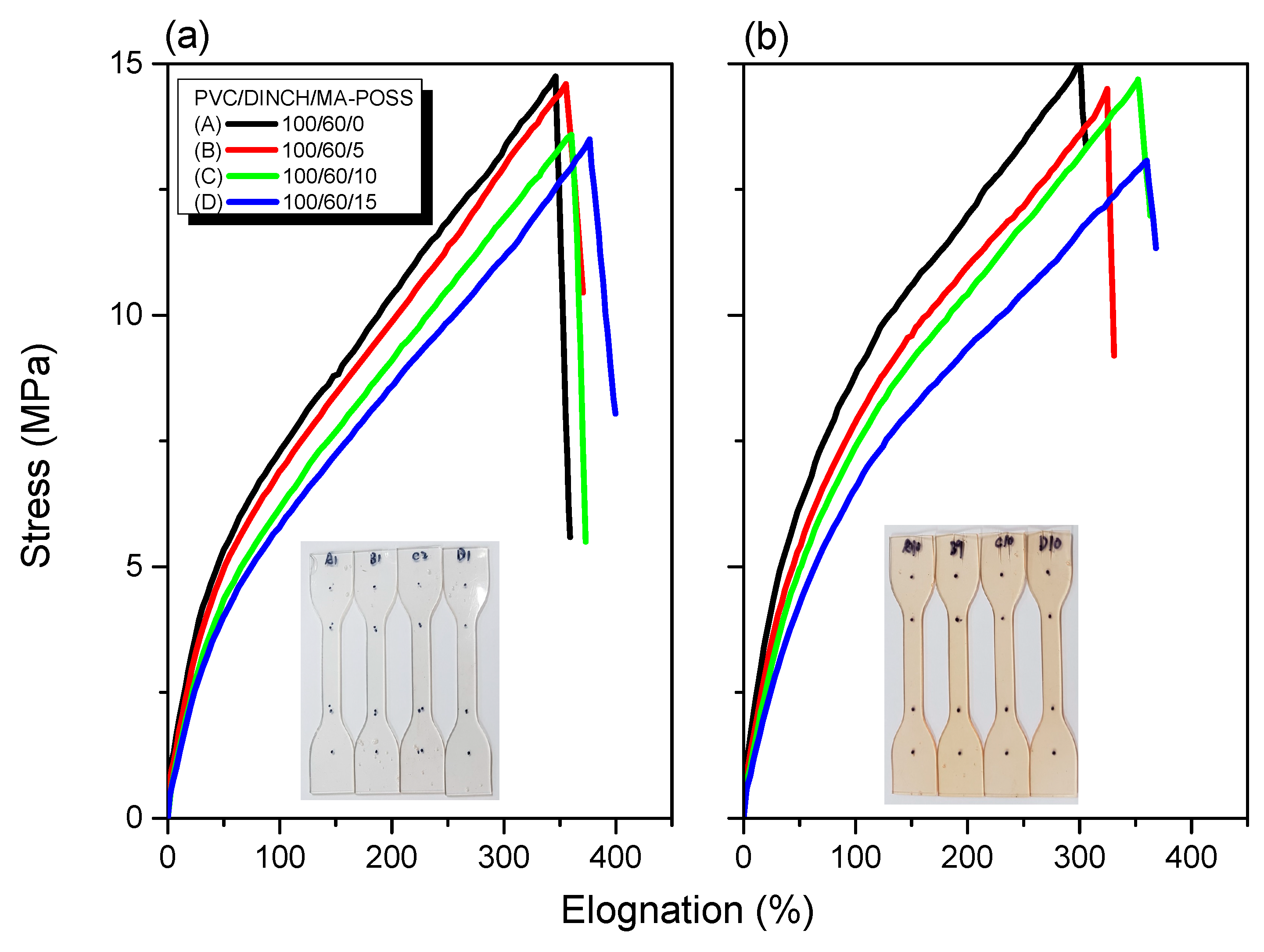
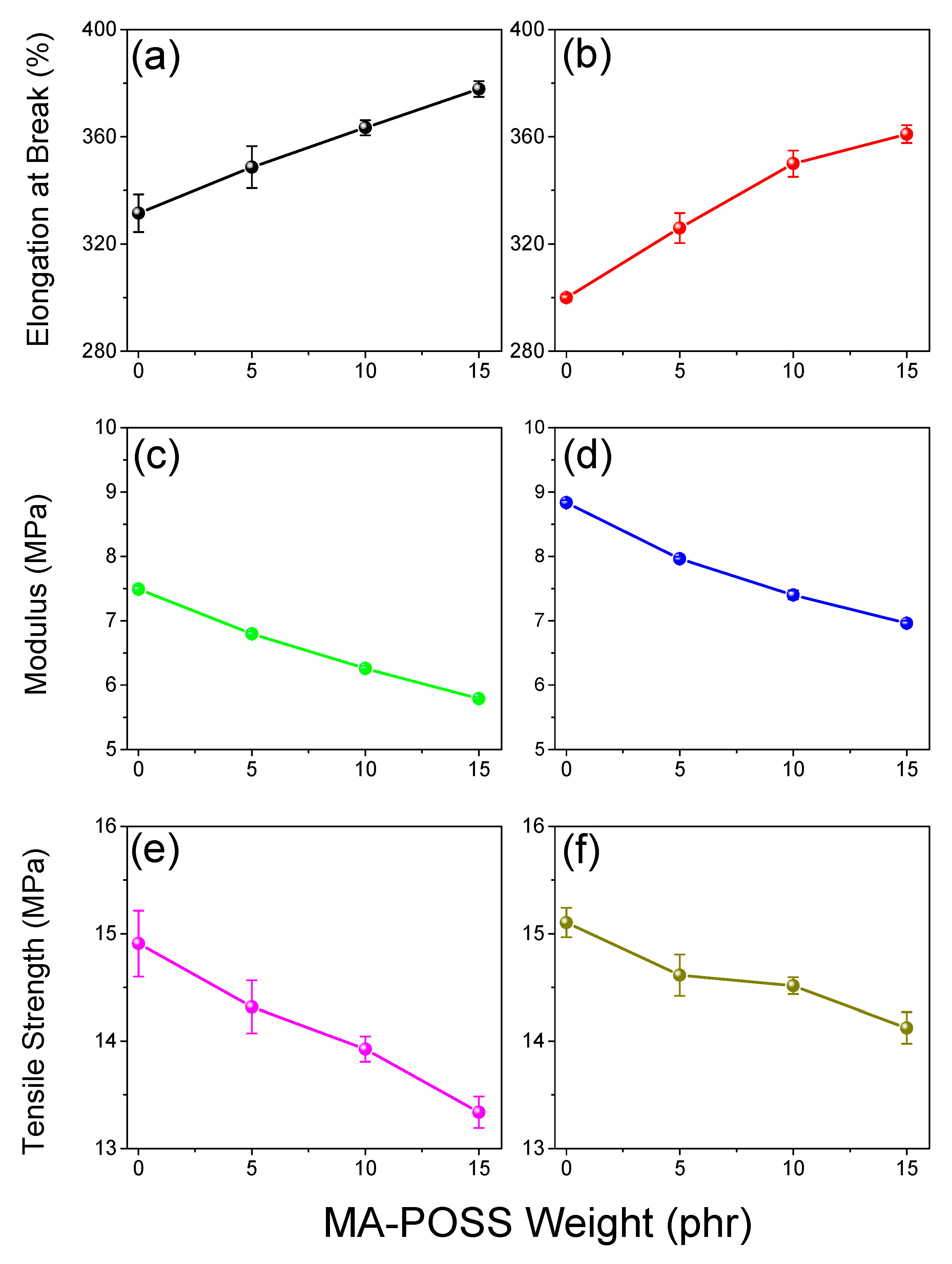
3.3. Thermal and Mechanical Properties of PVC/DINCH/MA-POSS Ternary Blends
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chiellni, F.; Ferri, M.; Morelli, A.; Dipaola, L.; Latini, G. Perspectives on alternatives to phthalate plasticized poly(vinyl chloride) in medical devices applications. Prog. Polym. Sci. 2013, 38, 1067–1088. [Google Scholar] [CrossRef] [Green Version]
- Huynh, N.M.N.; Boeva, Z.A.; Smatt, J.H.; Pesonen, M.P.; Lindfors, T. Reduced graphene oxide as a water, carbon dioxide and oxygen barrier in plasticized poly(vinyl chloride) films. RSC Adv. 2018, 8, 17645–17655. [Google Scholar] [CrossRef] [Green Version]
- Jia, P.Y.; Xia, H.Y.; Tang, K.H.; Zhou, Y.H. Plasticizers derived from biomass resources: A short review. Polymers 2018, 10, 1303. [Google Scholar] [CrossRef] [PubMed]
- Suresh, S.S.; Mohanty, S.; Nayak, S.K. Bio-based epoxidised oil for compatibilization and value addition of poly (vinyl chloride) (PVC) and poly(methyl methacrylate) (PMMA) in recycled blend. J. Polym. Res. 2017, 24, 120. [Google Scholar] [CrossRef]
- Li, D.Y.; Panchal, K.; Mafi, R.; Xi, L. An Atomistic Evaluation of the Compatibility and Plasticization Efficacy of Phthalates in Poly(vinyl chloride). Macromolecules 2018, 51, 6997–7012. [Google Scholar] [CrossRef]
- Jaeger, R.J.; Rubin, R.J. Migration of a Phthalate Ester Plasticizer from Polyvinyl Chloride Blood Bags into Stored Human Blood and Its Localization in Human Tissues. N. Engl. J. Med. 1972, 287, 1114–1118. [Google Scholar] [CrossRef]
- Ozeren, H.O.; Balcik, M.; Ahunbay, M.G.; Elliott, J.R. In Silico Screening of Green Plasticizers for Poly(vinyl chloride). Macromolecules 2019, 52, 2421–2430. [Google Scholar] [CrossRef]
- Deshmukh, K.; Khatake, S.M.; Joshi, G.M. Surface properties of graphene oxide reinforced polyvinyl chloride nanocomposites. J. Polym. Res. 2013, 20, 286. [Google Scholar] [CrossRef]
- Mansour, S.A.; Elsad, R.A. Dielectric spectroscopic analysis of polyvinyl chloride nanocomposites loaded with Fe2O3 nanocrystals. Polym. Adv. Tech. 2018, 29, 2477–2485. [Google Scholar] [CrossRef]
- Hajibeygi, M.; Maleki, M.; Shabanian, M.; Ducos, F.; Vahab, H. New polyvinyl chloride (PVC) nanocomposite consisting of aromatic polyamide and chitosan modified ZnO nanoparticles with enhanced thermal stability, low heat release rate and improved mechanical properties. Appl. Sur. Sci. 2018, 439, 1163–1179. [Google Scholar] [CrossRef]
- Kuo, S.W.; Chang, F.C. POSS related polymer nanocomposites. Prog. Polym. Sci. 2011, 36, 1649–1696. [Google Scholar] [CrossRef]
- Cordes, D.B.; Lickiss, P.D.; Rataboul, F. Recent developments in the chemistry of cubic polyhedral oligosilsesquioxanes. Chem. Rev. 2010, 110, 2081–2173. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Mather, P.T. POSS polymers: Physical properties and biomaterials applications. Polym. Rev. 2009, 49, 25–63. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Kuo, S.W. Functional Polyimide/Polyhedral Oligomeric Silsesquioxane Nanocomposites. Polymers 2019, 11, 26. [Google Scholar] [CrossRef]
- Mohamed, G.M.; Kuo, S.W. Functional Silica and Carbon Nanocomposites Based on Polybenzoxazines. Macromol. Chem. Phys. 2019, 220, 1800306. [Google Scholar] [CrossRef]
- Kuo, S.W. Building Blocks Precisely from Polyhedral Oligomeric Silsesquioxane Nanoparticles. ACS Cent. Sci. 2016, 2, 62–64. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, M.G.; Jheng, Y.R.; Yeh, S.L.; Chen, T.; Kuo, S.W. Unusual Emission of Polystyrene-Based Alternating Copolymers Incorporating Aminobutyl Maleimide Fluorophore-Containing Polyhedral Oligomeric Silsesquioxane NPs. Polymers 2017, 9, 103. [Google Scholar] [CrossRef]
- Li, Z.; Kong, J.; Wang, F.; He, C. Polyhedral oligomeric silsesquioxanes (POSSs): An important building block for organic optoelectronic materials. J. Mater. Chem. C 2017, 5, 5283–5298. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, X.; Sun, Y.; Xie, W. POSS Dental Nanocomposite Resin: Synthesis, Shrinkage, Double Bond Conversion, Hardness, and Resistance Properties. Polymers 2018, 10, 369. [Google Scholar] [CrossRef]
- Blanco, I. The Rediscovery of POSS: A Molecule Rather than a Filler. Polymers 2018, 10, 904. [Google Scholar] [CrossRef]
- Yang, X.M.; Li, Y.Y.; Wang, Y.L.; Yang, Y.G.; Hao, J.W. Nitrocellulose-based hybrid materials with T7-POSS as a modifier: Effective reinforcement for thermal stability, combustion safety, and mechanical properties. J. Polym. Res. 2018, 24, 50. [Google Scholar] [CrossRef]
- Liao, Y.T.; Lin, Y.C.; Kuo, S.W. Highly Thermally Stable, Transparent, and Flexible Polybenzoxazine Nanocomposites by Combination of Double-Decker-Shaped Polyhedral Silsesquioxanes and Polydimethylsiloxane. Macromolecules 2017, 50, 5739–5747. [Google Scholar] [CrossRef]
- Yu, C.Y.; Kuo, S.W. Phenolic Functionality of Polyhedral Oligomeric Silsesquioxane Nanoparticles Affects Self-Assembly Supramolecular Structures of Block Copolymer Hybrid Complexes. Ind. Eng. Chem. Res. 2018, 57, 2546–2559. [Google Scholar] [CrossRef]
- Zhou, H.; Ye, Q.; Xu, J. Polyhedral oligomeric silsesquioxane-based hybrid materials and their applications. Mater. Chem. Front. 2017, 1, 212–230. [Google Scholar] [CrossRef]
- Li, Y.; Dong, X.H.; Zou, Y.; Wang, Z.; Yue, K.; Huang, M.; Liu, H.; Feng, X.; Lin, Z.; Zhang, W.; et al. Polyhedral oligomeric silsesquioxane meets “click” chemistry: Rational design and facile preparation of functional hybrid materials. Polymer 2017, 125, 303–329. [Google Scholar] [CrossRef]
- Huang, K.W.; Tsai, L.W.; Kuo, S.W. Influence of Octakis-Functionalized Polyhedral Oligomeric Silsesquioxanes on the Physical Properties of Their Polymer Nanocomposites. Polymer 2009, 50, 4876–4887. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Kuo, S.W. Polybenzoxazine/Polyhedral Oligomeric Silsesquioxane (POSS) Nanocomposites. Polymers 2016, 8, 225. [Google Scholar] [CrossRef]
- Ayandele, E.; Sarkar, B.; Alexandridis, P. Polyhedral Oligomeric Silsesquioxane (POSS)-Containing Polymer Nanocomposites. Nanomaterials 2012, 2, 445–475. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.C.; Lin, R.C.; Tseng, S.M.; Kuo, S.W. Minimizing the Strong Screening Effect of Polyhedral Oligomeric Silsesquioxane Nanoparticles in Hydrogen-Bonded Random Copolymers. Polymers 2018, 10, 303. [Google Scholar] [CrossRef]
- Yeh, S.L.; Zhu, C.Y.; Kuo, S.W. Strong Hydrogen Bonding with Inorganic Pendant Polyhedral Oligomeric Silsesquioxane Nanoparticles Provides High Glass Transition Temperature Poly (methyl methacrylate) Copolymers. J. Nanosci. Nanotechnol. 2018, 18, 188–194. [Google Scholar] [CrossRef]
- Chen, W.C.; Kuo, S.W. Othro-Imide and Allyl Groups Effect on Highly Thermal Stable Polybenzoxazine/Double-Decker–Shaped Polyhedral Silsesquioxanes (DDSQ) Nanocomposites. Macromolecules 2018, 51, 9602–9612. [Google Scholar] [CrossRef]
- Sterzynski, T.; Tomaszewska, J.; Andrzejewski, J.; Skorczewska, K. Evaluation of glass transition temperature of PVC/POSS nanocomposites. Compos. Sci. Tech. 2015, 117, 398–403. [Google Scholar] [CrossRef]
- Soong, S.Y.; Cohen, R.E.; Boyce, M.C. Polyhedral oligomeric silsesquioxane as a novel plasticizer for oly(vinyl chloride). Polymer 2007, 48, 1410–1418. [Google Scholar] [CrossRef]
- Soong, S.Y.; Cohen, R.E.; Boyce, M.C.; Mulliken, A.D. Rate-dependent deformation behavior of POSS-filled and plasticized poly(vinyl chloride). Macromolecules 2006, 39, 2900–2908. [Google Scholar] [CrossRef]
- Yang, S.; Wu, W.; Jiao, Y.; Fan, H.; Cai, Z. Poly(ethylene glycol)–polyhedral oligomeric sesquioxane as a novel plasticizer and thermal stabilizer for poly(vinyl chloride) nanocomposites. Polym. Inter. 2016, 65, 1172–1178. [Google Scholar] [CrossRef]
- Kwei, T. The effect of hydrogen bonding on the glass transition temperatures of polymer mixtures. J. Polym. Sci. Polym. Lett. Ed. 1984, 22, 307–313. [Google Scholar] [CrossRef]
- Kuo, S.W. Hydrogen Bonding in Polymeric Materials; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Pan, C.T.; Yen, C.K.; Wang, S.Y.; Fan, S.K.; Chiou, F.Y.; Lin, L.; Huang, J.C.; Kuo, S.W. Energy Harvester and Cell Proliferation from Biocompatible PMLG Nanofibers Prepared Using Near-Field Electrospinning and Electrospray Technology. J. Nanosci. Nanotechnol. 2018, 18, 156–164. [Google Scholar] [CrossRef]
- Jheng, Y.R.; Mohamed, M.G.; Kuo, S.W. Supramolecular Interactions Induce Unexpectedly Strong Emissions from Triphenylamine-Functionalized Polytyrosine Blended with Poly (4-vinylpyridine). Polymers 2017, 9, 503. [Google Scholar] [CrossRef]
- Lin, R.C.; Mohamed, M.G.; Kuo, S.W. Coumarin-and Carboxyl-Functionalized Supramolecular Polybenzoxazines Form Miscible Blends with Polyvinylpyrrolidone. Polymers 2017, 9, 146. [Google Scholar] [CrossRef]
- Kuo, S.W.; Huang, W.J.; Huang, C.F.; Chan, S.C.; Chang, F.C. Miscibility, Specific Interactions, and Spherulite Growth Rates of Binary Poly(acetoxystyrene)/Poly(ethylene oxide) Blends. Macromolecules 2004, 37, 4164–4173. [Google Scholar] [CrossRef]
- Kuo, S.W.; Huang, C.F.; Tung, P.H.; Huang, W.J.; Huang, J.M.; Chang, F.C. Synthesis, Thermal Properties and Specific Interactions of High Tg Increase in Poly(2,6-dimethyl-1,4-phenylene oxide)-block-Polystyrene Copolymers. Polymer 2005, 46, 9348–9361. [Google Scholar] [CrossRef]
- Mao, B.H.; El-Mahdy, A.F.M.; Kuo, S.W. Miscibility Enhancement of Conjugated Polymers Blending with Polystyrene Derivatives through Bio-Inspired Multiple Complementary Hydrogen Bonds. J. Polym. Res. 2019, 26, 208. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-K.; Tsai, F.-C.; Ma, C.-C.; Wang, M.-L.; Kuo, S.-W. Using Methacryl-Polyhedral Oligomeric Silsesquioxane as the Thermal Stabilizer and Plasticizer in Poly(vinyl chloride) Nanocomposites. Polymers 2019, 11, 1711. https://doi.org/10.3390/polym11101711
Wang Y-K, Tsai F-C, Ma C-C, Wang M-L, Kuo S-W. Using Methacryl-Polyhedral Oligomeric Silsesquioxane as the Thermal Stabilizer and Plasticizer in Poly(vinyl chloride) Nanocomposites. Polymers. 2019; 11(10):1711. https://doi.org/10.3390/polym11101711
Chicago/Turabian StyleWang, Yu-Kai, Fang-Chang Tsai, Chao-Chen Ma, Min-Ling Wang, and Shiao-Wei Kuo. 2019. "Using Methacryl-Polyhedral Oligomeric Silsesquioxane as the Thermal Stabilizer and Plasticizer in Poly(vinyl chloride) Nanocomposites" Polymers 11, no. 10: 1711. https://doi.org/10.3390/polym11101711