Evolution of Dielectric Behavior of Regenerated Cellulose Film during Isothermal Dehydration Monitored in Real Time via Dielectric Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Cellulose Film
2.3. Real-Time Dielectric Monitoring
2.4. Dielectric Spectra Analysis
3. Results and Discussion
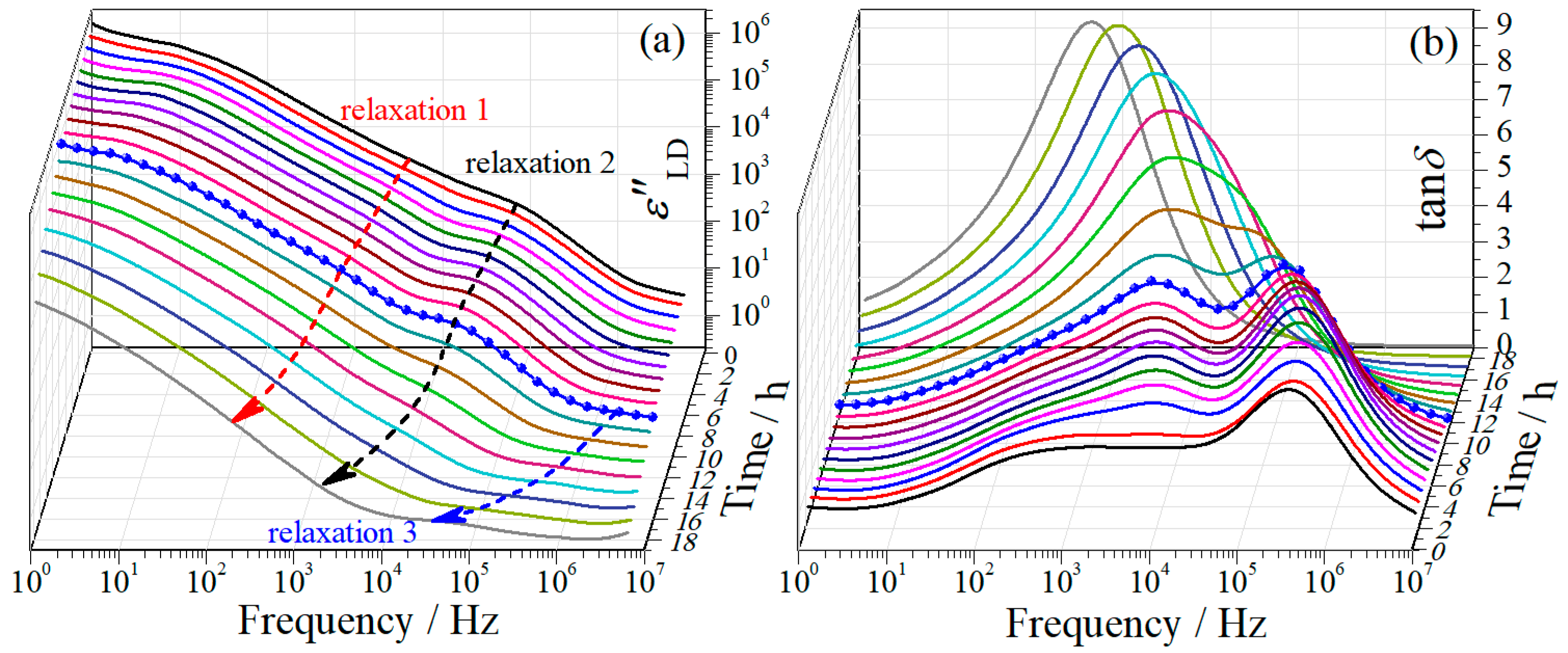
3.1. Evolution of the Dielectric Relaxation Behavior of RC Film during Isothermal Drying
3.1.1. Dielectric Behavior at the First Drying Stage (0–18 h)
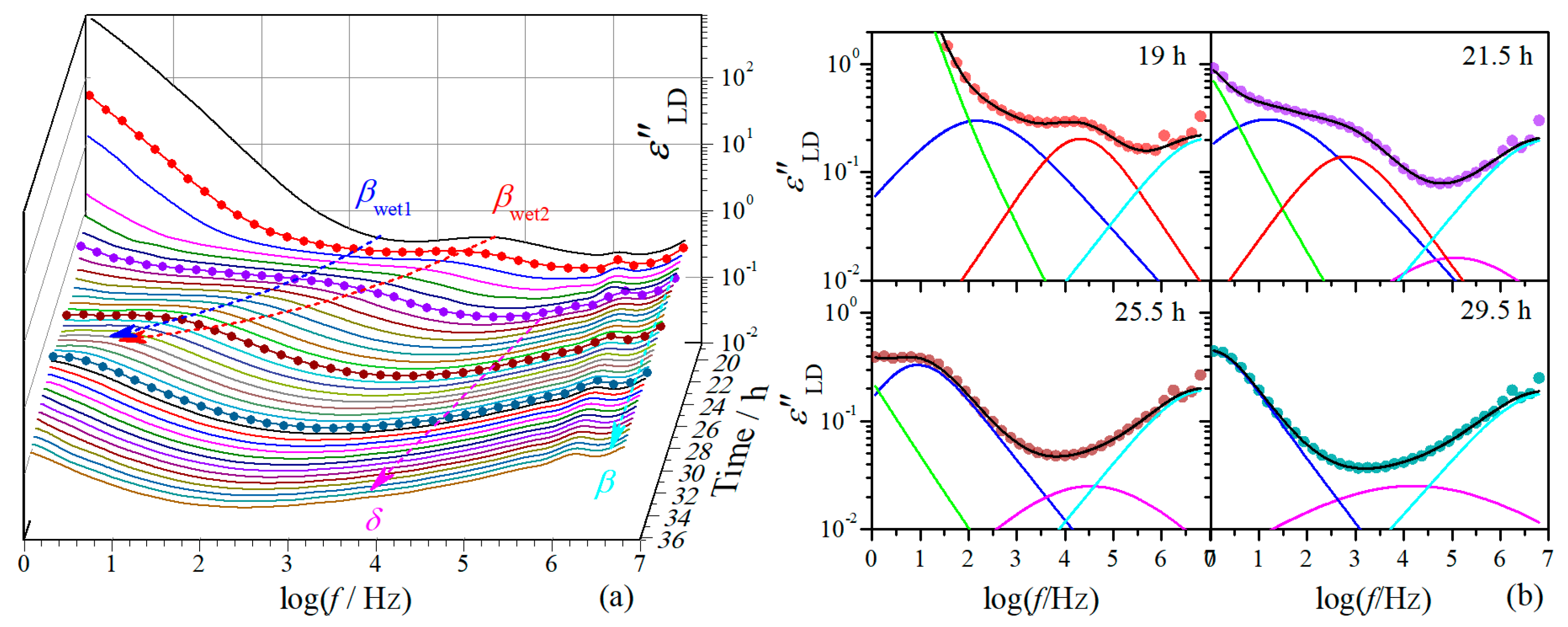
3.1.2. Dielectric Behavior at the Second Drying Stage (18.5–36 h)
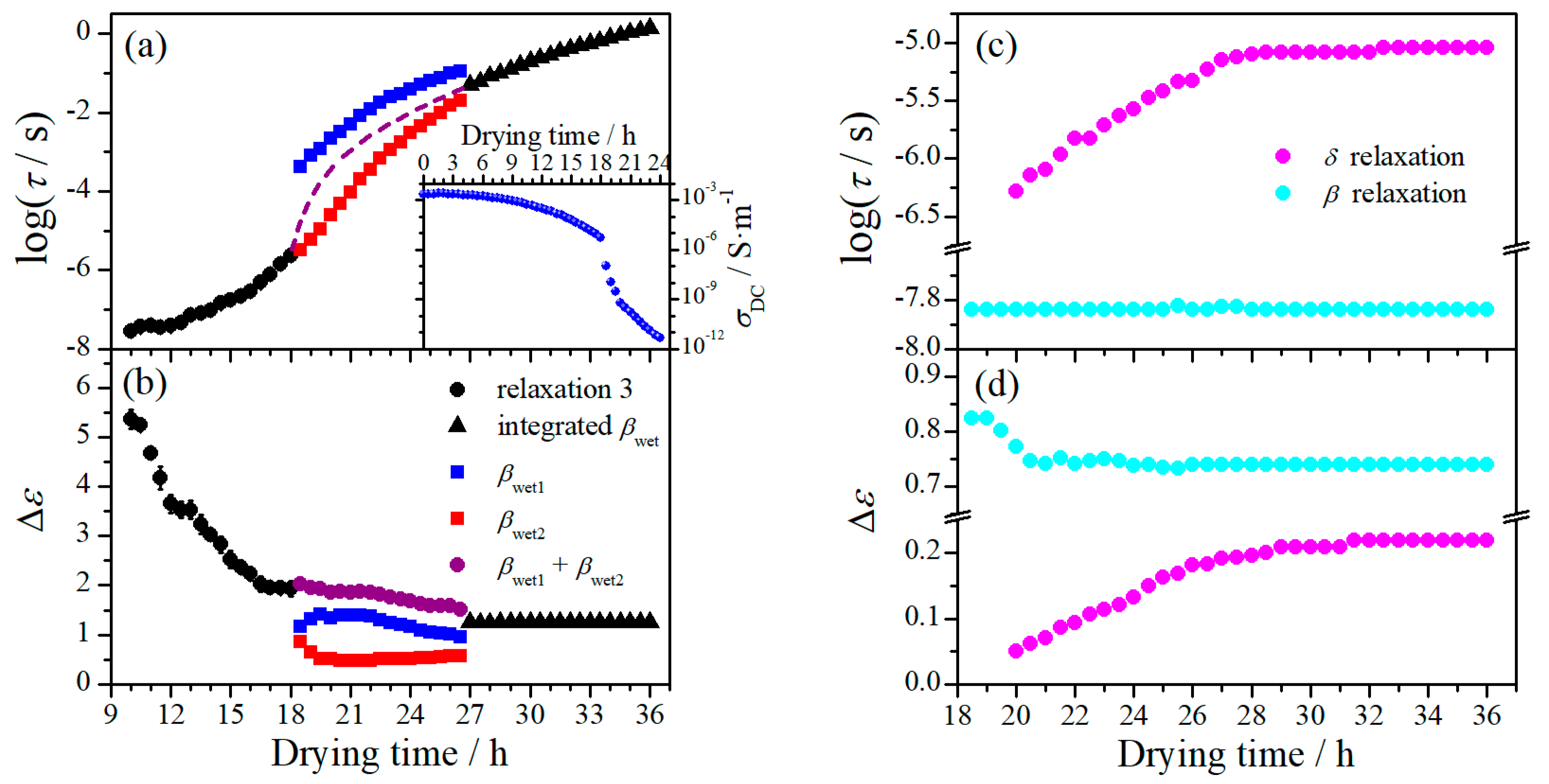
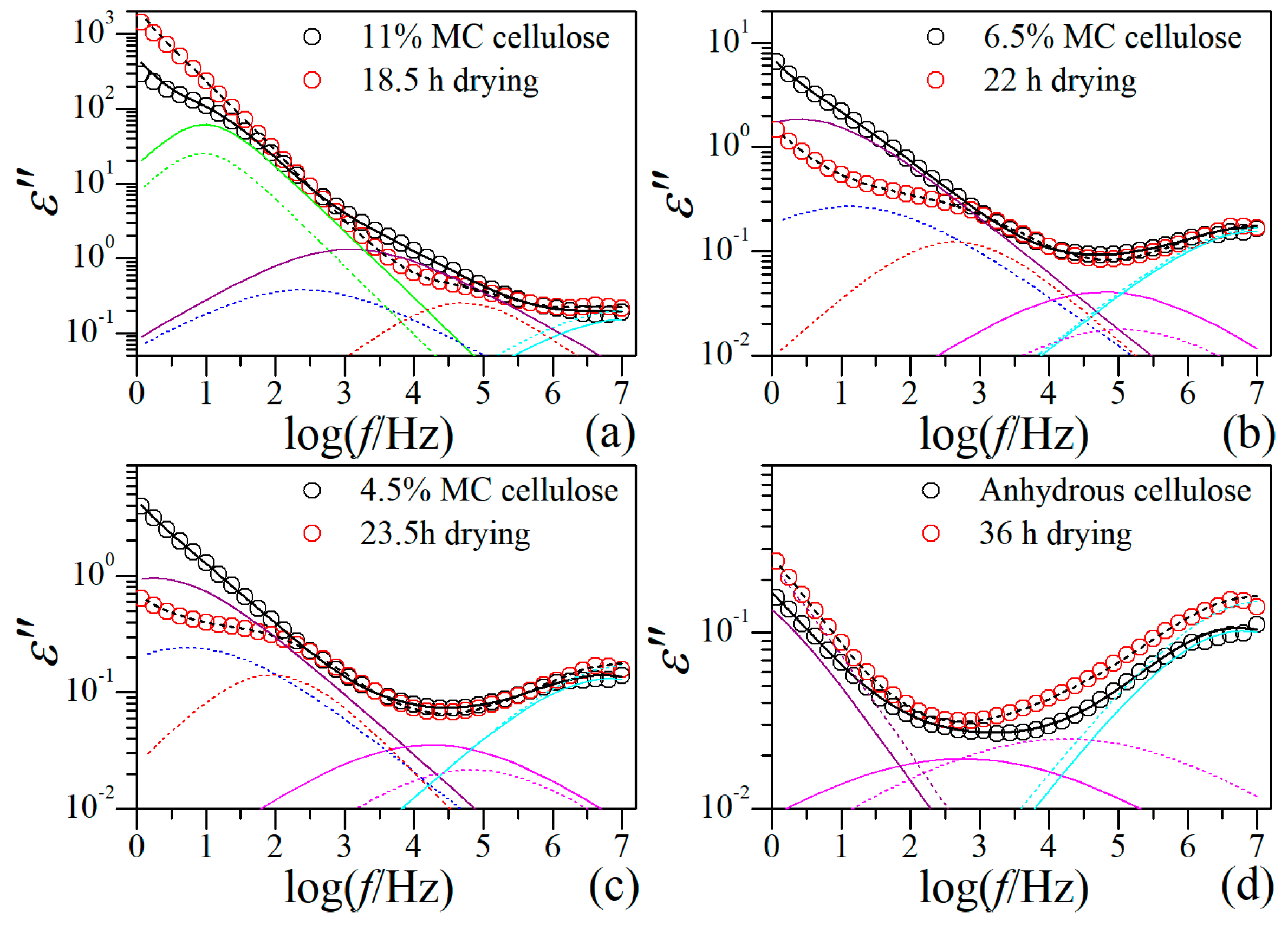
3.2. Progression of Water Loss and Its Influence on the Dielectric Relaxation Behavior of RC Film
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, J.M.; Zhang, J. Advanced Functional Materials Based on Cellulose. Acta Polym. Sin. 2010, 1376–1398. [Google Scholar] [CrossRef]
- Wang, S.; Lu, A.; Zhang, L. Recent advances in regenerated cellulose materials. Prog. Polym. Sci. 2016, 53, 169–206. [Google Scholar] [CrossRef]
- Qi, H. Novel Functional Materials Based on Cellulose; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. Engl. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Ritcey, A.M.; Gray, D.G. Cholesteric order in gels and films of regenerated cellulose. Biopolymers 1988, 27, 1363–1374. [Google Scholar] [CrossRef]
- Togawa, E.; Kondo, T. Change of morphological properties in drawing water-swollen cellulose films prepared from organic solutions. A view of molecular orientation in the drawing process. J. Polym. Sci. Part B Polym. Phys. 1999, 37, 451–459. [Google Scholar] [CrossRef]
- Kondo, T.; Kasai, W.; Brown, R.M. Formation of nematic ordered cellulose and chitin. Cellulose 2004, 11, 463–474. [Google Scholar] [CrossRef]
- Kondo, T. Nematic Ordered Cellulose: Its Structure and Properties. In Cellulose: Molecular and Structural Biology; Springer: Dordrecht, The Netherlands, 2007; pp. 285–305. [Google Scholar] [CrossRef]
- Einfeldt, J.; Meißner, D.; Kwasniewski, A. Polymerdynamics of cellulose and other polysaccharides in solid state-secondary dielectric relaxation processes. Prog. Polym. Sci. 2001, 26, 1419–1472. [Google Scholar] [CrossRef]
- Einfeldt, J.; Kwasniewski, A. Characterization of different types of cellulose by dielectric spectroscopy. Cellulose 2002, 9, 225–238. [Google Scholar] [CrossRef]
- Einfeldt, J.; Meißner, D.; Kwasniewski, A. Molecular interpretation of the main relaxations found in dielectric spectra of cellulose—Experimental arguments. Cellulose 2004, 11, 137–150. [Google Scholar] [CrossRef]
- Rachocki, A.; Markiewicz, E.; Tritt-Goc, J. Dielectric relaxation in cellulose and its derivatives. Acta Phys. Pol. A 2005, 108, 137–145. [Google Scholar] [CrossRef]
- Kaminski, K.; Kaminska, E.; Ngai, K.L.; Paluch, M.; Wlodarczyk, P.; Kasprzycka, A.; Szeja, W. Identifying the origins of two secondary relaxations in polysaccharides. J. Phys. Chem. B 2009, 113, 10088–10096. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Chen, Z.; Du, X.; Chen, L. Contribution of different state of adsorbed water to the sub-Tg dynamics of cellulose. Carbohydr. Polym. 2019, 210, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Kremer, F.; Schönhals, A. Broadband Dielectric Spectroscopy; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar] [CrossRef]
- Deshmukh, K.; Sankaran, S.; Ahamed, B.; Sadasivuni, K.K.; Pasha, K.S.K.; Ponnamma, D.; Rama Sreekanth, P.S.; Chidambaram, K. Dielectric Spectroscopy. In Spectroscopic Methods for Nanomaterials Characterization; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 237–299. [Google Scholar] [CrossRef]
- Chen, Z.; Sepulveda, A.; Ediger, M.D.; Richert, R. Dynamics of glass-forming liquids. XVI. Observation of ultrastable glass transformation via dielectric spectroscopy. J. Chem. Phys. 2013, 138, 12A519. [Google Scholar] [CrossRef]
- Minoguchi, A.; Nozaki, R. Broadband complex permittivity measurements of sorbitol during isothermal crystallization. J. Non Cryst. Sol. 2002, 307, 246–251. [Google Scholar] [CrossRef]
- Bras, A.R.; Viciosa, M.T.; Wang, Y.M.; Dionisio, M.; Mano, J.F. Crystallization of poly(L-lactic acid) probed with dielectric relaxation spectroscopy. Macromolecules 2006, 39, 6513–6520. [Google Scholar] [CrossRef]
- Asami, K.; Gheorghiu, E.; Yonezawa, T. Real-time monitoring of yeast cell division by dielectric spectroscopy. Biophys. J. 1999, 76, 3345–3348. [Google Scholar] [CrossRef]
- Lu, P.; Hsieh, Y.-L. Preparation and characterization of cellulose nanocrystals from rice straw. Carbohydr. Polym. 2012, 87, 564–573. [Google Scholar] [CrossRef]
- Steeman, P.A.M.; van Turnhout, J. Fine Structure in the Parameters of Dielectric and Viscoelastic Relaxations. Macromolecules 1994, 27, 5421–5427. [Google Scholar] [CrossRef]
- Wubbenhorst, M.; van Turnhout, J. Analysis of complex dielectric spectra. I. One-dimensional derivative techniques and three-dimensional modelling. J. Non-Cryst. Sol. 2002, 305, 40–49. [Google Scholar] [CrossRef]
- Chen, Z.; Li, X.W.; Zhao, K.S.; Xiao, J.X.; Yang, L.K. Dielectric spectroscopy investigation on the interaction of poly(diallyldimethylammonium chloride) with sodium decyl sulfate in aqueous solution. J. Phys. Chem. B 2011, 115, 5766–5774. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Tatsumi, D.; Tamai, N.; Takaki, T. Solution properties of celluloses from different biological origins in LiCl.DMAc. Cellulose 2001, 8, 275–282. [Google Scholar] [CrossRef]
- Dyre, J.C. The random free-energy barrier model for ac conduction in disordered solids. J. Appl. Phys. 1988, 64, 2456–2468. [Google Scholar] [CrossRef]
- Namikawa, H. Characterization of the diffusion process in oxide glasses based on the correlation between electric conduction and dielectric relaxation. J. Non-Cryst. Sol. 1975, 18, 173–195. [Google Scholar] [CrossRef]
- Sidebottom, D.L. Universal Approach for Scaling the ac Conductivity in Ionic Glasses. Phys. Rev. Lett. 1999, 82, 3653–3656. [Google Scholar] [CrossRef]
- Sangoro, J.; Iacob, C.; Serghei, A.; Naumov, S.; Galvosas, P.; Karger, J.; Wespe, C.; Bordusa, F.; Stoppa, A.; Hunger, J.; et al. Electrical conductivity and translational diffusion in the 1-butyl-3-methylimidazolium tetrafluoroborate ionic liquid. J. Chem. Phys. 2008, 128, 214509. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Nozaki, R. Dielectric spectroscopy study on ionic liquid microemulsion composed of water, TX-100, and BmimPF6. J. Chem. Phys. 2012, 136, 244505. [Google Scholar] [CrossRef] [Green Version]
- Maxwell, J.C. A Treatise on Electricity and Magnetism; Clarendon Press: Wotton-under-Edge, UK, 1873. [Google Scholar]
- Wagner, K.W. Dielektrische Eigenschaften von verschiedenen Isolierstoffen. Arch. Elektrotech. 1914, 3, 67–106. [Google Scholar] [CrossRef] [Green Version]
- Kuttich, B.; Grefe, A.-K.; Kröling, H.; Schabel, S.; Stühn, B. Molecular mobility in cellulose and paper. RSC Adv. 2016, 6, 32389–32399. [Google Scholar] [CrossRef] [Green Version]
- Dawsey, T.R.; McCormick, C.L. The Lithium Chloride/Dimethylacetamide Solvent for Cellulose: A Literature Review. J. Macromol. Sci. Part C 1990, 30, 405–440. [Google Scholar] [CrossRef]
- Ishii, D.; Tatsumi, D.; Matsumoto, T.; Murata, K.; Hayashi, H.; Yoshitani, H. Investigation of the structure of cellulose in LiCl/DMAc solution and its gelation behavior by small-angle X-ray scattering measurements. Macromol. Biosci. 2006, 6, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Medronho, B.; Lindman, B. Brief overview on cellulose dissolution/regeneration interactions and mechanisms. Adv. Colloid Interface Sci. 2015, 222, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Togawa, E.; Brown, R.M., Jr. “Nematic ordered cellulose”: A concept of glucan chain association. Biomacromolecules 2001, 2, 1324–1330. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, X.; Zheng, W.; Zhu, J. Regenerated cellulose/graphene nanocomposite films prepared in DMAC/LiCl solution. Carbohydr. Polym. 2012, 88, 26–30. [Google Scholar] [CrossRef]
- Zhang, B.-X.; Azuma, J.-I.; Uyama, H. Preparation and characterization of a transparent amorphous cellulose film. RSC Adv. 2015, 5, 2900–2907. [Google Scholar] [CrossRef]
- Yudianti, R.; Syampurwadi, A.; Onggo, H.; Karina, M.; Uyama, H.; Azuma, J. Properties of bacterial cellulose transparent film regenerated from dimethylacetamide-LiCl solution. Polym. Adv. Technol. 2016, 27, 1102–1107. [Google Scholar] [CrossRef]
- Han, Q.; Gao, X.; Zhang, H.; Chen, K.; Peng, L.; Jia, Q. Preparation and comparative assessment of regenerated cellulose films from corn (Zea mays) stalk pulp fines in DMAc/LiCl solution. Carbohydr. Polym. 2019. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Chen, Z.; Du, X. Evolution of Dielectric Behavior of Regenerated Cellulose Film during Isothermal Dehydration Monitored in Real Time via Dielectric Spectroscopy. Polymers 2019, 11, 1749. https://doi.org/10.3390/polym11111749
Zhao H, Chen Z, Du X. Evolution of Dielectric Behavior of Regenerated Cellulose Film during Isothermal Dehydration Monitored in Real Time via Dielectric Spectroscopy. Polymers. 2019; 11(11):1749. https://doi.org/10.3390/polym11111749
Chicago/Turabian StyleZhao, Hao, Zhen Chen, and Xianfeng Du. 2019. "Evolution of Dielectric Behavior of Regenerated Cellulose Film during Isothermal Dehydration Monitored in Real Time via Dielectric Spectroscopy" Polymers 11, no. 11: 1749. https://doi.org/10.3390/polym11111749




