A Quarterthiophene-Based Dye as an Efficient Interface Modifier for Hybrid Titanium Dioxide/Poly(3-hexylthiophene)(P3HT) Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tountas, M.; Topal, Y.; Verykios, A.; Soultati, A.; Kaltzoglou, A.; Papadopoulos, T.A.; Tsikritzis, D. A silanol-functionalized polyoxometalate with excellent electron transfer mediating behavior to ZnO and TiO2cathode interlayers for highly efficient and extremely stable polymer solar cells. J. Mater. Chem. C 2018, 6, 1459–1469. [Google Scholar] [CrossRef]
- Wright, M.; Uddin, A. Organic—inorganic hybrid solar cells: A comparative review. Sol. Energy Mater. Sol. Cells 2012, 107, 87–111. [Google Scholar] [CrossRef]
- Loheeswaran, S.; Thanihaichelvan, M.; Ravirajan, P.; Nelson, J. Controlling recombination kinetics of hybrid poly-3-hexylthiophene (P3HT)/titanium dioxide solar cells by self-assembled monolayers. J. Mater. Sci. Mater. Electron. 2017, 28, 4732–4737. [Google Scholar] [CrossRef]
- Eom, S.H.; Baek, M.J.; Park, H.; Yan, L.; Liu, S.; You, W.; Lee, S.H. Roles of interfacial modifiers in hybrid solar cells: Inorganic/polymer bilayer vs inorganic/polymer:Fullerene bulk heterojunction. ACS Appl. Mater. Interfaces 2014, 6, 803–810. [Google Scholar] [CrossRef]
- Ravirajan, P.; Haque, S.A.; Durrant, J.R.; Bradley, D.D.C.; Nelson, J. The effect of polymer optoelectronic properties on the performance of multilayer hybrid polymer/TiO2solar cells. Adv. Funct. Mater. 2005, 15, 609–618. [Google Scholar] [CrossRef]
- Balashangar, K.; Paranthaman, S.; Thanihaichelvan, M.; Amalraj, P.A.; Velauthapillai, D.; Ravirajan, P. Multi-walled carbon nanotube incorporated nanoporous titanium dioxide electrodes for hybrid polymer solar cells. Mater. Lett. 2018, 219, 265–268. [Google Scholar] [CrossRef]
- Ishwara, T.; Bradley, D.D.C.; Nelson, J.; Ravirajan, P.; Vanseveren, I.; Cleij, T.; Vanderzande, D.; Lutsen, L.; Tierney, S.; Heeney, M.; et al. Influence of polymer ionization potential on the open-circuit voltage of hybrid polymer/ Ti O2 solar cells. Appl. Phys. Lett. 2008, 92, 48–51. [Google Scholar] [CrossRef]
- Prashanthan, K.; Thivakarasarma, T.; Ravirajan, P.; Planells, M.; Robertson, N.; Nelson, J. Enhancement of hole mobility in hybrid titanium dioxide/poly(3-hexylthiophene) nanocomposites by employing an oligothiophene dye as an interface modifier. J. Mater. Chem. C 2017, 5, 11758–11762. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Zhu, Y.; Ye, X.; Li, X.; Tong, Y.; Xu, J. Balanced Dipole Effects on Interfacial Engineering for Polymer/TiO2 Array Hybrid Solar Cells. Nanoscale Res. Lett. 2017, 12, 85. [Google Scholar] [CrossRef] [PubMed]
- Mecking, S.; Schmidt-Mende, L.; Ehrenreich, P.; Groh, A.; Huster, J.; Goodwin, H.; Deschler, F. Tailored Interface Energetics for Efficient Charge Separation in Metal Oxide-Polymer Solar Cells. Sci. Rep. 2019, 9, 1–11. [Google Scholar]
- Peiró, A.M.; Ravirajan, P.; Govender, K.; Boyle, D.S.; O’Brien, P.; Bradley, D.D.C.; Nelson, J.; Durrant, J.R. The effect of zinc oxide nanostructure on the performance of hybrid polymer/zinc oxide solar cells. Proc. SPIE - Int. Soc. Opt. Eng. 2005, 5938, 593819. [Google Scholar]
- Armstrong, C.L.; Price, M.B.; Munoz-Rojas, D.; Davis, N.J.K.L.; Abdi-Jalebi, M.; Friend, R.H.; Greenham, N.C.; MacManus-Driscoll, J.L.; Boehm, M.L.; Musselman, K.P. Influence of an Inorganic Inter layer on Exciton Separation in Hybrid Solar Cells. ACS Nano 2015, 9, 11863–11871. [Google Scholar] [CrossRef] [PubMed]
- Bouclé, J.; Ravirajan, P.; Nelson, J. Hybrid polymer-metal oxide thin films for photovoltaic applications. J. Mater. Chem. 2007, 17, 3141–3153. [Google Scholar] [CrossRef]
- Ravirajan, P.; Atienzar, P.; Nelson, J. Post-Processing Treatments in Hybrid Polymer/Titanium Dioxide Multilayer Solar Cells. J. Nanoelectron. Optoelectron. 2012, 7, 498–502. [Google Scholar] [CrossRef]
- Liao, W.; Hsu, S.; Lin, W.; Wu, J. Hierarchical TiO2 Nanostructured Array/P3HT Hybrid Solar Cells with Interfacial Modification. J. Phys. Chem. C 2012, 116, 15938–15945. [Google Scholar] [CrossRef]
- Planells, M.; Abate, A.; Snaith, H.J.; Robertson, N. Oligothiophene interlayer effect on photocurrent generation for hybrid TiO2/P3HT Solar Cells. ACS Appl. Mater. Interfaces 2014, 6, 17226–17235. [Google Scholar] [CrossRef] [PubMed]
- Lo, S.; Liu, Z.; Li, J.; Chan, H.L.; Yan, F. Progress in Natural Science: Materials International Hybrid solar cells based on poly ( 3-hexylthiophene ) and electrospun TiO2 nano fibers modi fied with CdS nanoparticles. Progress Nat. Sc.: Mater. Int. 2013, 23, 514–518. [Google Scholar] [CrossRef]
- Thanihaichelvan, M.; Sockiah, K.; Balashangar, K.; Ravirajan, P. Cadmium sulfide interface layer for improving the performance of titanium dioxide/poly (3-hexylthiophene) solar cells by extending the spectral response. J. Mater. Sci. Mater. Electron. 2015, 26, 3558–3563. [Google Scholar] [CrossRef]
- Thanihaichelvan, M.; Sri Kodikara, M.M.P.; Ravirajan, P.; Velauthapillai, D. Enhanced performance of nanoporous titanium dioxide solar cells using cadmium sulfide and poly(3-hexylthiophene) co-sensitizers. Polymers 2017, 9, 467. [Google Scholar] [CrossRef]
- Senthil, T.S.; Muthukumarasamy, N.; Velauthapillai, D.; Agilan, S.; Thambidurai, M.; Balasundaraprabhu, R. Natural dye (cyanidin 3-O-glucoside) sensitized nanocrystalline TiO2 solar cell fabricated using liquid electrolyte/quasi-solid-state polymer electrolyte. Renew. Energy 2011, 36, 2484–2488. [Google Scholar] [CrossRef]
- Gokilamani, N.; Muthukumarasamy, N.; Thambidurai, M.; Ranjitha, A.; Velauthapillai, D. Utilization of natural anthocyanin pigments as photosensitizers for dye-sensitized solar cells. J. Sol.-Gel Sci. Technol. 2013, 66, 212–219. [Google Scholar] [CrossRef]
- Prabavathy, N.; Shalini, S.; Balasundaraprabhu, R.; Velauthapillai, D.; Prasanna, S.; Muthukumarasamy, N. Enhancement in the photostability of natural dyes for dye-sensitized solar cell (DSSC) applications: A review. Int. J. Energy Res. 2017, 41, 1372–1396. [Google Scholar] [CrossRef]
- Akila, Y.; Muthukumarasamy, N.; Agilan, S.; Mallick, T.K.; Senthilarasu, S.; Velauthapillai, D. Enhanced performance of natural dye sensitised solar cells fabricated using rutile TIO2 nanorods. Opt. Mater. (Amst) 2016, 58, 76–83. [Google Scholar] [CrossRef]
- Ramakrishnan, V.M.; Natarajan, M.; Santhanam, A.; Asokan, V.; Velauthapillai, D. Size controlled synthesis of TiO2nanoparticles by modified solvothermal method towards effective photo catalytic and photovoltaic applications. Mater. Res. Bull. 2018, 97, 351–360. [Google Scholar] [CrossRef]
- Wang, P.; Zakeeruddin, S.M.; Humphry-Baker, R.; Moser, J.E.; Grätzel, M. Molecular-Scale Interface Engineering of TiO2 Nanocrystals: Improving the Efficiency and Stability of Dye-Sensitized Solar Cells. Adv. Mater. 2003, 15, 2101–2104. [Google Scholar] [CrossRef]
- Wang, Z.S.; Kawauchi, H.; Kashima, T.; Arakawa, H. Significant influence of TiO2 photoelectrode morphology on the energy conversion efficiency of N719 dye-sensitized solar cell. Coord. Chem. Rev. 2004, 248, 1381–1389. [Google Scholar] [CrossRef]
- Zhong, M.; Yang, D.; Zhang, J.; Shi, J.; Wang, X.; Li, C. Improving the performance of CdS/P3HT hybrid inverted solar cells by interfacial modification. Sol. Energy Mater. Sol. Cells 2012, 96, 160–165. [Google Scholar] [CrossRef]
- Reeja-Jayan, B.; Koen, K.A.; Ono, R.J.; Vanden Bout, D.A.; Bielawski, C.W.; Manthiram, A. Oligomeric interface modifiers in hybrid polymer solar cell prototypes investigated by fluorescence voltage spectroscopy. Phys. Chem. Chem. Phys. 2015, 17, 10640–10647. [Google Scholar] [CrossRef]
- Kan, B.; Li, M.; Zhang, Q.; Liu, F.; Wan, X.; Wang, Y.; Ni, W.; Long, G.; Yang, X.; Feng, H.; et al. A series of simple oligomer-like small molecules based on oligothiophenes for solution-processed solar cells with high efficiency. J. Am. Chem. Soc. 2015, 137, 3886–3893. [Google Scholar] [CrossRef]
- Hu, Y.; Ivaturi, A.; Planells, M.; Boldrini, C.L.; Biroli, A.O.; Robertson, N. “Donor-free” oligo(3-hexylthiophene) dyes for efficient dye-sensitized solar cells. J. Mater. Chem. A 2016, 4, 2509–2516. [Google Scholar] [CrossRef]
- Wang, P.; Klein, C.; Humphry-Baker, R.; Zakeeruddin, S.M.; Graetzel, M. A high molar extinction coefficient sensitizer for stable dye-sensitized solar cells. J. Am. Chem. Soc. 2005, 127, 808–809. [Google Scholar] [CrossRef] [PubMed]
- Abate, A.; Planells, M.; Hollman, D.J.; Stranks, S.D.; Petrozza, A.; Kandada, A.R.S.; Vaynzof, Y.; Pathak, S.K.; Robertson, N.; Snaith, H.J. An organic “donor-free” dye with enhanced open-circuit voltage in solid-state sensitized solar cells. Adv. Energy Mater. 2014, 4, 1400166. [Google Scholar] [CrossRef]
- Pitchaiya, S.; Natarajan, M.; Santhanam, A.; Asokan, V.; Madurai Ramakrishnan, V.; Selvaraj, Y.; Yuvapragasam, A.; Rangasamy, B.; Sundaram, S.; Velauthapillai, D. The Performance of CH3NH3PbI3 - Nanoparticles based – Perovskite Solar Cells Fabricated by Facile Powder press Technique. Mater. Res. Bull. 2018, 108, 61–72. [Google Scholar] [CrossRef]
- Uthayaraj, S.; Karunarathne, D.; Kumara, G.; Murugathas, T.; Rasalingam, S.; Rajapakse, R.; Ravirajan, P.; Velauthapillai, D. Powder Pressed Cuprous Iodide (CuI) as A Hole Transporting Material for Perovskite Solar Cells. Materials 2019, 12, 2037. [Google Scholar] [CrossRef] [PubMed]
- Ishwara, T. Comparison of TiO2 Nanoporous Films in Hybrid Organic-inorganic Solar Cells. Energy Procedia 2017, 110, 109–114. [Google Scholar] [CrossRef]
- Xu, B.; Sai-Anand, G.; Gopalan, A.I.; Qiao, Q.; Kang, S.W. Improving photovoltaic properties of P3HT:IC60BA through the incorporation of small molecules. Polymers 2018, 10, 121. [Google Scholar] [CrossRef]
- Mahmood, A.; Khan, S.U.D.; Rana, U.A.; Janjua, M.R.S.A.; Tahir, M.H.; Nazar, M.F.; Song, Y. Effect of thiophene rings on UV/visible spectra and non-linear optical (NLO) properties of triphenylamine based dyes: A quantum chemical perspective. J. Phys. Org. Chem. 2015, 28, 418–422. [Google Scholar] [CrossRef]
- Loheeswaran, S.; Balashangar, K.; Jevirshan, J.; Ravirajan, P. Controlling Recombination Kinetics of Hybrid Nanocrystalline Titanium Dioxide/Polymer Solar Cells by Inserting an Alumina Layer at the Interface. J. Nanoelectron. Optoelectron. 2014, 8, 484–488. [Google Scholar] [CrossRef]
- Ravirajan, P.; Peir, A.M.; Nazeeruddin, M.K.; Graetzel, M.; Bradley, D.D.C.; Durrant, J.R.; Nelson, J.; Peiro, A.M. Hybrid Polymer/Zinc Oxide Photovoltaic Devices with Vertically Oriented ZnO Nanorods and an Amphiphilic Molecular Interface Layer Hybrid Polymer/Zinc Oxide Photovoltaic Devices with Vertically Oriented ZnO Nanorods and an Amphiphilic Molecular Interfa. J. Phys. Chem. 2006, 110, 7635–7639. [Google Scholar] [CrossRef]
- Wang, D.; Tao, H.; Zhao, X.; Ji, M.; Zhang, T. Enhanced photovoltaic performance in TiO2/P3HT hybrid solar cell by interface modification. J. Semicond. 2015, 36, 023006. [Google Scholar] [CrossRef]
- Ehrenreich, P.; Pfadler, T.; Paquin, F.; Dion-Bertrand, L.I.; Paré-Labrosse, O.; Silva, C.; Weickert, J.; Schmidt-Mende, L. Role of charge separation mechanism and local disorder at hybrid solar cell interfaces. Phys. Rev. B - Condens. Matter Mater. Phys. 2015, 91, 035304. [Google Scholar] [CrossRef]
- Wang, D.; Han, J.; Zhang, T.; Zhao, X.; Tao, H. TiO2/P3HT Hybrid Solar Cell with Efficient Interface Modification by Organic and Inorganic Materials: A Comparative Study. J. Nanosci. Nanotechnol. 2015, 16, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Tai, Q.; Zhao, X.; Yan, F. Hybrid solar cells based on poly(3-hexylthiophene) and electrospun TiO2 nanofibers with effective interface modification. J. Mater. Chem. 2010, 20, 7366–7371. [Google Scholar] [CrossRef]
- Hsu, S.; Liao, W.; Lin, W.; Wu, J. Modulation of Photocarrier Dynamics in Indoline Dye-Modified TiO 2 Nanorod Array/P3HT Hybrid Solar Cell with 4- tert -Butylpridine. J. Phys. Chem. C 2012, 116, 25721–25726. [Google Scholar] [CrossRef]
- Pei, J.; Hao, Y.Z.; Lv, H.J.; Sun, B.; Li, Y.P.; Guo, Z.M. Optimizing the performance of TiO2/P3HT hybrid solar cell by effective interfacial modification. Chem. Phys. Lett. 2016, 644, 127–131. [Google Scholar] [CrossRef]
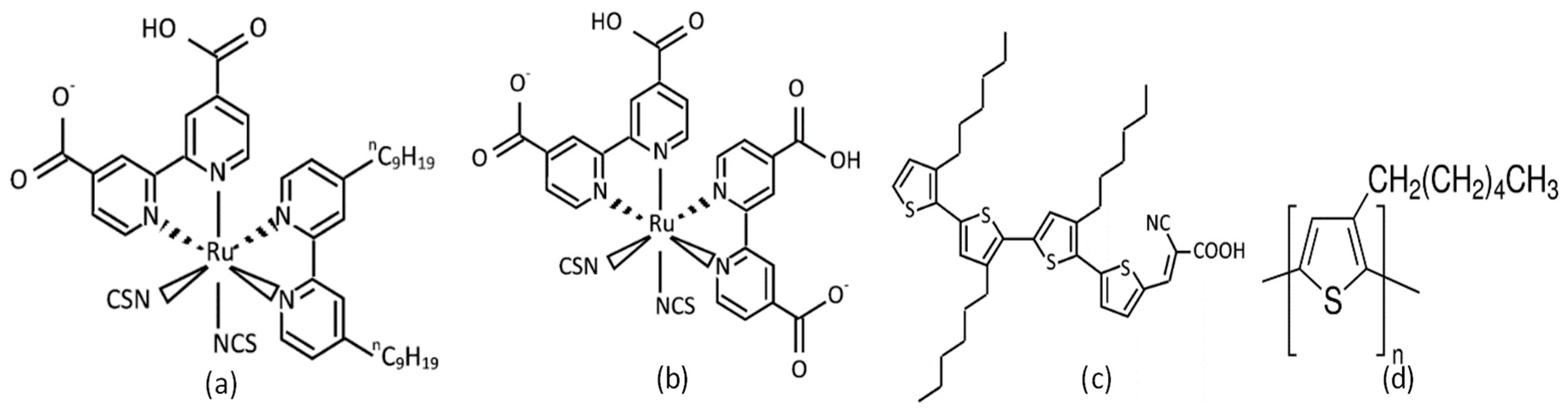





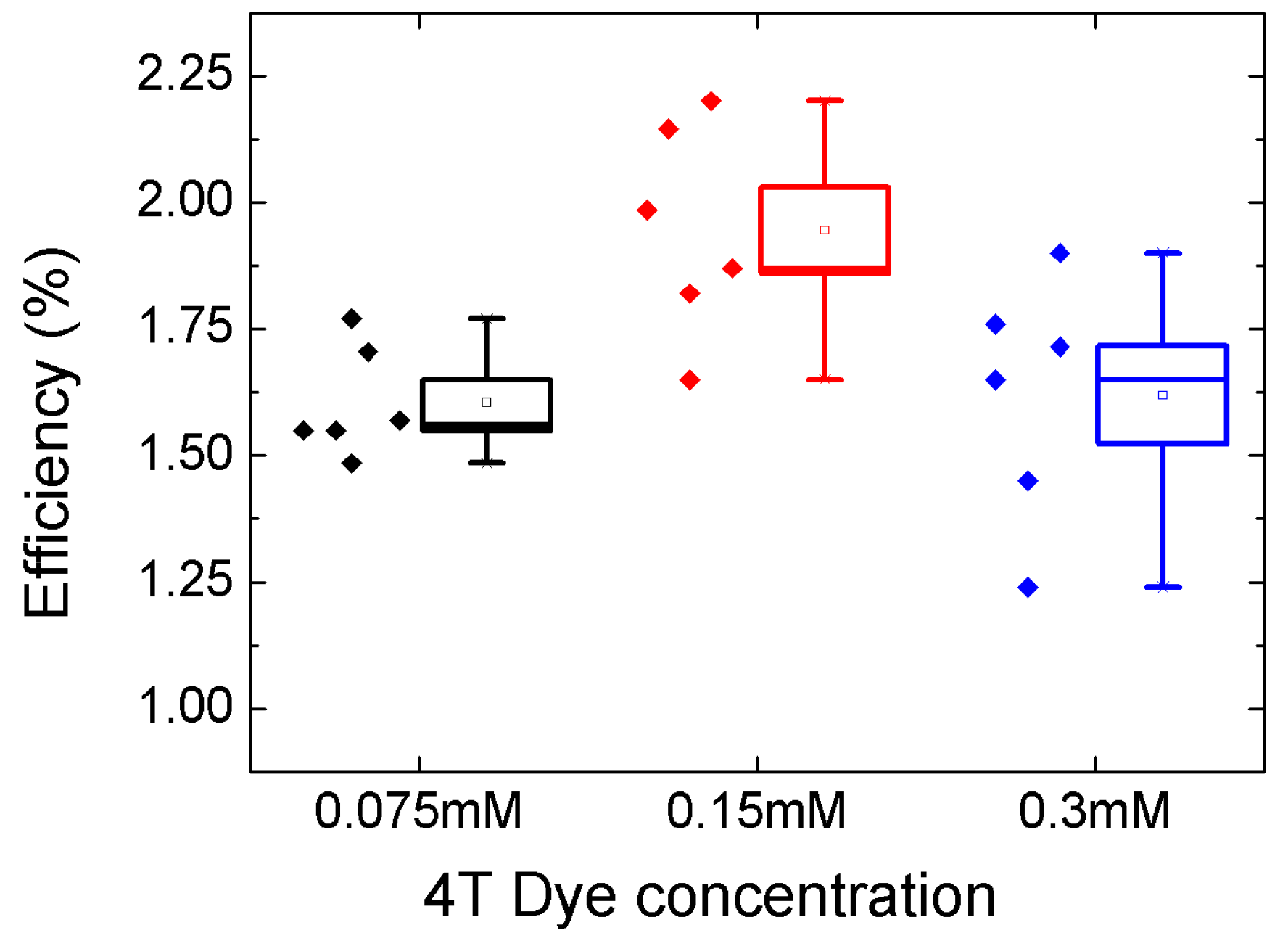
| Condition | ||||
|---|---|---|---|---|
| Without dye (control) | 2.09 | 0.44 | 44 | 0.41 |
| N719 | 3.33 | 0.65 | 39 | 0.86 |
| Z907 | 3.70 | 0.71 | 38 | 1.01 |
| 4T | 7.30 | 0.57 | 49 | 2.04 |
| Device Structure—Different Interface Modifiers | Efficiency % | Year | Reference |
|---|---|---|---|
| TiO2/carboxylated oligothiophene/P3HT | 0.11 | 2015 | [28] |
| TiO2/BT5 oligomer/P3HT | 0.21 | 2019 | [10] |
| TiCl4 treatment/TiO2 nanorod/ACA/P3HT | 0.28 | 2015 | [40] |
| TiO2/TiCl4 treatment/[6,6]-Phenyl C61 butyric acid/P3HT | 0.37 | 2015 | [41] |
| TiO2/TiCl4 treatment/D131/P3HT | 1.53 | 2015 | [41] |
| TiO2/TiCl4 treatment/squaraine dye SQ2/P3HT | 2.22 | 2015 | [41] |
| TiO2 nanorod/P3HT/PEDOT:PSS | 0.43 | 2012 | [15] |
| TiO2 nanorod(650 nm)/D149/P3HT/PEDOT:PSS | 1.58 | 2012 | [15] |
| TiO2 nanorod(1.5 μm)/D149/P3HT/PEDOT:PSS | 3.12 | 2012 | [15] |
| TiO2 nanorod/Z907/P3HT/PEDOT:PSS | 0.94 | 2012 | [15] |
| TiO2 nanowires/Pyridine/P3HT | 0.45 | 2015 | [42] |
| TiO2/Z907/P3HT/PEDOT:PSS | 0.53 | 2017 | [35] |
| TiO2 nanowires/TiCl4 treatment/CdS/P3HT | 0.7 | 2015 | [42] |
| TiO2 nanofibers/N719/P3HT | 0.90 | 2010 | [43] |
| TiO2/Nitro Benzoic Acid treatment/P3HT/PEDOT:PSS | 1.05 | 2017 | [3] |
| TiO2 nanofibers/N719 + PPA/P3HT | 1.09 | 2010 | [43] |
| TiO2/Methoxy Benzoic Acid treatment/P3HT/PEDOT:PSS | 1.24 | 2017 | [3] |
| TiO2/Al2O3/N719/P3HT/PEDOT:PSS | 1.4 | 2014 | [38] |
| TiO2/TiCl4 treatment/4T/doped P3HT | 1.54 | 2014 | [16] |
| TiO2/TiCl4 treatment/5T/doped P3HT | 2.32 | 2014 | [16] |
| TiO2 nanorod/TiCl4 treatment/D149/TBP/P3HT/PEDOT:PSS | 1.83 | 2012 | [44] |
| TiO2/triphenylamine dye/P3HT | 2.01 | 2016 | [45] |
| TiO2/Z907/P3HT | 1.01 | 2019 | Current work |
| TiO2/N719/P3HT | 0.86 | 2019 | Current work |
| TiO2/4T/P3HT | 2.04 | 2019 | Current work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirashanthan, A.; Murugathas, T.; Robertson, N.; Ravirajan, P.; Velauthapillai, D. A Quarterthiophene-Based Dye as an Efficient Interface Modifier for Hybrid Titanium Dioxide/Poly(3-hexylthiophene)(P3HT) Solar Cells. Polymers 2019, 11, 1752. https://doi.org/10.3390/polym11111752
Pirashanthan A, Murugathas T, Robertson N, Ravirajan P, Velauthapillai D. A Quarterthiophene-Based Dye as an Efficient Interface Modifier for Hybrid Titanium Dioxide/Poly(3-hexylthiophene)(P3HT) Solar Cells. Polymers. 2019; 11(11):1752. https://doi.org/10.3390/polym11111752
Chicago/Turabian StylePirashanthan, Arumugam, Thanihaichelvan Murugathas, Neil Robertson, Punniamoorthy Ravirajan, and Dhayalan Velauthapillai. 2019. "A Quarterthiophene-Based Dye as an Efficient Interface Modifier for Hybrid Titanium Dioxide/Poly(3-hexylthiophene)(P3HT) Solar Cells" Polymers 11, no. 11: 1752. https://doi.org/10.3390/polym11111752






