The Influence of Mucin-Based Artificial Saliva on Properties of Polycaprolactone and Polylactide
Abstract
:1. Introduction
2. Materials and Methods
2.1. PCL and PLA Preparation for Degradation Studies
2.2. Degree of Swelling, Weight Loss, and CLSM Observations
2.3. Molecular Weight
2.4. Differential Scanning Calorimetry (DSC) and Degree of Crystallinity
2.5. Thermogravimetry (TG) and Activation Energy
2.6. Hardness and Tensile Strength
2.7. Atomic Force Microscopy Measurments
3. Results and Discussion
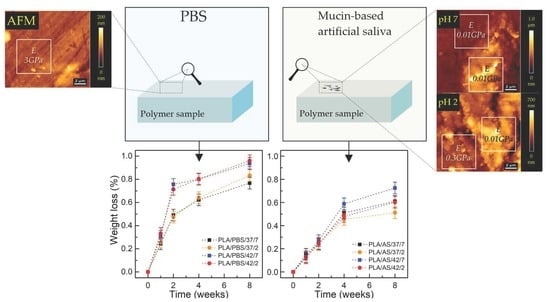
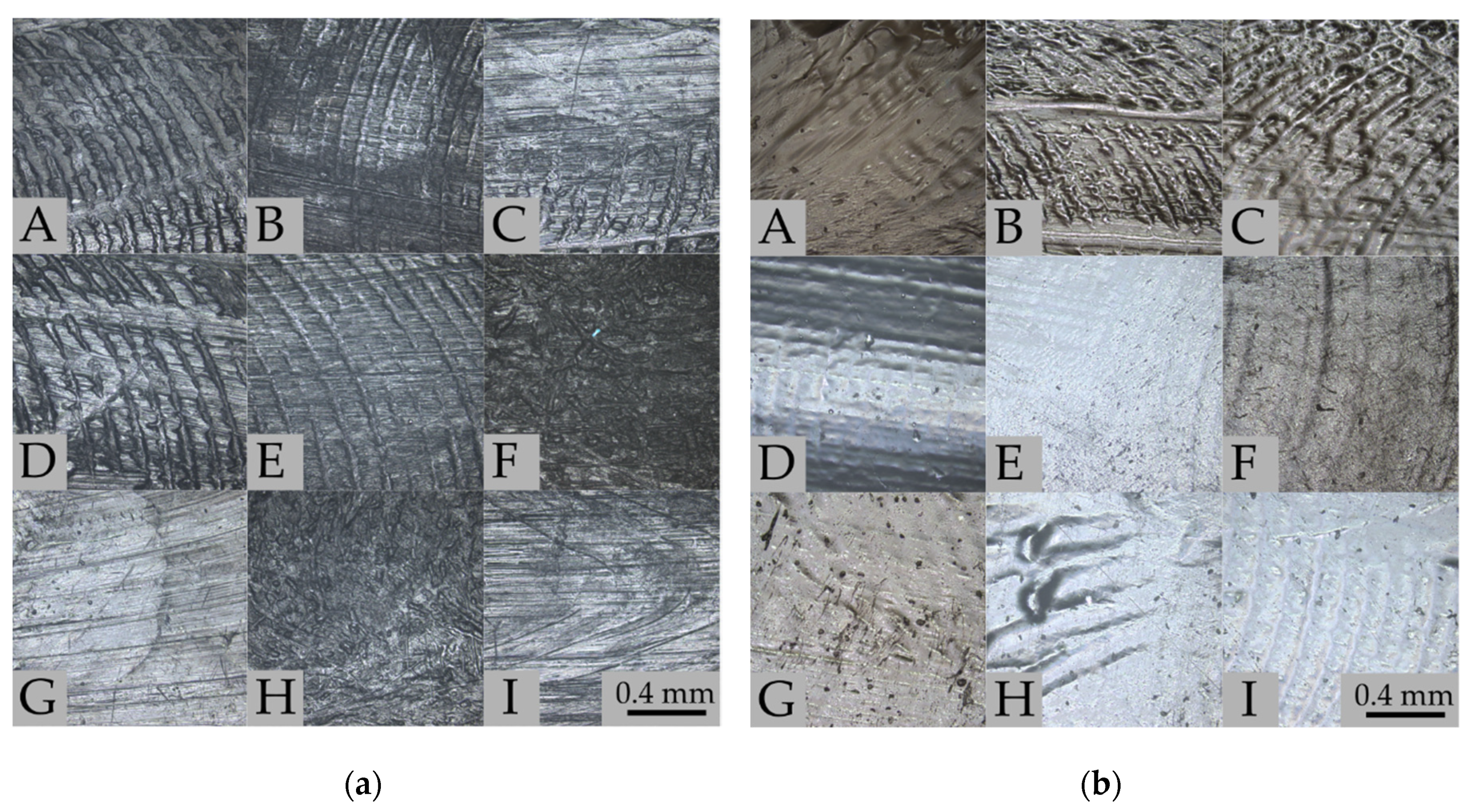
3.1. Degree of Swelling, Weight Loss, and CLSM Observations
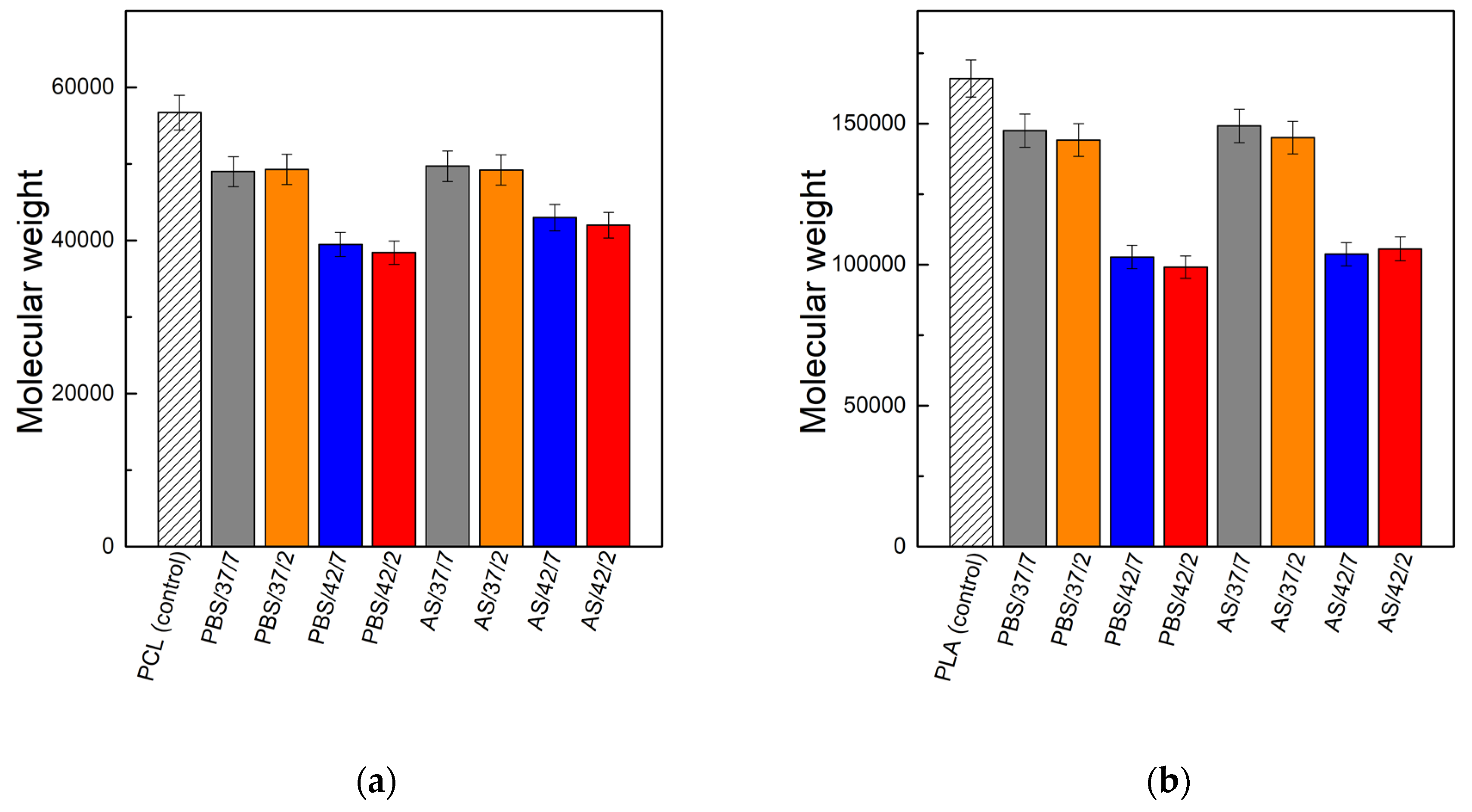
3.2. Molecular Weight
3.3. Thermal Studies
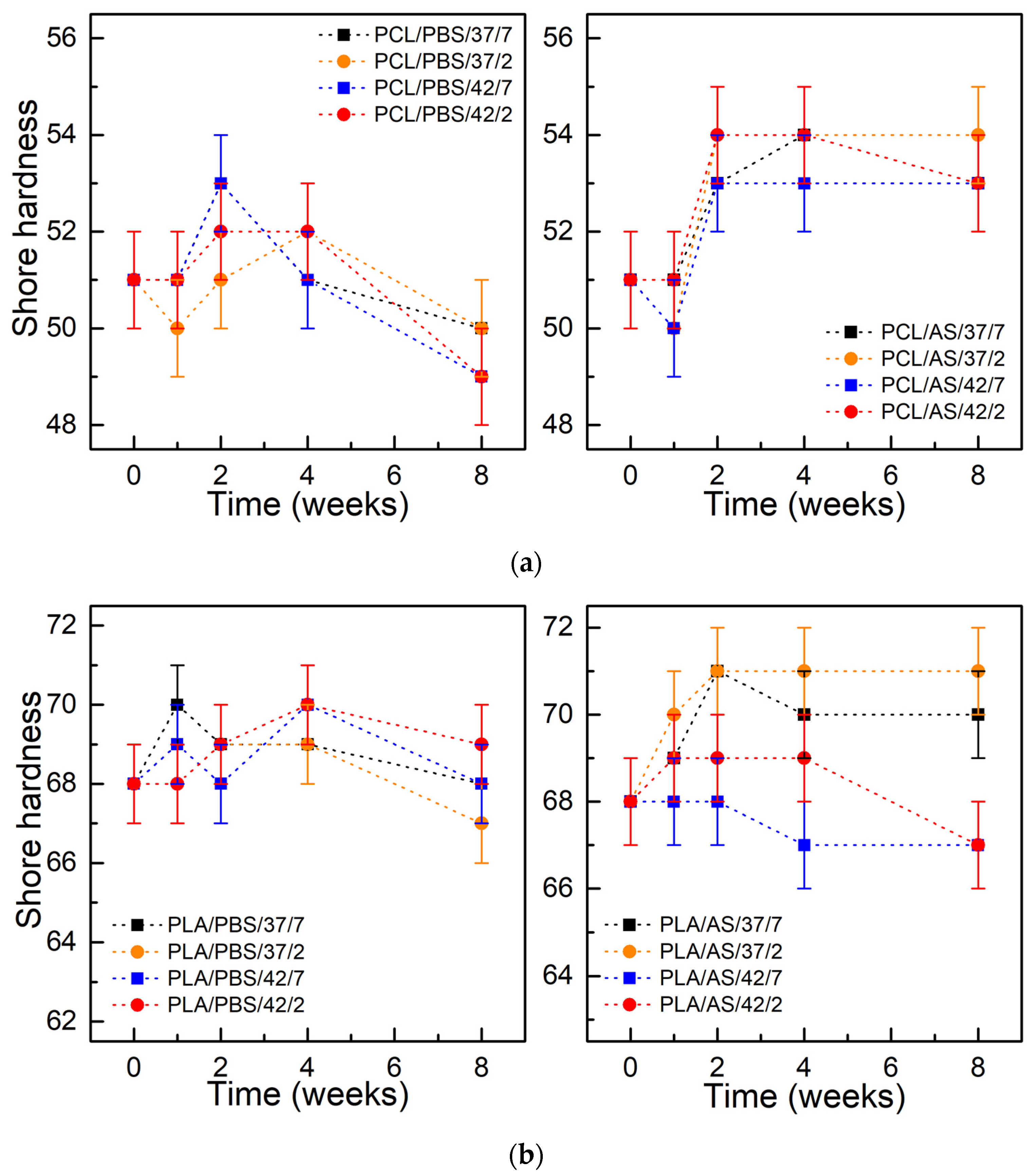
3.4. Mechanical Tests
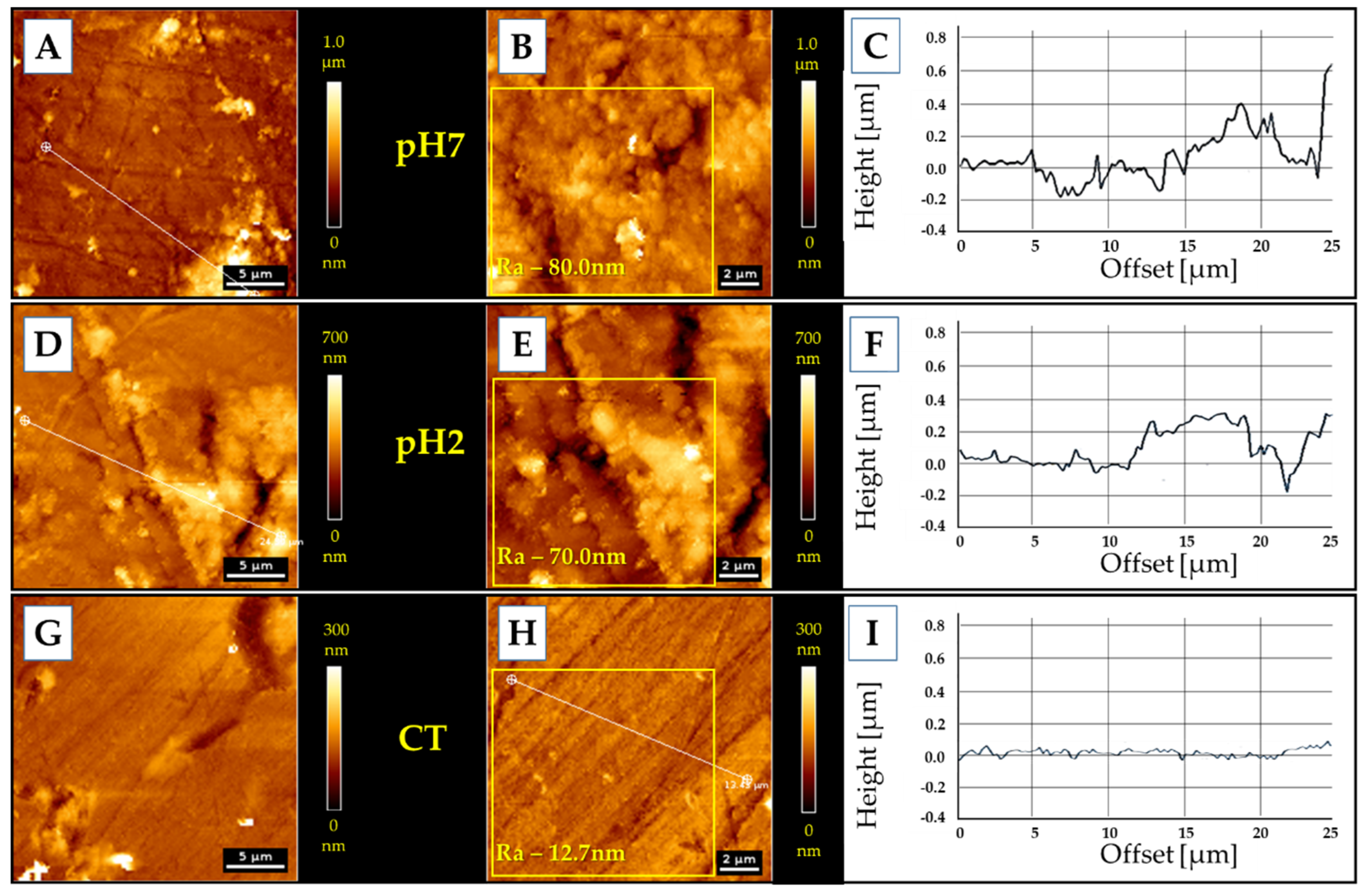
3.5. AFM Measurments
4. Summary and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tian, H.; Tang, Z.; Zhuang, X.; Chen, X.; Jing, X. Biodegradable synthetic polymers: Preparation, functionalization and biomedical application. Prog. Polym. Sci. 2012, 37, 237–280. [Google Scholar] [CrossRef]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Teo, A.J.T.; Mishra, A.; Park, I.; Kim, Y.J.; Park, W.T.; Yoon, Y.J. Polymeric Biomaterials for Medical Implants and Devices. ACS Biomater. Sci. Eng. 2016, 2, 454–472. [Google Scholar] [CrossRef]
- Mystkowska, J.; Mazurek-Budzyńska, M.; Piktel, E.; Niemirowicz, K.; Karalus, W.; Deptuła, P.; Pogoda, K.; Łysik, D.; Dąbrowski, J.R.; Rokicki, G.; et al. Assessment of aliphatic poly(ester-carbonate-urea-urethane)s potential as materials for biomedical application. J. Polym. Res. 2017, 24, 144. [Google Scholar] [CrossRef]
- Lyu, S.; Untereker, D. Degradability of polymers for implantable biomedical devices. Int. J. Mol. Sci. 2009, 10, 4033–4065. [Google Scholar] [CrossRef]
- Han, X.; Pan, J. A model for simultaneous crystallisation and biodegradation of biodegradable polymers. Biomaterials 2009, 30, 423–430. [Google Scholar] [CrossRef]
- Bikiaris, D.N.; Papageorgiou, G.Z.; Achilias, D.S.; Pavlidou, E.; Stergiou, A. Miscibility and enzymatic degradation studies of poly(ε-caprolactone)/poly(propylene succinate) blends. Eur. Polym. J. 2007, 43, 2491–2503. [Google Scholar] [CrossRef]
- Metzmacher, I.; Radu, F.; Bause, M.; Knabner, P.; Friess, W. A model describing the effect of enzymatic degradation on drug release from collagen minirods. Eur. J. Pharm. Biopharm. 2007, 67, 349–360. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Ren, K.; Wang, Y.; Sun, T.; Yue, W.; Zhang, H. Electrospun PCL/gelatin composite nanofiber structures for effective guided bone regeneration membranes. Mater. Sci. Eng. C 2017, 78, 324–332. [Google Scholar] [CrossRef]
- Hayami, J.W.S.; Surrao, D.C.; Waldman, S.D.; Amsden, B.G. Design and characterization of a biodegradable composite scaffold for ligament tissue engineering. J. Biomed. Mater. Res. Part A 2010, 92, 1407–1420. [Google Scholar] [CrossRef] [PubMed]
- Li, W.J.; Tuli, R.; Okafor, C.; Derfoul, A.; Danielson, K.G.; Hall, D.J.; Tuan, R.S. A three-dimensional nanofibrous scaffold for cartilage tissue engineering using human mesenchymal stem cells. Biomaterials 2005, 26, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, A.A.; Vig, K.; Baganizi, D.R.; Sahu, R.; Dixit, S.; Dennis, V.; Singh, S.R.; Pillai, S.R. Future prospects for scaffolding methods and biomaterials in skin tissue engineering: A review. Int. J. Mol. Sci. 2016, 17, 1974. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi-Mobarakeh, L.; Prabhakaran, M.P.; Morshed, M.; Nasr-Esfahani, M.H.; Ramakrishna, S. Electrospun poly(ε-caprolactone)/gelatin nanofibrous scaffolds for nerve tissue engineering. Biomaterials 2008, 29, 4532–4539. [Google Scholar] [CrossRef]
- Ju, Y.M.; Choi, J.S.; Atala, A.; Yoo, J.J.; Lee, S.J. Bilayered scaffold for engineering cellularized blood vessels. Biomaterials 2010, 31, 4313–4321. [Google Scholar] [CrossRef]
- Dash, T.K.; Konkimalla, V.B. Poly-ε-caprolactone based formulations for drug delivery and tissue engineering: A review. J. Control. Release 2012, 158, 15–33. [Google Scholar] [CrossRef]
- Tsuji, H. Poly(lactide) stereocomplexes: Formation, structure, properties, degradation, and applications. Macromol. Biosci. 2005, 5, 569–597. [Google Scholar] [CrossRef]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical applications of biodegradable polymers. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef]
- Middleton, J.C.; Tipton, A.J. Synthetic biodegradable polymers as orthopedic devices. Biomaterials 2000, 21, 2335–2346. [Google Scholar] [CrossRef]
- Shim, I.K.; Jung, M.R.; Kim, K.H.; Seol, Y.J.; Park, Y.J.; Park, W.H.; Lee, S.J. Novel three-dimensional scaffolds of poly(L -lactic acid) microfibers using electrospinning and mechanical expansion: Fabrication and bone regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 95, 150–160. [Google Scholar] [CrossRef]
- Cui, F.Z.; Li, Y.; Ge, J. Self-assembly of mineralized collagen composites. Mater. Sci. Eng. R Rep. 2007, 57, 1–27. [Google Scholar] [CrossRef]
- Barber, J.G.; Handorf, A.M.; Allee, T.J.; Li, W.J. Braided nanofibrous scaffold for tendon and ligament tissue engineering. Tissue Eng. Part A 2013, 19, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Yamaoka, H.; Nishizawa, S.; Nagata, S.; Ogasawara, T.; Asawa, Y.; Fujihara, Y.; Takato, T.; Hoshi, K. The optimization of porous polymeric scaffolds for chondrocyte/atelocollagen based tissue-engineered cartilage. Biomaterials 2010, 31, 4506–4516. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Murugan, R.; Ramakrishna, S.; Wang, X.; Ma, Y.X.; Wang, S. Fabrication of nano-structured porous PLLA scaffold intended for nerve tissue engineering. Biomaterials 2004, 25, 1891–1900. [Google Scholar] [CrossRef]
- Hu, J.; Sun, X.; Ma, H.; Xie, C.; Chen, Y.E.; Ma, P.X. Porous nanofibrous PLLA scaffolds for vascular tissue engineering. Biomaterials 2010, 31, 7971–7977. [Google Scholar] [CrossRef]
- Ma, H.; Hu, J.; Ma, P.X. Polymer scaffolds for small-diameter vascular tissue engineering. Adv. Funct. Mater. 2010, 20, 2833–2841. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly(lactic acid) modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Lu, L.; Garcia, C.A.; Mikos, A.G. In vitro degradation of thin poly(DL-lactic-co-glycolic acid) films. J. Biomed. Mater. Res. 1999, 46, 236–244. [Google Scholar] [CrossRef]
- McDonald, P.F.; Lyons, J.G.; Geever, L.M.; Higginbotham, C.L. In vitro degradation and drug release from polymer blends based on poly(dl-lactide), poly(l-lactide-glycolide) and poly(ε-caprolactone). J. Mater. Sci. 2010, 45, 1284–1292. [Google Scholar] [CrossRef]
- Vey, E.; Rodger, C.; Booth, J.; Claybourn, M.; Miller, A.F.; Saiani, A. Degradation kinetics of poly(lactic-co-glycolic) acid block copolymer cast films in phosphate buffer solution as revealed by infrared and Raman spectroscopies. Polym. Degrad. Stab. 2011, 96, 1882–1889. [Google Scholar] [CrossRef]
- Xue, Y.N.; Huang, Z.Z.; Zhang, J.T.; Liu, M.; Zhang, M.; Huang, S.W.; Zhuo, R.X. Synthesis and self-assembly of amphiphilic poly(acrylic acid-b-dl-lactide) to form micelles for pH-responsive drug delivery. Polymer 2009, 50, 3706–3713. [Google Scholar] [CrossRef]
- Bao, M.; Lou, X.; Zhou, Q.; Dong, W.; Yuan, H.; Zhang, Y. Electrospun biomimetic fibrous scaffold from shape memory polymer of PDLLA-co-TMC for bone tissue engineering. ACS Appl. Mater. Interfaces 2014, 6, 2611–2621. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Det-Amornrat, U.; Wang, J.; Basit, A.W.; Gaisford, S. 3D scanning and 3D printing as innovative technologies for fabricating personalized topical drug delivery systems. J. Control. Release 2016, 234, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Rios, H.F.; Jin, Q.; Bland, M.E.; Flanagan, C.L.; Hollister, S.J.; Giannobile, W.V. Biomimetic hybrid scaffolds for engineering human tooth-ligament interfaces. Biomaterials 2010, 31, 5945–5952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.S.; Lee, S.J.; Jo, H.H.; Lee, J.H.; Kim, W.D.; Lee, J.Y.; Park, S.A. Fabrication and characterization of 3D-printed bone-like β-tricalcium phosphate/polycaprolactone scaffolds for dental tissue engineering. J. Ind. Eng. Chem. 2017, 46, 175–181. [Google Scholar] [CrossRef]
- Xiong, X.Y.; Li, Q.H.; Li, Y.P.; Guo, L.; Li, Z.L.; Gong, Y.C. Pluronic P85/poly(lactic acid) vesicles as novel carrier for oral insulin delivery. Colloids Surf. B Biointerfaces 2013, 111, 282–288. [Google Scholar] [CrossRef]
- Lin, C.H.; Su, J.M.; Hsu, S.H. Evaluation of type II collagen scaffolds reinforced by poly(ε- caprolactone) as tissue-engineered trachea. Tissue Eng. Part C Methods 2008, 14, 69–77. [Google Scholar] [CrossRef]
- Mäkitie, A.A.; Korpela, J.; Elomaa, L.; Reivonen, M.; Kokkari, A.; Malin, M.; Korhonen, H.; Wang, X.; Salo, J.; Sihvo, E.; et al. Novel additive manufactured scaffolds for tissue engineered trachea research. Acta Otolaryngol. 2013, 133, 412–417. [Google Scholar] [CrossRef]
- Gao, M.; Zhang, H.; Dong, W.; Bai, J.; Gao, B.; Xia, D.; Feng, B.; Chen, M.; He, X.; Yin, M.; et al. Tissue-engineered trachea from a 3D-printed scaffold enhances whole-segment tracheal repair. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.Y.; Xiong, X.Y.; Tian, Y.; Li, Z.L.; Gong, Y.C.; Li, Y.P. A review of biodegradable polymeric systems for oral insulin delivery. Drug Deliv. 2016, 23, 1882–1891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyu, S.; Schley, J.; Loy, B.; Lind, D.; Hobot, C.; Sparer, R.; Untereker, D. Kinetics and time-temperature equivalence of polymer degradation. Biomacromolecules 2007, 8, 2301–2310. [Google Scholar] [CrossRef] [PubMed]
- Mathisen, T.; Lewis, M.; Albertsson, A.-C. Hydrolytic degradation of nonoriented poly(β-propiolactone). J. Appl. Polym. Sci. 1991, 42, 2365–2370. [Google Scholar] [CrossRef]
- Tsuji, H.; Mizuno, A.; Ikada, Y. Properties and morphology of poly(L-lactide). III. Effects of initial crystallinity on long-term in vitro hydrolysis of high molecular weight poly(L-lactide) film in phosphate-buffered solution. J. Appl. Polym. Sci. 2000, 77, 1452–1464. [Google Scholar] [CrossRef]
- Wiggins, J.S.; Hassan, M.K.; Mauritz, K.A.; Storey, R.F. Hydrolytic degradation of poly(d,l-lactide) as a function of end group: Carboxylic acid vs. hydroxyl. Polymer 2006, 47, 1960–1969. [Google Scholar] [CrossRef]
- Lyu, S.; Sparer, R.; Untereker, D. Analytical solutions to mathematical models of the surface and bulk erosion of solid polymers. J. Polym. Sci. Part B Polym. Phys. 2005, 43, 383–397. [Google Scholar] [CrossRef]
- Pitt, C.G.; Chasalow, F.I.; Hibionada, Y.M.; Klimas, D.M.; Schindler, A. Aliphatic polyesters. I. The degradation of poly(ε-caprolactone) in vivo. J. Appl. Polym. Sci. 1981, 26, 3779–3787. [Google Scholar] [CrossRef]
- Pitt, G.G.; Gratzl, M.M.; Kimmel, G.L.; Surles, J.; Sohindler, A. Aliphatic polyesters II. The degradation of poly (DL-lactide), poly (ε-caprolactone), and their copolymers in vivo. Biomaterials 1981, 2, 215–220. [Google Scholar] [CrossRef]
- Vert, M.; Li, S.; Garreau, H. More about the degradation of LA/GA-derived matrices in aqueous media. J. Control. Release 1991, 16, 15–26. [Google Scholar] [CrossRef]
- Göpferich, A. Polymer bulk erosion. Macromolecules 1997, 30, 2598–2604. [Google Scholar] [CrossRef]
- Tsuji, H.; Nakahara, K.; Ikarashi, K. Poly(L-lactide), 8 high-temperature hydrolysis of poly(L-lactide) films with different crystallinities and crystalline thicknesses in phosphate-buffered solution. Macromol. Mater. Eng. 2001, 286, 398–406. [Google Scholar] [CrossRef]
- Deng, M.; Zhou, J.; Chen, G.; Burkley, D.; Xu, Y.; Jamiolkowski, D.; Barbolt, T. Effect of load and temperature on in vitro degradation of poly(glycolide-co-L-lactide) multifilament braids. Biomaterials 2005, 26, 4327–4336. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.R.; Bei, J.Z.; Wang, S.G. Polycaprolactone microparticles and their biodegradation. Polym. Degrad. Stab. 2000, 67, 455–459. [Google Scholar] [CrossRef]
- Sekosan, G.; Vasanthan, N. Morphological changes of annealed poly-ε-caprolactone by enzymatic degradation with lipase. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 202–211. [Google Scholar] [CrossRef]
- Castilla-Cortázar, I.; Más-Estellés, J.; Meseguer-Dueñas, J.M.; Ivirico, J.L.E.; Marí, B.; Vidaurre, A. Hydrolytic and enzymatic degradation of a poly(ε-caprolactone) network. Polym. Degrad. Stab. 2012, 97, 1241–1248. [Google Scholar] [CrossRef]
- Reeve, M.S.; McCarthy, S.P.; Downey, M.J.; Gross, R.A. Polylactide Stereochemistry: Effect on Enzymic Degradability. Macromolecules 1994, 27, 825–831. [Google Scholar] [CrossRef]
- Gan, Z.; Yu, D.; Zhong, Z.; Liang, Q.; Jing, X. Enzymatic degradation of poly(ε-caprolactone)/poly(DL-lactide) blends in phosphate buffer solution. Polymer 1999, 40, 2859–2862. [Google Scholar] [CrossRef]
- Gibbins, H.L.; Yakubov, G.E.; Proctor, G.B.; Wilson, S.; Carpenter, G.H. What interactions drive the salivary mucosal pellicle formation? Colloids Surf. B Biointerfaces 2014, 120, 184–192. [Google Scholar] [CrossRef] [Green Version]
- Mystkowska, J.; Niemirowicz-Laskowska, K.; Łysik, D.; Tokajuk, G.; Dąbrowski, J.R.; Bucki, R. The role of oral cavity biofilm on metallic biomaterial surface destruction–corrosion and friction aspects. Int. J. Mol. Sci. 2018, 19, 743. [Google Scholar] [CrossRef] [Green Version]
- Łysik, D.; Niemirowicz-Laskowska, K.; Bucki, R.; Tokajuk, G.; Mystkowska, J. Artificial Saliva: Challenges and Future Perspectives for the Treatment of Xerostomia. Int. J. Mol. Sci. 2019, 20, 3199. [Google Scholar] [CrossRef] [Green Version]
- Mystkowska, J.; Dabrowski, J.R.; Romanowska, J.; Klekotka, M.; Car, H.; Milewska, A.J. Artificial mucin-based saliva preparations—Physicochemical and tribological properties. Oral Health Prev. Dent. 2018, 16, 183–193. [Google Scholar] [PubMed]
- Mystkowska, J.; Łysik, D.; Klekotka, M. Effect of saliva and mucin-based saliva substitutes on fretting processes of 316 austenitic stainless steel. Metals 2019, 9, 178. [Google Scholar] [CrossRef] [Green Version]
- Mystkowska, J.; Jałbrzykowski, M.; Dabrowski, J.R. Tribological properties of selected self-made solutions of synthetic saliva. Solid State Phenom. 2013, 199, 567–572. [Google Scholar] [CrossRef]
- Kouparitsas, I.K.; Mele, E.; Ronca, S. Synthesis and electrospinning of polycaprolactone from an aluminium-based catalyst: Influence of the ancillary ligand and initiators on catalytic efficiency and fibre structure. Polymers 2019, 11, 677. [Google Scholar] [CrossRef] [Green Version]
- Rak, J.; Ford, J.L.; Rostron, C.; Walters, V. The preparation and characterization of poly(D,L-lactic acid) for use as a biodegradable drug carrier. Pharm. Acta Helv. 1985, 60, 162–169. [Google Scholar]
- Jia, S.; Yu, D.; Zhu, Y.; Wang, Z.; Chen, L.; Fu, L. Morphology, crystallization and thermal behaviors of PLA-based composites: Wonderful effects of hybrid GO/PEG via dynamic impregnating. Polymers 2017, 9, 528. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.Z.; Zhou, L.; Tang, C.Y.; Tsui, C.P. Crystallization properties of polycaprolactone composites filled with nanometer calcium carbonate. J. Appl. Polym. Sci. 2013, 128, 2940–2944. [Google Scholar] [CrossRef]
- Bansil, R.; Turner, B.S. Mucin structure, aggregation, physiological functions and biomedical applications. Curr. Opin. Colloid Interface Sci. 2006, 11, 164–170. [Google Scholar] [CrossRef]
- Xu, L.; Crawford, K.; Gorman, C.B. Effects of temperature and pH on the degradation of poly(lactic acid) brushes. Macromolecules 2011, 44, 4777–4782. [Google Scholar] [CrossRef]
- Li, S.; McCarthy, S. Further investigations on the hydrolytic degradation of poly (DL-lactide). Biomaterials 1999, 20, 35–44. [Google Scholar] [CrossRef]
- Proikakis, C.S.; Mamouzelos, N.J.; Tarantili, P.A.; Andreopoulos, A.G. Swelling and hydrolytic degradation of poly(d,l-lactic acid) in aqueous solutions. Polym. Degrad. Stab. 2006, 91, 614–619. [Google Scholar] [CrossRef]
- Loh, X.J. The effect of pH on the hydrolytic degradation of poly(ε-caprolactone) -block-poly(ethylene glycol) copolymers. J. Appl. Polym. Sci. 2013, 127, 2046–2056. [Google Scholar] [CrossRef]
- Reich, G. Use of DSC to study the degradation behavior of PLA and PLGA microparticles. Drug Dev. Ind. Pharm. 1997, 23, 1177–1189. [Google Scholar] [CrossRef]
- Paul, M.A.; Delcourt, C.; Alexandre, M.; Degée, P.; Monteverde, F.; Dubois, P. Polylactide/montmorillonite nanocomposites: Study of the hydrolytic degradation. Polym. Degrad. Stab. 2005, 87, 535–542. [Google Scholar] [CrossRef]
- França, D.C.; Bezerra, E.B.; Morais, D.D.S.; Araújo, E.M.; Wellen, R.M.R. Effect of hydrolytic degradation on mechanical properties of PCL. Mater. Sci. Forum 2016, 869, 342–345. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Ito, Y.; Zhang, P.; Chen, X. A comparative study on the in vivo degradation of poly(L-lactide) based composite implants for bone fracture fixation. Sci. Rep. 2016, 6, 20770. [Google Scholar] [CrossRef] [Green Version]
- Sotres, J.; Jankovskaja, S.; Wannerberger, K.; Arnebrant, T. Ex-Vivo Force Spectroscopy of Intestinal Mucosa Reveals the Mechanical Properties of Mucus Blankets. Sci. Rep. 2017, 7, 7270. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Bansil, R.; Bhaskar, K.R.; Turner, B.S.; LaMont, J.T.; Niu, N.; Afdhal, N.H. pH-dependent conformational change of gastric mucin leads to sol-gel transition. Biophys. J. 1999, 76, 1250–1258. [Google Scholar] [CrossRef] [Green Version]
- Bhaskar, K.R.; Gong, D.H.; Bansil, R.; Pajevic, S.; Hamilton, J.A.; Turner, B.S.; LaMont, J.T. Profound increase in viscosity and aggregation of pig gastric mucin at low pH. Am. J. Physiol. 1991, 261, G827–G832. [Google Scholar] [CrossRef]
- Celli, J.P.; Turner, B.S.; Afdhal, N.H.; Ewoldt, R.H.; McKinley, G.H.; Bansil, R.; Erramilli, S. Rheology of gastric mucin exhibits a pH-dependent sol-gel transition. Biomacromolecules 2007, 8, 1580–1586. [Google Scholar] [CrossRef] [Green Version]

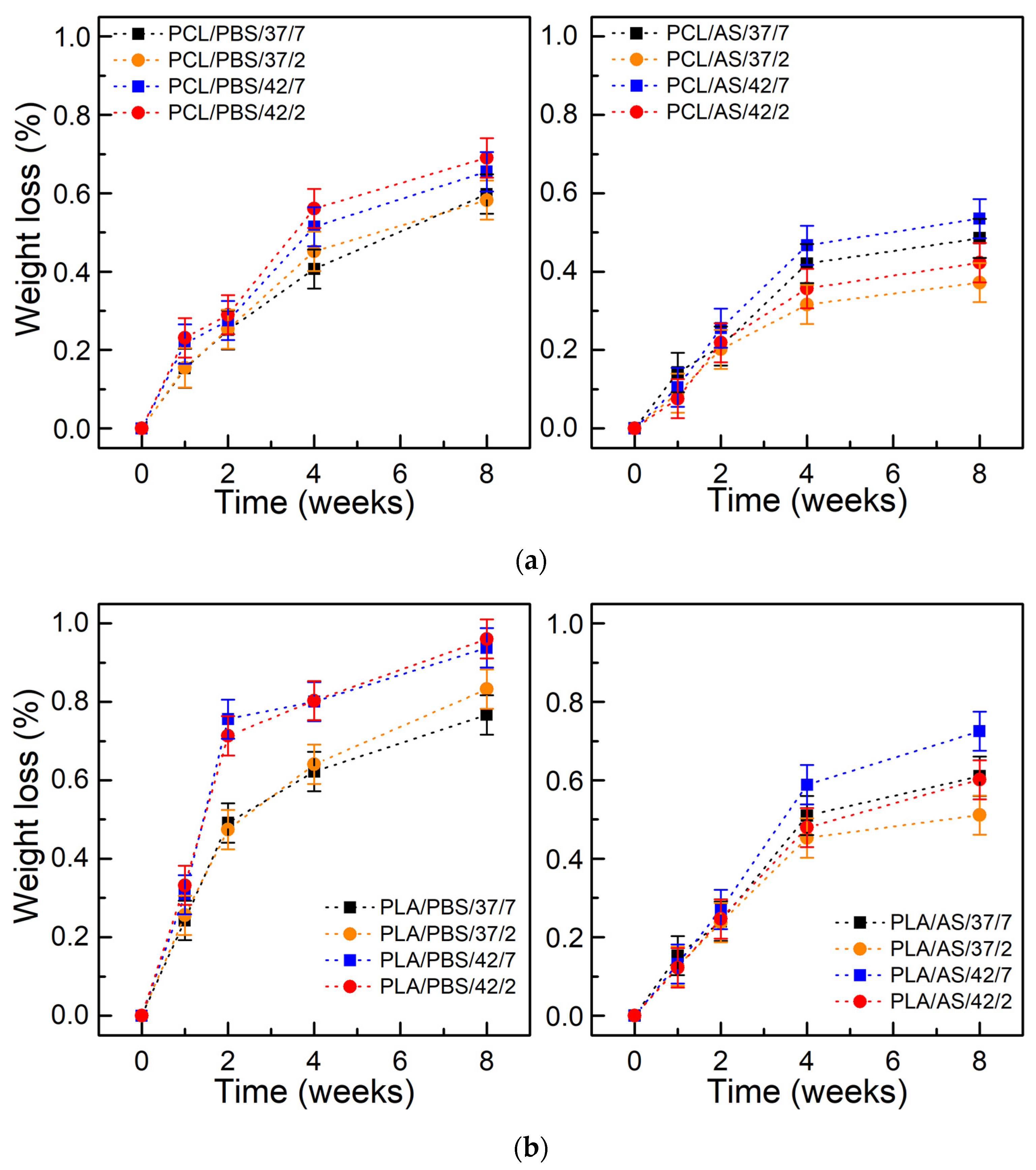


| Designation | Material | Medium | Temperature (°C) | Ph |
|---|---|---|---|---|
| PCL/PBS/37/7 | PCL | PBS | 37 | 7.4 |
| PCL/PBS/37/2 | PCL | PBS | 37 | 2 |
| PCL/PBS/42/7 | PCL | PBS | 42 | 7.4 |
| PCL/PBS/42/2 | PCL | PBS | 42 | 2 |
| PCL/AS/37/7 | PCL | Artificial saliva | 37 | 7.4 |
| PCL/AS/37/2 | PCL | Artificial saliva | 37 | 2 |
| PCL/AS/42/7 | PCL | Artificial saliva | 42 | 7.4 |
| PCL/AS/42/2 | PCL | Artificial saliva | 42 | 2 |
| PLA/PBS/37/7 | PLA | PBS | 37 | 7.4 |
| PLA/PBS/37/2 | PLA | PBS | 37 | 2 |
| PLA/PBS/42/7 | PLA | PBS | 42 | 7.4 |
| PLA/PBS/42/2 | PLA | PBS | 42 | 2 |
| PLA/AS/37/7 | PLA | Artificial saliva | 37 | 7.4 |
| PLA/AS/37/2 | PLA | Artificial saliva | 37 | 2 |
| PLA/AS/42/7 | PLA | Artificial saliva | 42 | 7.4 |
| PLA/AS/42/2 | PLA | Artificial saliva | 42 | 2 |
| Sample | Tg (°C) | Tc (°C) | Tm (°C) | Xc (%) | TDS (°C) | TDE (°C) | Ea (kJ/mol) |
|---|---|---|---|---|---|---|---|
| PCL | −65.2 | 37.5 | 54 | 59.3 | 360.6 | 450.9 | 221.2 |
| PCL/PBS/37/7 | −70.6 | 36.4 | 54.8 | 59.6 | 361.4 | 450.6 | 218.8 |
| PCL/PBS/37/2 | −65.3 | 36.1 | 53.5 | 59.9 | 365.2 | 436.7 | 174.4 |
| PCL/PBS/42/7 | −65.3 | 35.5 | 53.2 | 65.5 | 365.6 | 433.3 | 183.8 |
| PCL/PBS/42/2 | −69.95 | 35.1 | 53.5 | 63.6 | 367.1 | 433.2 | 160.3 |
| PCL/AS/37/7 | −78.3 | 36.7 | 53.8 | 57.8 | 364.3 | 426.8 | 162.7 |
| PCL/AS/37/2 | −79.7 | 36.8 | 53.8 | 59.0 | 366.4 | 434.9 | 181.3 |
| PCL/AS/42/7 | −79.4 | 36.4 | 53.4 | 60.0 | 365.3 | 434.1 | 177.6 |
| PCL/AS/42/2 | −82.2 | 36.7 | 53.1 | 61.5 | 366.4 | 434.4 | 177.9 |
| PLA | 48.5 | 107.7 | 146.7 | 2.6 | 324.2 | 388.7 | 190.2 |
| PLA/PBS/37/7 | 46.8 | 96.9 | 143.7 | 3.2 | 319.8 | 385.9 | 163.0 |
| PLA/PBS/37/2 | 47.1 | 97.6 | 145.6 | 3.1 | 319.7 | 385.2 | 140.4 |
| PLA/PBS/42/7 | 41.1 | 103.5 | 134.5 | 2.8 | 316.9 | 384.7 | 154.2 |
| PLA/PBS/42/2 | 41.6 | 100 | 135.5 | 2.6 | 316.6 | 384.4 | 144.0 |
| PLA/AS/37/7 | 47.3 | 96.6 | 144.2 | 3.1 | 308.9 | 378.5 | 158.2 |
| PLA/AS/37/2 | 46.5 | 99.2 | 141.7 | 3.5 | 318.0 | 385.1 | 170.1 |
| PLA/AS/42/7 | 45.7 | 99.3 | 140.2 | 4.2 | 314.1 | 382.1 | 157.1 |
| PLA/AS/42/2 | 47.5 | 95 | 145.3 | 4.6 | 310.7 | 383.0 | 177.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łysik, D.; Mystkowska, J.; Markiewicz, G.; Deptuła, P.; Bucki, R. The Influence of Mucin-Based Artificial Saliva on Properties of Polycaprolactone and Polylactide. Polymers 2019, 11, 1880. https://doi.org/10.3390/polym11111880
Łysik D, Mystkowska J, Markiewicz G, Deptuła P, Bucki R. The Influence of Mucin-Based Artificial Saliva on Properties of Polycaprolactone and Polylactide. Polymers. 2019; 11(11):1880. https://doi.org/10.3390/polym11111880
Chicago/Turabian StyleŁysik, Dawid, Joanna Mystkowska, Grzegorz Markiewicz, Piotr Deptuła, and Robert Bucki. 2019. "The Influence of Mucin-Based Artificial Saliva on Properties of Polycaprolactone and Polylactide" Polymers 11, no. 11: 1880. https://doi.org/10.3390/polym11111880