A Novel Application of Electroactive Polyimide Doped with Gold Nanoparticles: As a Chemiresistor Sensor for Hydrogen Sulfide Gas
Abstract
1. Introduction
2. Experimental
2.1. Chemicals and Equipment
2.2. Synthesis and Characterization of PI and PI/AuNPs
2.3. Evaluation of Fabricated Sensors Toward H2S
3. Results and Discussion
3.1. Synthesis and Characterization of PI-Based Materials
3.1.1. Synthesis of electroactive polyimide-based materials
3.1.2. Structural and Morphological Characterization
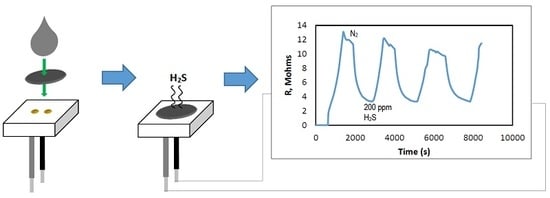
3.2. Evaluation of the Sensor′s Performance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- OSHA Fact Sheet; U.S. Department of Labor: Washington, DC, USA. Available online: https://www.osha.gov/SLTC/hydrogensulfide/hazards.html (accessed on 3 November 2019).
- Occupational Safety and Health Administration Technical Center. Hydrogen Sulfide Backup Data Report (ID-141); Occupational Safety and Health Administration Technical Center: Salt Lake City, UT, USA, 1989.
- Rubright, S.; Pearce, L.; Peterson, J. Environmental toxicology of hydrogen sulfide. Nitric Oxide 2017, 71, 1–13. [Google Scholar] [CrossRef]
- Guidotti, T.L. Hydrogen Sulfide Intoxication. In Handbook of Clinical Neurology, 3rd ed.; Lotti, M., Bleecker, M.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; Chapter 8; Volume 131, pp. 111–133. [Google Scholar]
- Roth, S.H. Handbook of Hazardous Materials; Academic Press: Cambridge, UK, 1993; pp. 367–376. [Google Scholar]
- Terrence, S. Hydrogen Sulphide in Agricultural Biogas Systems; Ministry of Agriculture Food and Rural Affairs: Centre Wellington, ON, Canada, 2011; pp. 1–7.
- Plaza, C.; Xu, Q.; Townsend, T.; Bitton, G.; Booth, M. Evaluation of alternative landfill cover soils for attenuating hydrogen sulfide from construction and demolition (C&D) debris landfills. J. Environ. Manag. 2007, 84, 314–322. [Google Scholar]
- Shen, D.S.; Du, Y.; Fang, Y.; Hu, Li.; Fang, Ch.; Long, Yu. Characteristics of H2S emission from aged refuse after excavation exposure. J. Environ. Manag. 2015, 154, 159–165. [Google Scholar] [CrossRef]
- Textor, C.; Graf, H.F.; Herzog, M.; Oberhuber, J.M. Injection of gases into the stratosphere by explosive volcanic eruptions. J. Geophys. Res. 2003, 108, 4606. [Google Scholar] [CrossRef]
- Oppenheimer, C.; Scaillet, B.; Martin, R.S. Sulfur Degassing from Volcanoes: Source Conditions, Surveillance, Plume Chemistry and Earth System Impacts. Sulfur Magmas Melts 2011, 73, 363–422. [Google Scholar]
- Edmonds, M.; Grattan, J.; Michnowicz, S. Volcanic Gases: Silent Killers. In Observing the Volcano World. Advances in Volcanology; Fearnley, C.J., Bird, D.K., Haynes, K., McGuire, W.J., Jolly, G., Eds.; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Jafarinejad, S. Odours emission and control in the petroleum refinery: A review. Curr. Sci. Perspect. 2016, 2, 78–82. [Google Scholar]
- Jafarinejad, S. Pollutions and Wastes from the Petroleum Industry. In Petroleum Waste Treatment and Pollution Control; Butterworth-Heinemann: Oxford, UK, 2017; pp. 19–83. [Google Scholar]
- Shivanthan, M.C.; Perera, H.; Jayasinghe, S.; Karunanayake, P.; Chang, T.; Ruwanpathirana, S.; Jayasinghe, N.; De Silva, Y.; Jayaweerabandara, D. Hydrogen sulphide inhalational toxicity at a petroleum refinery in Sri Lanka: A case series of seven survivors following an industrial accident and a brief review of medical literature. J. Occup. Med. Toxicol. (Lond. UK) 2013, 8, 9. [Google Scholar] [CrossRef]
- Kangas, J.; Jappinen, P.; Savolainen, H. Exposure to Hydrogen Sulfide, Mercaptans and Sulfur Dioxide in Pulp Industry. Am. Ind. Hyg. Assoc. J. 1984, 45, 787–790. [Google Scholar] [CrossRef]
- Veluchamy, C.; Kalamdhad, A.S. Enhancement of hydrolysis of lignocellulose waste pulp and paper mill sludge through different heating processes on thermal pretreatment. J. Clean. Prod. 2017, 168, 219–226. [Google Scholar] [CrossRef]
- Crundwell, F.; Moats, M.; Ramachandran, V.; Robinson, T.; Davenport, W.G. Extractive Metallurgy of Nickel, Cobalt and Platinum Group Metals; Elsevier Ltd.: Oxford, UK, 2011; pp. 443–455. ISBN 9780080968094. [Google Scholar]
- Chaulya, S.K.; Prasad, G.M. Chapter 3—Gas Sensors for Underground Mines and Hazardous Areas. In Sensing and Monitoring Technologies for Mines and Hazardous Areas Monitoring and Prediction Technologies; Elsevier: Amsterdam, The Netherlands, 2016; pp. 161–212. [Google Scholar]
- Jiang, G.; Keller, J.; Bond, P.L. Determining the long-term effects of H2S concentration, relative humidity and air temperature on concrete sewer corrosion. Water Res. 2014, 65, 157–169. [Google Scholar] [CrossRef]
- Andriamanohiarisoamanana, F.J.; Sakamoto, Y.; Yamashiro, T.; Yasui, S.; Iwasaki, M.; Ihara, I.; Tsuji, O.; Umetsu, K. Effects of handling parameters on hydrogen sulfide emission from stored dairy manure. J. Environ. Manag. 2015, 154, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Maurer, D.L.; Koziel, J.A.; Bruning, K.; Parker, D.B. Farm-scale testing of soybean peroxidase and calcium peroxide for surficial swine manure treatment and mitigation of odorous VOCs, ammonia and hydrogen sulfide emissions. Atmos. Environ. 2017, 166, 467–478. [Google Scholar] [CrossRef]
- Kim, K.H. Performance characterization of the GC/PFPD for H2S, CH3SH, CH3SCH3, and CH3SSCH3 in air. Atmos. Environ. 2005, 39, 2235–2242. [Google Scholar] [CrossRef]
- Vitvitsky, V.; Banerjee, R. H2S analysis in biological samples using gas chromatography with sulfur chemiluminescence detection. Methods Enzym. 2015, 554, 111–123. [Google Scholar]
- Varlet, V.; Giuliani, N.; Palmiere, C.; Maujean, G.; Augsburger, M. Hydrogen Sulfide Measurement by Headspace-Gas Chromatography-Mass Spectrometry (HS-GC-MS): Application to Gaseous Samples and Gas Dissolved in Muscle. J. Anal. Toxicol. 2015, 39, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Larsen, E.S.; Hong, W.W.; Spartz, M.L. Hydrogen Sulfide Detection by UV-Assisted Infrared Spectrometry. Appl. Spectrosc. 1997, 51, 1656–1667. [Google Scholar] [CrossRef]
- Chen, R.; Morris, H.R.; Whitmore, P.M. Fast detection of hydrogen sulfide gas in the ppmv range with silver nanoparticle films at ambient conditions. Sens. Actuators B Chem. 2013, 186, 431–438. [Google Scholar] [CrossRef]
- Gersen, S.; Van Essen, M.; Visser, P.; Ahmad, M.; Mokhov, A.; Sepman, A.; Alberts, R.; Douma, A.; Levinsky, H. Detection of H2S, SO2 and NO2 in CO2 at pressures ranging from 1-40 bar by using broadband absorption spectroscopy in the UV/VIS range. Energy Procedia 2014, 63, 2570–2582. [Google Scholar] [CrossRef]
- Shariati-Rad, M.; Irandoust, M.; Jalilvand, F. Spectrophotometric determination of hydrogen sulfide in environmental samples using sodium 1,2-naphthoquinone-4-sulfonate and response surface methodology. Int. J. Environ. Sci. Technol. 2016, 13, 1347–1356. [Google Scholar] [CrossRef]
- Wallace, K.J.; Cordero, S.R.; Tan, C.P.; Lynch, V.M.; Anslyn, E.V. A colorimetric response to hydrogen sulfide. Sens. Actuators B Chem. 2007, 120, 362–367. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Niu, Q.; Gao, P.; Zhang, G.; Dong, C.; Shuang, S. Gold nanoclusters as fluorescent sensors for selective and sensitive hydrogen sulfide detection. Talanta 2017, 171, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, N.S.; Deo, R.P.; Wang, J. Electrochemical determination of hydrogen sulfide at carbon nanotube modified electrodes. Anal. Chim. Acta 2004, 517, 131–137. [Google Scholar] [CrossRef]
- Zeng, L.; He, M.; Yu, H.; Li, D. An H2S Sensor Based on Electrochemistry for Chicken Coops. Sensors 2016, 16, 1398. [Google Scholar] [CrossRef]
- Ke, Z.J.; Tang, D.L.; Lai, X.; Dai, Z.Y.; Zhang, Q. Optical fiber evanescent-wave sensing technology of hydrogen sulfide gas concentration in oil and gas fields. Optik 2018, 157, 1094–1100. [Google Scholar] [CrossRef]
- Zhou, H.; Wen, J.Q.; Zhang, X.Z.; Wang, W.; Feng, D.Q.; Wang, Q.; Jia, F. A Study on Fiber-optic Hydrogen Sulfide Gas Sensor. Phys. Procedia 2014, 56, 1102–1106. [Google Scholar] [CrossRef]
- He, H.; Dong, C.; Fu, Y.; Han, W.; Zhao, T.; Xing, L.; Xue, X. Self-powered smelling electronic-skin based on the piezo-gas-sensor matrix for real-time monitoring the mining environment. Sens. Actuators B Chem. 2018, 267, 392–402. [Google Scholar] [CrossRef]
- Kuchmenko, T.A.; Kochetova, Z.Y.; Silina, Y.E.; Korenman, Y.I.; Kulin, L.A.; Lapitski, I.V. Determination of Trace Amounts of Hydrogen Sulfide in a Gas Flow Using a Piezoelectric Detector. J. Anal. Chem. 2007, 62, 781–787. [Google Scholar] [CrossRef]
- Berahman, M.; Sheikhi, M. Hydrogen sulfide gas sensor based on decorated zigzag graphene nanoribbon with copper. Sens. Actuators B Chem. 2015, 219, 338–345. [Google Scholar] [CrossRef]
- Tomchenko, A.A.; Harmer, G.P.; Marquis, B.T.; Allen, J.W. Semiconducting metal oxide sensor array for the selective detection of combustion gases. Sens. Actuators B Chem. 2003, 93, 126–134. [Google Scholar] [CrossRef]
- MalekAlaie, M.; Jahangiri, M.; Rashidi, A.; HaghighiAsl, A.; Izadi, N.; Rashidi, A. Selective hydrogen sulfide (H2S) sensors based on molybdenum trioxide (MoO3) nanoparticle decorated reduced graphene oxide. Mater. Sci. Semicond. Process. 2015, 38, 93–100. [Google Scholar] [CrossRef]
- Kaur, M.; Jain, N.; Sharma, K.; Bhattacharya, S.; Roy, M.; Tyagi, A.; Gupta, S.; Yakhmi, J.V. Room-temperature H2S gas sensing at ppb level by single crystal In2O3 whiskers. Sens. Actuators B Chem. 2008, 133, 456–461. [Google Scholar] [CrossRef]
- Alaie, M.M.; Jahangiri, M.; Rashidi, A.; Asl, A.H.; Izadi, N.; Rashidi, A. A novel selective H2S sensor using dodecylamine and ethylenediamine functionalized graphene oxide. J. Ind. Eng. Chem. 2015, 29, 97–103. [Google Scholar] [CrossRef]
- Sarfraz, J.; Ihalainen, P.; Määttänen, A.; Peltonen, J.; Linden, M. Printed hydrogen sulfide gas sensor on paper substrate based on polyaniline composite. Thin Solid Film. 2013, 534, 621–628. [Google Scholar] [CrossRef]
- Li, M.; Zhou, D.; Zhao, J.; Zheng, Z.; He, J.; Hu, L.; Xia, Z.; Tang, J.; Liu, H. Resistive gas sensors based on colloidal quantum dot (CQD) solids for hydrogen sulfide detection. Sens. Actuators B Chem. 2015, 217, 198–201. [Google Scholar] [CrossRef]
- Kumar, V.; Sunny; Rawal, I.; Mishra, V.; Dwivedi, R.; Das, R. Fabrication and characterization of gridded Pt/SiO2/Si MOS structure for hydrogen and hydrogen sulphide sensing. Mater. Chem. Phys. 2014, 146, 418–424. [Google Scholar] [CrossRef]
- Emelin, E.V.; Nikolaev, I.N. Sensitivity of mos sensors to hydrogen, hydrogen sulfide, and nitrogen dioxide in different gas atmospheres. Meas. Tech. 2006, 49, 524–528. [Google Scholar] [CrossRef]
- Crowley, K.; Morrin, A.; Sheperd, R.; Panhuis, M.; Wallace, G.; Smyth, M.; Killard, A. Fabrication of Polyaniline-Based Gas Sensors using Piezoelectric Inkjet and Silkscreen Printing for the Detection of Hydrogen Sulfide. IEEE Sens. J. 2010, 10, 1419–1426. [Google Scholar] [CrossRef]
- Liu, C.; Hayashi, K.; Toko, K. Au nanoparticles decorated polyaniline nanofiber sensor for detecting volatile sulfur compounds in expired breath. Sens. Actuators B Chem. 2012, 161, 504–509. [Google Scholar] [CrossRef]
- Chen, R.; Whitmore, P.M. Chapter 6: Silver Nanoparticle Films as Hydrogen Sulfide Gas Sensors with Applications in Art Conservation. In The Science and Function of Nanomaterials: From Synthesis to Application; Harper-Leatherman, A.S., Solbrig, C.M., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2014. [Google Scholar]
- Yuan, Z.; Lu, F.; Peng, M.; Wang, C.-W.; Tseng, Y.T.; Du, Y.; Cai, N.; Lien, C.W.; Chang, H.T.; He, Y.; et al. Selective Colorimetric Detection of Hydrogen Sulfide Based on Primary Amine-Active Ester Cross-Linking of Gold Nanoparticles. Anal. Chem. 2015, 87, 7267–7273. [Google Scholar] [CrossRef]
- Ji, W.F.; Chu, C.M.; Hsu, S.C.; Lu, Y.D.; Yu, Y.C.; Santiago, K.S.; Yeh, J.M. Synthesis and characterization of organo-soluble aniline oligomer-based electroactive doped with gold nanoparticles, and application to electrochemical sensing of ascorbic acid. Polymers 2017, 128, 218–228. [Google Scholar] [CrossRef]
- Vanherck, K.; Vankelecom, I.; Verbiest, T. Improving fluxes of polyimide membranes containing gold nanoparticles by photothermal heating. J. Membr. Sci. 2011, 373, 5–13. [Google Scholar] [CrossRef]
- Weng, C.J.; Jhuo, Y.S.; Chen, Y.L.; Feng, C.F.; Chang, C.H.; Chen, S.W.; Yeh, J.M.; Wei, Y. Intrinsically electroactive polyimide microspheres fabricated by electrospraying technology for ascorbic acid detection. J. Mater. Chem. 2011, 21, 15666–15672. [Google Scholar] [CrossRef]
- Weng, C.J.; Huang, K.Y.; Jhuo, Y.S.; Chen, Y.L.; Feng, C.F.; Cho-Ming, C.; Yeh, J.M. Controllable Electroactive Polyimide Particles Size Generated by Electrospraying. Polym. Int. 2012, 61, 205–212. [Google Scholar] [CrossRef]
- Huang, T.C.; Lin, S.T.; Yeh, L.C.; Chen, C.A.; Huang, H.Y.; Nian, Z.Y.; Chen, H.H.; Yeh, J.M. Aniline pentamer-based electroactive polyimide prepared from oxidation coupling polymerization for electrochemical sensing application. Polymers 2012, 53, 4373–4379. [Google Scholar] [CrossRef]
- Huang, T.C.; Yeh, L.C.; Huang, H.Y.; Nian, Z.Y.; Yeh, Y.C.; Chou, Y.C.; Yeh, J.M.; Tsai, M.H. The use of a carbon paste electrode mixed with multiwalled carbon nanotube/electroactive polyimide composites as an electrode for sensing ascorbic acid. Polym. Chem. 2014, 5, 630–637. [Google Scholar] [CrossRef]
- Huang, K.Y.; Jhuo, Y.S.; Wu, P.S.; Lin, C.H.; Yu, Y.H.; Yeh, J.M. Electrochemical Studies for the Content of Amine-Capped Aniline Trimers on the Corrosion Protection Effect of as-prepared Electroactive Polyimide Coatings. Eur. Polym. J. 2009, 45, 485–493. [Google Scholar] [CrossRef]
- Weng, C.J.; Huang, J.Y.; Huang, K.Y.; Jhuo, Y.S.; Tsai, M.H.; Yeh, J.M. Advanced anticorrosive coatings prepared from electroactive polyimide–TiO2 hybrid nanocomposite materials. Electrochim. Acta 2010, 55, 8430–8438. [Google Scholar] [CrossRef]
- Huang, H.Y.; Huang, T.C.; Yeh, T.C.; Tsai, C.Y.; Lai, C.L.; Tsai, M.H.; Yeh, J.M.; Chou, Y.C. Advanced anticorrosive materials prepared from amine-capped aniline trimer-based electroactive polyimide-clay nanocomposite materials with synergistic effects of redox catalytic capability and gas barrier properties. Polymers 2011, 52, 2391–2400. [Google Scholar] [CrossRef]
- Huang, T.C.; Yeh, T.C.; Huang, H.Y.; Ji, W.F.; Chou, Y.C.; Hung, W.I.; Yeh, J.M.; Tsai, M.H. Electrochemical Studies on aniline-pentamer-Based Electroactive Polyimide Coating: Corrosion Protection and Electrochromic Properties. Electrochim. Acta 2011, 56, 10151–10158. [Google Scholar] [CrossRef]
- Chang, K.C.; Lu, H.I.; Peng, C.W.; Lai, M.C.; Hsu, S.C.; Hsu, M.H.; Tsai, Y.K.; Chang, C.H.; Hung, W.I.; Wei, Y.; et al. Nanocasting Technique to Prepare Lotus-leaf-like Superhydrophobic Electroactive Polyimide as Advanced Anticorrosive Coatings. ACS Appl. Mater. Interfaces 2013, 5, 1460–1467. [Google Scholar] [CrossRef]
- Chou, Y.C.; Lee, P.C.; Hsu, T.F.; Huang, W.Y.; Zi-Han, L.; Chuang, C.Y.; Yang, T.I.; Yeh, J.M. Synthesis and anticorrosive properties of electroactive polyimide/SiO2 composites. Polym. Compos. 2014, 35, 617–625. [Google Scholar] [CrossRef]
- Chang, K.C. Advanced anticorrosive coatings prepared from electroactive polyimide/graphene nanocomposites with synergistic effects of redox catalytic capability and gas barrier properties. Express Polym. Lett. 2014, 8, 243–255. [Google Scholar] [CrossRef]
- Huang, T.C.; Yeh, L.C.; Lai, G.H.; Huang, B.S.; Yang, T.I.; Hsu, S.C.; Lo, A.Y.; Yeh, J.M. Advanced Superhydrophobic Electroactive Fluorinated Polyimide and Its Application in Anticorrosion Coating. Int. J. Green Energy 2016, 14, 113–120. [Google Scholar] [CrossRef]
- Ji, W.F.; Li, C.W.; Yu, S.K.; Chen, P.J.; Chen, H.L.; Chen, R.D.; Chen, B.H.; Hsu, C.L.; Yeh, J.M. Biomimetic electroactive polyimide with rose petal-like surface structure prepared from nanocasting technique for anticorrosive coating application. Express Polym. Lett. 2017, 11, 635–644. [Google Scholar] [CrossRef]
- Weng, C.J.; Jhuo, Y.S.; Huang, K.Y.; Feng, C.F.; Yeh, J.M.; Wei, Y.; Tsai, M.H. Mechanically and Thermally Enhanced Intrinsically Dopable Polyimide Membrane with Advanced Gas Separation Capabilities. Macromolecules 2011, 44, 6067–6076. [Google Scholar] [CrossRef]
- Weng, C.J.; Jhuo, Y.S.; Chang, C.H.; Feng, C.F.; Peng, C.W.; Dai, C.F.; Yeh, J.M.; Wei, Y. A smart surface prepared using the switchable superhydrophobicity of neat electrospun intrinsically electroactive polyimide fiber mats. Soft Matter 2011, 7, 10313–10318. [Google Scholar] [CrossRef]
- Chang, K.C.; Huang, K.Y.; Hsu, C.H.; Ji, W.F.; Lai, M.C.; Hung, W.I.; Chuang, T.L.; Yeh, J.M. Synthesis of ultra-high-strength electroactive polyimide membranes containing oligoaniline in the main chain by thermal imidization reaction. Eur. Polym. J. 2014, 56, 26–32. [Google Scholar] [CrossRef]
- Chang, K.C.; Lu, H.I.; Lai, M.C.; Hsu, C.H.; Hsiao, Y.R.; Huang, K.Y.; Chuang, T.L.; Yeh, J.M.; Liu, W.R. Enhanced Physical Properties of Electroactive Polyimide Nanocomposites by Addition of Graphene Nanosheets. Polym. Int. 2014, 63, 1011–1017. [Google Scholar] [CrossRef]
- Jeon, H.; Lee, K. Effect of gold nanoparticle morphology on thermal properties of polyimide nanocomposite films. Colloids Surf. A Physicochem. Eng. Asp. 2019, 579, 123651. [Google Scholar] [CrossRef]
- Wei, Y.; Yang, C.; Ding, T. A one-step method to synthesize n,n’-bis(4’-aminopiienyl)- 1,4-quinonenediimine and its derivatives. Tetrahedron Lett. 1996, 37, 731–734. [Google Scholar] [CrossRef]
- Krishnamurthy, S.; Esterle, A.; Sharma, N.C.; Sahi, S.V. Yucca-derived synthesis of gold nanomaterial and their catalytic potential. Nanoscale Res. Lett. 2014, 9, 627. [Google Scholar] [CrossRef]
- Sneha, K.; Sathishkumar, M.; Kim, S.; Yun, Y.S. Counter ions and temperature incorporated tailoring of biogenic gold nanoparticles. Process. Biochem. 2010, 45, 1450–1458. [Google Scholar] [CrossRef]















© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padua, L.M.G.; Yeh, J.-M.; Santiago, K.S. A Novel Application of Electroactive Polyimide Doped with Gold Nanoparticles: As a Chemiresistor Sensor for Hydrogen Sulfide Gas. Polymers 2019, 11, 1918. https://doi.org/10.3390/polym11121918
Padua LMG, Yeh J-M, Santiago KS. A Novel Application of Electroactive Polyimide Doped with Gold Nanoparticles: As a Chemiresistor Sensor for Hydrogen Sulfide Gas. Polymers. 2019; 11(12):1918. https://doi.org/10.3390/polym11121918
Chicago/Turabian StylePadua, Lee Marvin G., Jui-Ming Yeh, and Karen S. Santiago. 2019. "A Novel Application of Electroactive Polyimide Doped with Gold Nanoparticles: As a Chemiresistor Sensor for Hydrogen Sulfide Gas" Polymers 11, no. 12: 1918. https://doi.org/10.3390/polym11121918
APA StylePadua, L. M. G., Yeh, J.-M., & Santiago, K. S. (2019). A Novel Application of Electroactive Polyimide Doped with Gold Nanoparticles: As a Chemiresistor Sensor for Hydrogen Sulfide Gas. Polymers, 11(12), 1918. https://doi.org/10.3390/polym11121918