Fabrication and Properties of Hybrid Coffee-Cellulose Aerogels from Spent Coffee Grounds
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Fabrication of Hybrid Coffee-Cotton Aerogels
2.3. Surface Modification
2.4. Characterization
3. Results and Discussion
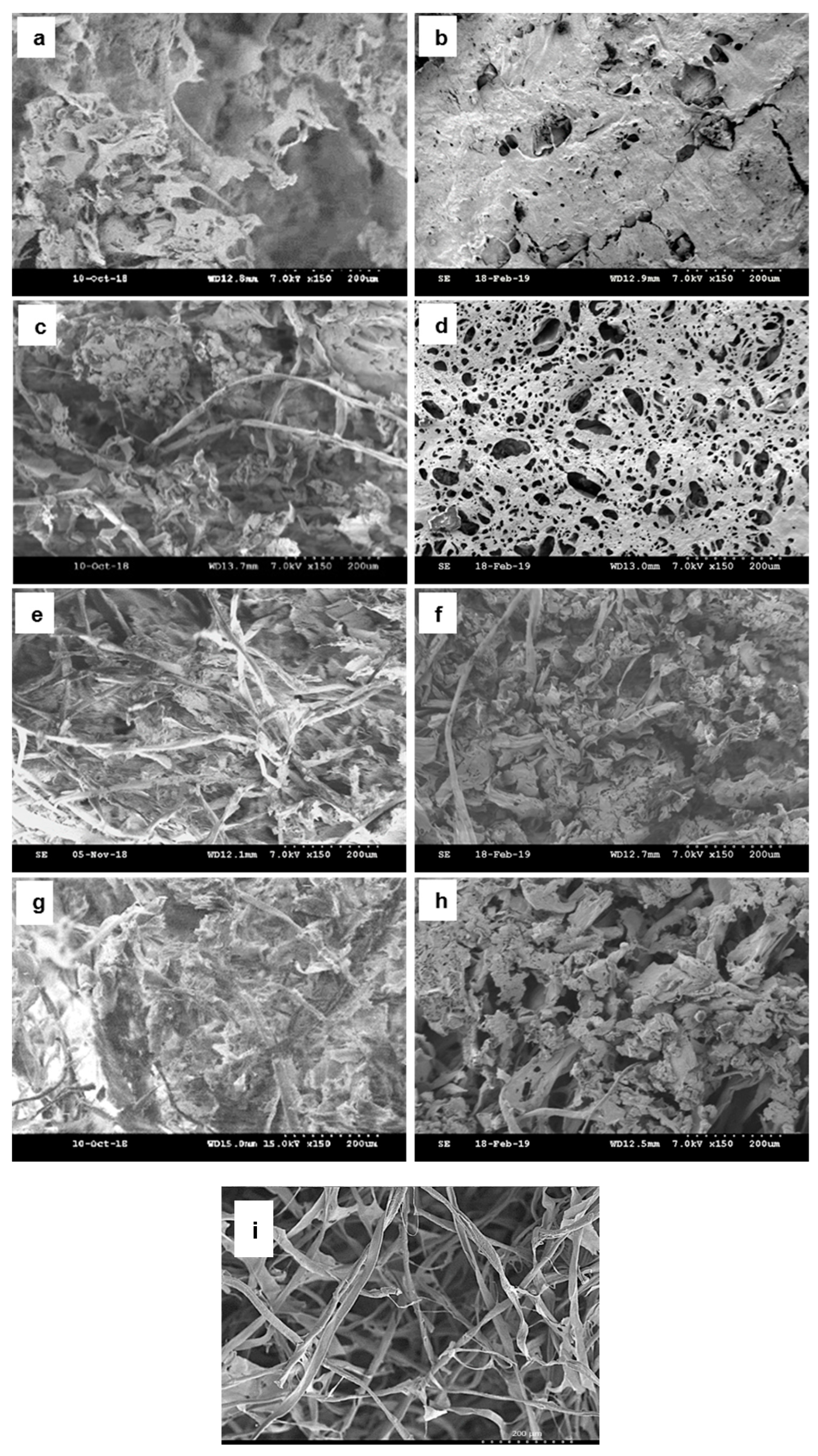
3.1. Morphologies and Structures of Hybrid Aerogels.
3.2. Oil Absorption Capacities and Kinetics of the Coffee and Hybrid Aerogels
3.3. Thermal Properties of the Hybrid Coffee-Cotton Aerogels
3.4. Mechanical Properties of the Hybrid Coffee-Cotton Aerogels
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- International Coffee Organization. Coffee Market Report; International Coffee Organization: London, UK, 2018; Available online: http://www.ico.org (accessed on 1 May 2019).
- Tokimoto, T.; Kawasaki, N.; Nakamura, T.; Akutagawa, J.; Tanada, S. Removal of lead ions in drinking water by coffee grounds as vegetable biomass. J. Colloid Interface Sci. 2005, 281, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Mussatto, S.I.; Machado, E.M.S.; Martins, S.; Teixeira, J.A. Production, composition, and application of coffee and its industrial residues. Food Bioprocess Technol. 2011, 4, 661–672. [Google Scholar] [CrossRef]
- Garvey, M. Coffee pollution: A comprehensive review of chemical and biological sources of food contamination and impact on human health. Nutrire 2019, 44, 1. [Google Scholar] [CrossRef]
- Pandey, A.; Soccol, C.R.; Larroche, C. Current Developments in Solid-State Fermantation. Biochem. Eng. J. 2013, 81, 146–161. [Google Scholar]
- Silva, M.A.; Nebra, S.A.; Machado Silva, M.J.; Sanchez, C.G. the use of biomass residues in the brazilian soluble coffee industry. Biomass Bioenergy 1998, 14, 457–467. [Google Scholar] [CrossRef]
- Woldesenbet, A.G.; Woldeyes, B.; Chandravanshi, B.S. Bio-ethanol production from wet coffee processing waste in Ethiopia. Springerplus 2016, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cameron, A.; O’Malley, S. Coffee Ground Recovery Program Summary Report; Planet Ark: Sydney, Australia, 2016. [Google Scholar]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, functional, and structural properties of spent coffee grounds and coffee silverskin. Food Bioprocess Technol. 2014, 7, 3493–3503. [Google Scholar] [CrossRef]
- Smith, M.J.; Flowers, T.H.; Lennard, F.J. Mechanical properties of wool and cotton yarns used in twenty-first century tapestry: Preparing for the future by understanding the present. Stud. Conserv. 2015, 60, 375–383. [Google Scholar] [CrossRef]
- Kiong, J.; Saparudin, K. Major Oil Spills in the Straits of Singapore; Infopedia; National Library Board Singapore: Singapore, 2010. Available online: http://eresources.nlb.gov.sg/infopedia/articles/SIP_1101_2010-09-06.html (accessed on 1 January 2019).
- Use of Sorbent Materials in Oil Spill Response. The International Tanker Owners Pollution Federation Limited. 2012. Available online: https://www.ukpandi.com/fileadmin/uploads/uk-pi/Knowledge_Base_-_International_Conventions/TIP%208%20Use%20of%20Sorbent%20Materials%20in%20Oil%20Spill%20Response.pdf (accessed on 1 April 2019).
- Sorbents. United States Environmental Protection Agency. Available online: https://archive.epa.gov/emergencies/content/learning/web/html/sorbents.html (accessed on 1 April 2019).
- Feng, J.; Nguyen, S.T.; Fan, Z.; Duong, H.M. Advanced fabrication and oil absorption properties of super-hydrophobic recycled cellulose aerogels. Chem. Eng. J. 2015, 270, 168–175. [Google Scholar] [CrossRef]
- Chen, X.I.; Liang, Y.N.; Tang, X.Z.; Shen, W.M.; Xu, X. Additive free poly(vinylidene fluoride) aerogel for oil/water separation and rapid oil absorption. Chem. Eng. J. 2017, 308, 182–186. [Google Scholar] [CrossRef]
- Mulyadi, A.; Zhang, Z.; Deng, Y.L. Fluorine free oil absorbents made from cellulose nanofibril aerogels. ACS Appl. Mater. Interfaces 2016, 8, 27322–27740. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Yang, S.D.; Ma, Q.; Jia, X.; Ma, P.C. Preparation of carbon nanotubes/graphene hybrid aerogel and its application for the absorption of organic compounds. Carbon 2017, 118, 7657–7671. [Google Scholar] [CrossRef]
- Wang, J.T.; Wang, H.F. Facile synthesis of flexible mesoporous aerogel with superhydrophobicity for efficient removal of layered and emulsified oil from water. J. Colloid Interf. Sci. 2018, 530, 3723–3782. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Gu, B.; Pennefather, M.P.; Nguyen, T.X.; Phan-Thien, N.; Duong, H.M. Cotton aerogels and cotton-cellulose aerogels from environmental waste for oil spillage cleanup. Mater. Des. 2017, 130, 452–458. [Google Scholar] [CrossRef]
- Bulk Material Density Guide. Hapman. Available online: https://hapman.com/education-support/bulk-material-density-guide/ (accessed on 1 December 2018).
- Cotton Fiber Properties. Barnhardt Purified Cotton. Available online: https://www.barnhardtcotton.net/technology/cotton-properties/ (accessed on 1 September 2018).
- Material Safety Data Sheet Poly (Vinyl Acetate). ACROS Organics. Available online: http://lattice.mse.ufl.edu/wp-content/uploads/2015/06/polyvinylacetate.pdf (accessed on 1 September 2018).
- Nguyen, S.T.; Feng, J.; Ng, S.K.; Wong, J.P.W.; Tan, V.B.V.; Duong, H.M. Advanced thermal insulation and absorption properties of recycled cellulose aerogels. Colloids Surf. A Physicochem. Eng. Asp. 2014, 445, 128–134. [Google Scholar] [CrossRef]
- Kim, S.; Kim, G.; Moon, J.; Ha, C. Mechanical properties of polypropylene/natural fiber composites: Comparison of wood fiber and cotton fiber. Polym. Test. 2008, 27, 801–806. [Google Scholar] [CrossRef]
- Hsieh, Y.L. Chemical structure and properties of cotton. In Cotton: Science and Technology; Gordon, S., Hsieh, Y.L., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2007; pp. 3–34. [Google Scholar]
- Li, X.; Strezov, V.; Kan, T. Energy recovery potential analysis of spent coffee grounds pyrolysis products. J. Anal. Appl. Pyrolysis 2014, 110, 79–87. [Google Scholar] [CrossRef]
- Brebu, M.; Vasile, C. Thermal degradation of lignin—A rewiew. Cell. Chem. Technol. 2009, 44, 353–363. [Google Scholar]
- Yang, H.; Yan, R.; Chen, H.; Zheng, C.; Lee, D.H.; Liang, D.T. In-depth investigation of biomass pyrolysis based on three major components: Hemicellulose, cellulose and lignin. Energy Fuels 2006, 20, 388–393. [Google Scholar] [CrossRef]
- Cagnon, B.; Py, X.; Guillot, A.; Stoeckli, F.; Chambat, G. Contributions of hemicellulose, cellulose and lignin to the mass and the porous properties of chars and steam activated carbons from various lignocellulosic precursors. Bioresour. Technol. 2009, 100, 292–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Samples | Spent Coffee Ground (wt.%) | Cotton Fiber (wt.%) | PVA (wt.%) | Density (mg/cm3) | Porosity (%) | Volume Change (%) |
|---|---|---|---|---|---|---|
| Co | 2.0 | 0 | 1.0 | 79.7 ± 10.7 | 92.1 ± 1.1 | 62.5 ± 5.0 |
| 0.25 Fib-Co | 2.0 | 0.25 | 1.0 | 57.3 ± 3.3 | 94.4 ± 0.3 | 43.6 ± 2.4 |
| 0.5 Fib-Co | 2.0 | 0.50 | 1.0 | 45.8 ± 6.1 | 95.5 ± 0.5 | 28.5 ± 6.5 |
| 1.0 Fib-Co | 2.0 | 1.00 | 1.0 | 55.3 ± 2.8 | 95.0 ± 0.3 | 28.5 ± 3.8 |
| Co | 0.25 Fib-Co | 0.5 Fib-Co | 1.0 Fib-Co | |
|---|---|---|---|---|
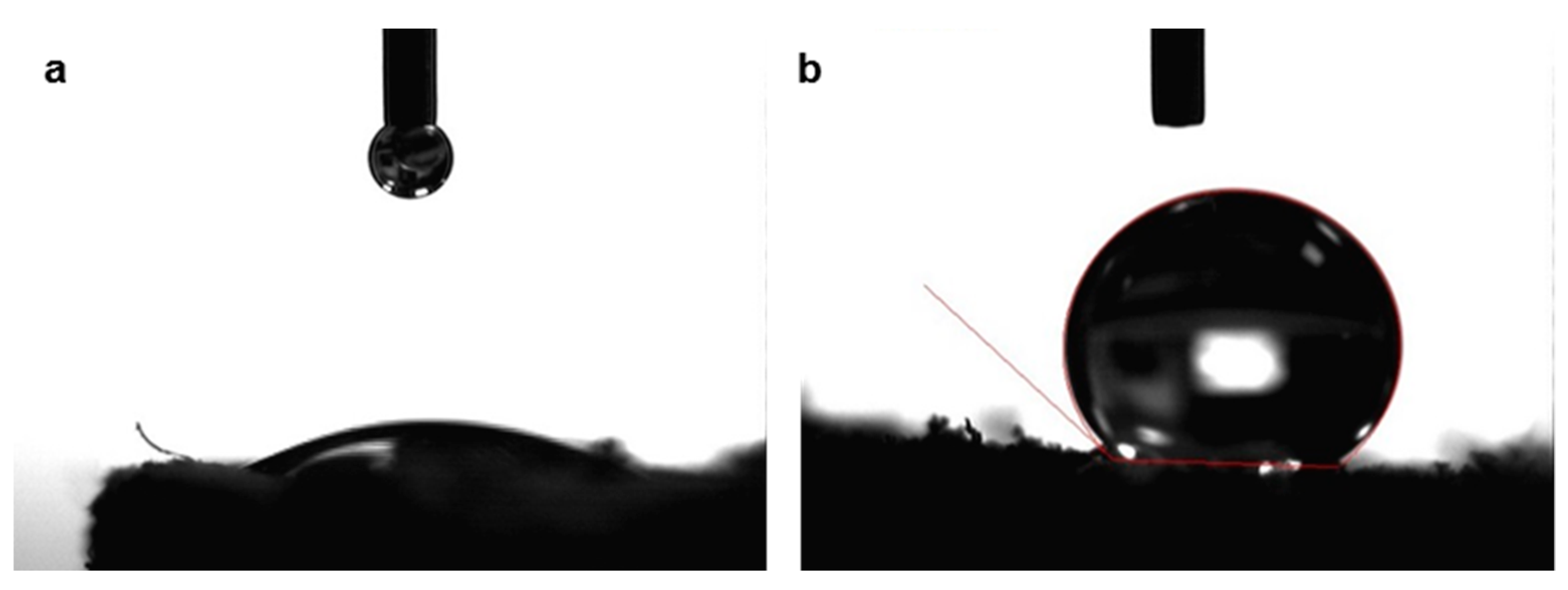
| Angle (°) | 136.8 ± 3.7 | 124.7 ± 2.5 | 139.1 ± 4.6 | 132.2 ± 3.7 |
| Co | 0.25 Fib-Co | 0.5 Fib-Co | 1.0 Fib-Co | ||
|---|---|---|---|---|---|
| Maximum absorption capacity | (g/g) | 11.6 | 13.8 | 16.0 | 15.6 |
| Pseudo first order | 0.8885 | 0.9595 | 0.9476 | 0.9912 | |
| 0.2954 | 0.3171 | 0.1564 | 0.2501 | ||
| Pseudo second order | 0.9993 | 0.9993 | 0.9990 | 0.9948 | |
| 0.0313 | 0.0396 | 0.0289 | 0.0217 |
| Thermal Conductivity, Kavg (W/mK) | Co | 0.25 Fib-Co | 0.5 Fib-Co | 1.0 Fib-Co |
|---|---|---|---|---|
| Top surface | 0.037 ± 0.001 | 0.038 ± 0.001 | 0.041 ± 0.001 | 0.042 ± 0.001 |
| Bottom surface | 0.062 ± 0.001 | 0.047 ± 0.002 | 0.045 ± 0.001 | 0.044 ± 0.002 |
| Avg. of both surfaces | 0.050 ± 0.001 | 0.043 ± 0.001 | 0.043 ± 0.001 | 0.043 ± 0.003 |
| Samples | Spent Coffee Ground (wt.%) | Cotton Fiber (wt.%) | Density (mg/cm3) | Compressive Young’s Modulus E (kPa) |
|---|---|---|---|---|
| Co | 2 | 0 | 79.9 ± 10.7 | 5.41 ± 0.09 |
| 0.25 Fib-Co | 2 | 0.25 | 57.3 ± 3.3 | 7.69 ± 0.01 |
| 0.5 Fib-Co | 2 | 0.50 | 45.8 ± 6.1 | 6.34 ± 0.06 |
| 1 Fib-Co | 2 | 1.00 | 55.3 ± 2.8 | 15.62 ± 0.06 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Kwek, L.P.; Le, D.K.; Tan, M.S.; Duong, H.M. Fabrication and Properties of Hybrid Coffee-Cellulose Aerogels from Spent Coffee Grounds. Polymers 2019, 11, 1942. https://doi.org/10.3390/polym11121942
Zhang X, Kwek LP, Le DK, Tan MS, Duong HM. Fabrication and Properties of Hybrid Coffee-Cellulose Aerogels from Spent Coffee Grounds. Polymers. 2019; 11(12):1942. https://doi.org/10.3390/polym11121942
Chicago/Turabian StyleZhang, Xiwen, Li Ping Kwek, Duyen K. Le, Men Shu Tan, and Hai Minh Duong. 2019. "Fabrication and Properties of Hybrid Coffee-Cellulose Aerogels from Spent Coffee Grounds" Polymers 11, no. 12: 1942. https://doi.org/10.3390/polym11121942





