Fabrication and Application of Black Phosphorene/Graphene Composite Material as a Flame Retardant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
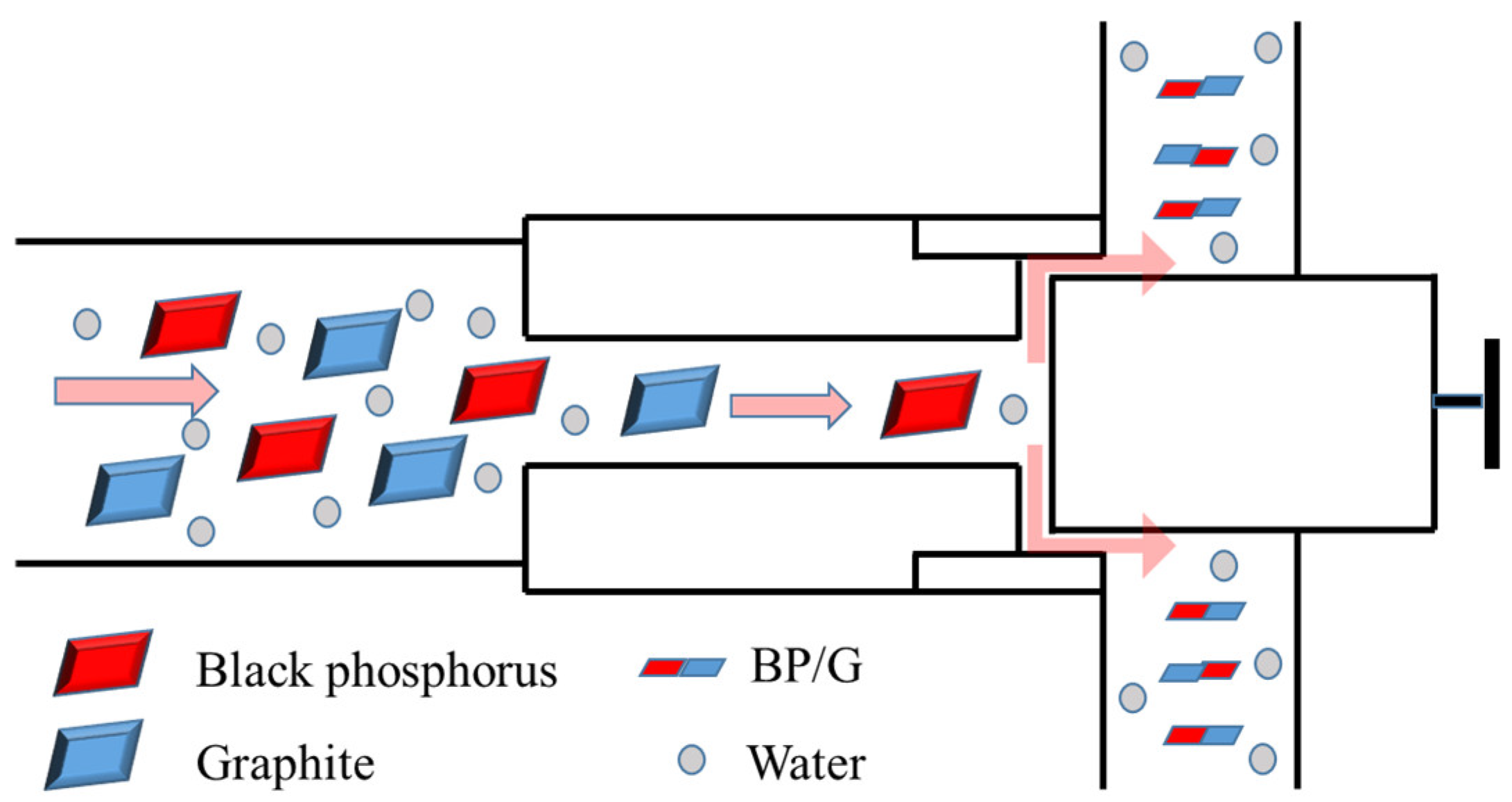
2.2. Fabrication of Black Phosphorene/Graphene (BP/G) Composite Materials
2.3. Preparation of Black Phosphorene/Graphene/WPU (BP/GWPU) Composite Materials
2.4. Analytical Test
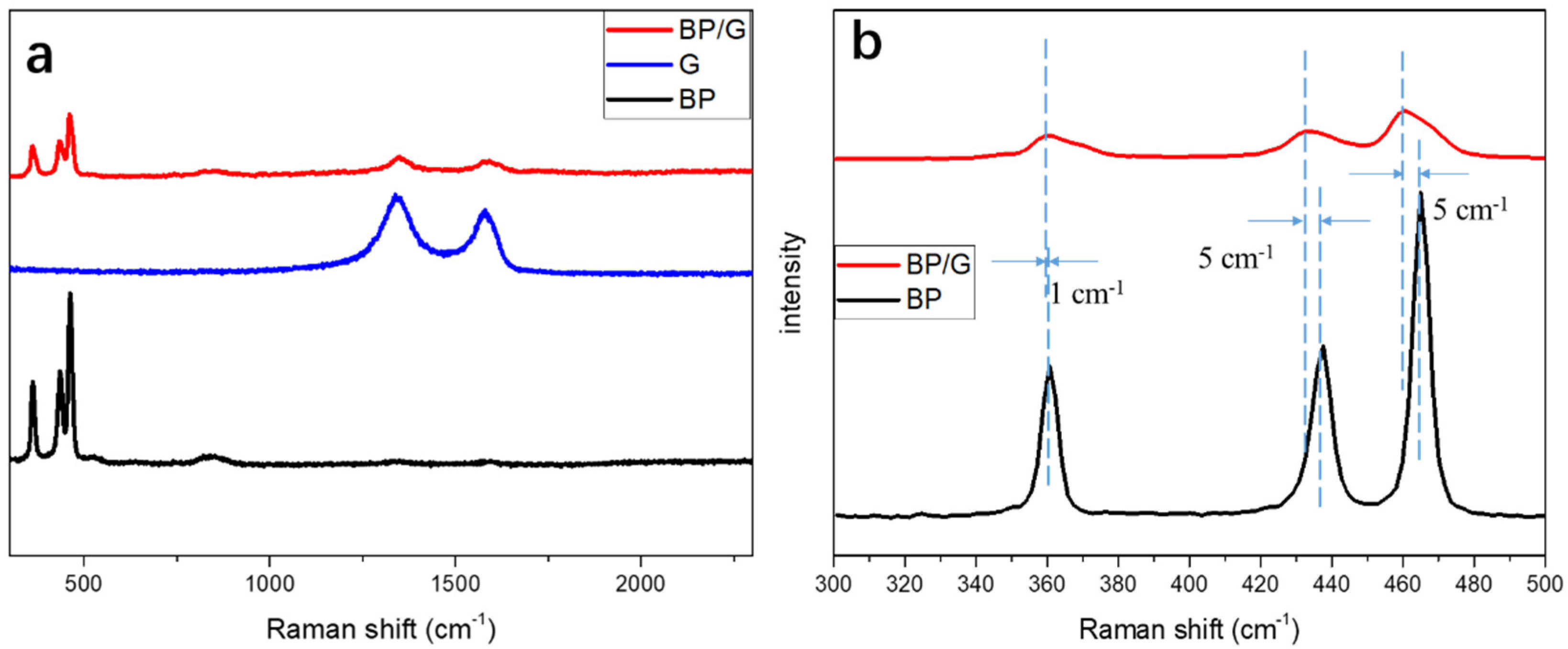
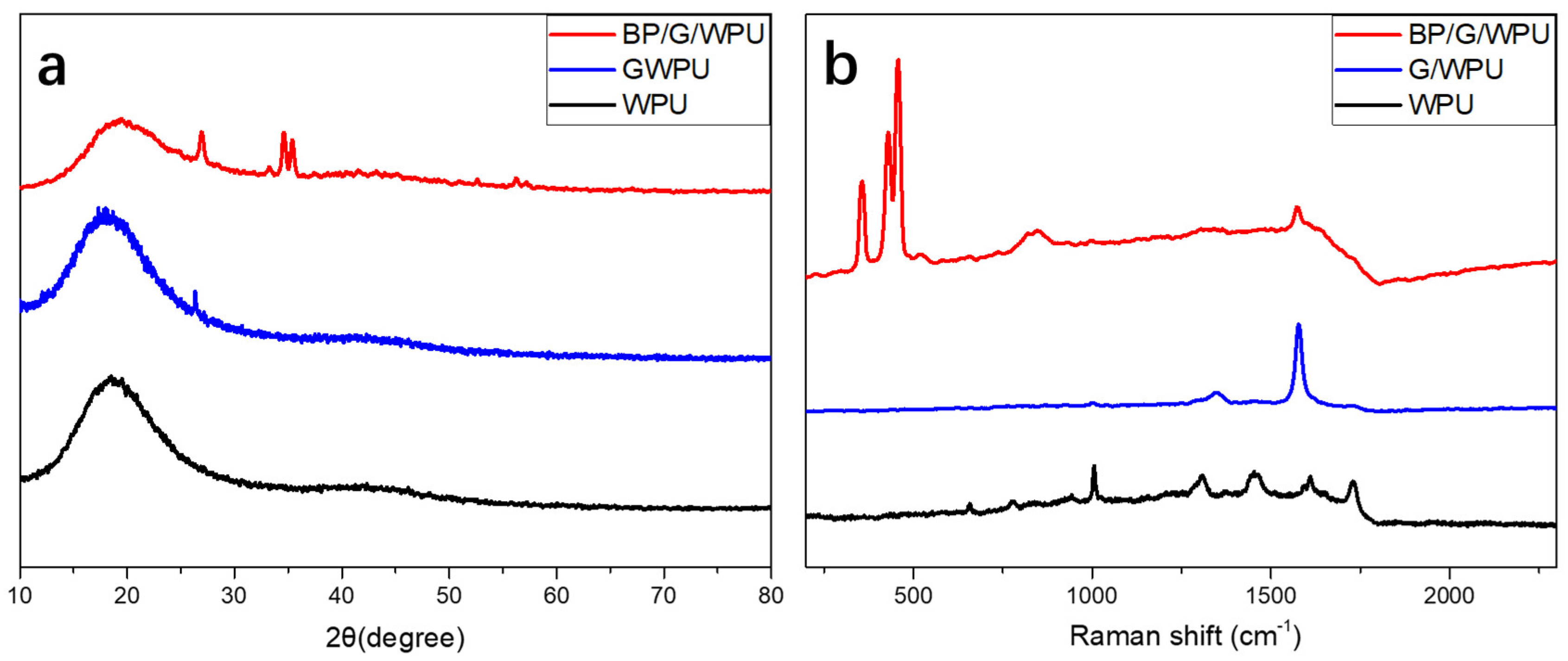
2.4.1. Structure Characterizations
2.4.2. Thermal Properties Measurement
2.4.3. Flammability Property Measurement
2.4.4. Mechanical Test
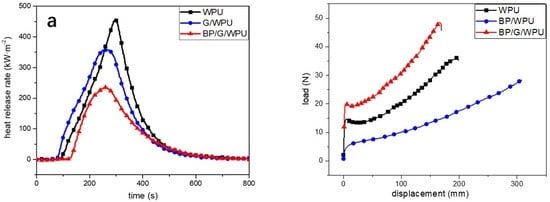
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Reich, E.S. Phosphorene excites materials scientists. Nature 2014, 506, 19. [Google Scholar] [CrossRef]
- Ren, X.L.; Lian, P.C.; Xie, D.L.; Yang, Y.; Mei, Y.; Huang, X.R.; Wang, Z.R.; Yin, X.T. Properties, preparation and application of black phosphorus/phosphorene for energy storage: A review. J. Mater. Sci. 2017, 52, 10364–10386. [Google Scholar] [CrossRef]
- Chen, Y.T.; Ren, R.; Pu, H.H.; Chang, J.B.; Mao, S.; Chen, J.H. Field-effect transistor biosensors with two-dimensional black phosphorus nanosheets. Biosens. Bioelectr. 2017, 89, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.; Soklaski, R.; Liang, Y.F.; Yang, L. Layer-controlled band gap and anisotropic excitons in few-layer black phosphorus. Phys. Rev. B 2014, 89, 817–824. [Google Scholar] [CrossRef]
- Late, D.J. Liquid exfoliation of black phosphorus nanosheets and its application as humidity sensor. Microporous Microporous Mat. 2016, 225, 494–503. [Google Scholar] [CrossRef]
- Castellanos-Gomez, A.; Vicarelli, L.; Prada, E.; Island, J.O.; Narasimha-Acharya, K.L.; Blanter, S.I.; Groenendijk, D.J.; Buscema, M.; Steele, G.A.; Alvarez, J.V. Isolation and characterization of few-layer black phosphorus. 2D Mater. 2014, 1, 025001. [Google Scholar] [CrossRef]
- Li, L.K.; Yu, Y.J.; Ye, G.J.; Ge, Q.Q.; Ou, X.D.; Wu, H.; Feng, D.L.; Chen, X.H.; Zhang, Y.B. Black phosphorus field-effect transistors. Nat. Nanotechnol. 2014, 9, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Esau, D.R.C.A.; Jussila, H.; Ye, G.; Ren, Z.; Bai, J. Black phosphorus polycarbonate polymer composite for pulsed fibre lasers. Appl. Mater. Today 2016, 4, 17–23. [Google Scholar] [CrossRef]
- Sun, Z.P. Optical Modulators with Two-dimensional Layered Materials. In Proceedings of the 2016 Progress in Electromagnetics Research Symposium (Piers), Shanghai, China, 8–11 August 2016; p. 3851. [Google Scholar]
- Ren, X.L.; Mei, Y.; Lian, P.C.; Xie, D.L.; Yang, Y.Y.; Wang, Y.Z.; Wang, Z.R. A Novel Application of Phosphorene as a Flame Retardant. Polymers 2018, 10, 227. [Google Scholar] [CrossRef]
- Titelman, G.I.; Gonen, Y.; Keidar, Y.; Bron, S. Discolouration of polypropylene-based compounds containing magnesium hydroxide. Polym. Degrad. Stabil. 2002, 77, 345–352. [Google Scholar] [CrossRef]
- Guo, Y.C.; Xue, Y.; Zuo, X.H.; Zhang, L.X.; Yang, Z.H.; Zhou, Y.C.; Marmorat, C.; He, S.; Rafailovich, M. Capitalizing on the molybdenum disulfide/graphene synergy to produce mechanical enhanced flame retardant ethylene-vinyl acetate composites with low aluminum hydroxide loading. Polym. Degrad. Stabil. 2017, 144, 155–166. [Google Scholar] [CrossRef]
- Shi, X.X.; Peng, X.F.; Zhu, J.Y.; Lin, G.Y.; Kuang, T.R. Synthesis of DOPO-HQ-functionalized graphene oxide as a novel and efficient flame retardant and its application on polylactic acid: Thermal property, flame retardancy, and mechanical performance. J. Colloid Interface Sci. 2018, 524, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.B.; Hu, W.Z.; Gui, Z.; Hu, Y. Bi2Se3 nanosheets: Advanced nanofillers for reinforcing and flame retarding polyethylene nanocomposites. Compos. Part A Appl. Sci. 2017, 100, 371–380. [Google Scholar] [CrossRef]
- Poveda, R.L.; Dorogokupets, G.; Gupta, N. Carbon nanofiber reinforced syntactic foams: Degradation mechanism for long term moisture exposure and residual compressive properties. Polym. Degrad. Stabil. 2013, 98, 2041–2053. [Google Scholar] [CrossRef]
- Dai, Z.H.; Wang, G.R.; Liu, L.Q.; Hou, Y.; Wei, Y.G.; Zhang, Z. Mechanical behavior and properties of hydrogen bonded graphene/polymer nano-interfaces. Compos. Sci. Technol. 2016, 136, 1–9. [Google Scholar] [CrossRef]
- Hu, K.S.; Kulkarni, D.D.; Choi, I.; Tsukruk, V.V. Graphene-polymer nanocomposites for structural and functional applications. Prog. Polym. Sci. 2014, 39, 1934–1972. [Google Scholar] [CrossRef]
- Nine, M.J.; Cole, M.A.; Tran, D.N.H.; Losic, D. Graphene: A multipurpose material for protective coatings. J. Mater. Chem. A 2015, 3, 12580–12602. [Google Scholar] [CrossRef]
- Salas, E.C.; Sun, Z.Z.; Luttge, A.; Tour, J.M. Reduction of graphene oxide via bacterial respiration. ACS Nano 2010, 4, 4852–4856. [Google Scholar] [CrossRef]
- Hou, Y.B.; Duan, L.J.; Gui, Z.; Hu, Y. An infiltration method to synthesize thermoplastic polyurethane composites based on size-controlled graphene foams. Compos. Part A Appl. Sci. 2017, 97, 67–75. [Google Scholar] [CrossRef]
- Guo, Y.Q.; Bao, C.L.; Song, L.; Yuan, B.H.; Hu, Y. In Situ polymerization of graphene, graphite oxide, and functionalized graphite oxide into epoxy resin and comparison study of on-the-flame behavior. Ind. Eng. Chem. Res. 2011, 50, 7772–7783. [Google Scholar] [CrossRef]
- Shahil, K.M.F.; Balandin, A.A. Thermal properties of graphene and multilayer graphene: Applications in thermal interface materials. Solid State Commun. 2012, 152, 1331–1340. [Google Scholar] [CrossRef]
- Gaska, K.; Kadar, R.; Rybak, A.; Siwek, A.; Gubanski, S. Gas barrier, thermal, mechanical and rheological properties of highly aligned graphene-LDPE nanocomposites. Polymers 2017, 9, 294. [Google Scholar] [CrossRef]
- Cai, W.; Feng, X.M.; Wang, B.B.; Hu, W.Z.; Yuan, B.H.; Hong, N.N.; Hu, Y. A novel strategy to simultaneously electrochemically prepare and functionalize graphene with a multifunctional flame retardant. Chem. Eng. J. 2017, 316, 514–524. [Google Scholar] [CrossRef]
- Mu, X.W.; Yuan, B.H.; Feng, X.M.; Qiu, S.L.; Song, L.; Hu, Y. The effect of doped heteroatoms (nitrogen, boron, phosphorus) on inhibition thermal oxidation of reduced graphene oxide. RSC Adv. 2016, 6, 105021–105029. [Google Scholar] [CrossRef]
- Yuan, B.H.; Hu, Y.; Chen, X.F.; Shi, Y.Q.; Niu, Y.; Zhang, Y.; He, S.; Dai, H.M. Dual modification of graphene by polymeric flame retardant and Ni(OH)2 nanosheets for improving flame retardancy of polypropylene. Compos. Part A Appl. Sci. 2017, 100, 106–117. [Google Scholar] [CrossRef]
- Dittrich, B.; Wartig, K.A.; Hofmann, D.; Mulhaupt, R.; Schartel, B. Flame retardancy through carbon nanomaterials: Carbon black, multiwall nanotubes, expanded graphite, multi-layer graphene and graphene in polypropylene. Polym. Degrad. Stabil. 2013, 98, 1495–1505. [Google Scholar] [CrossRef]
- Liu, S.; Fang, Z.P.; Yan, H.Q.; Chevali, V.S.; Wang, H. Synergistic flame retardancy effect of graphene nanosheets and traditional retardants on epoxy resin. Compos. Part A 2016, 89, 26–32. [Google Scholar] [CrossRef]
- Shi, Y.Q.; Xing, W.Y.; Wang, B.B.; Hong, N.N.; Zhu, Y.L.; Wang, C.M.; Gui, Z.; Yuen, R.K.K.; Hu, Y. Synergistic effect of graphitic carbon nitride and ammonium polyphosphate for enhanced thermal and flame retardant properties of polystyrene. Mater. Chem. Phys. 2016, 177, 283–292. [Google Scholar] [CrossRef]
- Nilges, T.; Kersting, M.; Pfeifer, T. A fast low-pressure transport route to large black phosphorus single crystals. J. Solid State Chem. 2008, 181, 1707–1711. [Google Scholar] [CrossRef]
- Kim, M.J.; Jeon, I.Y.; Seo, J.M.; Dai, L.M.; Baek, J.B. Graphene phosphonic acid as an efficient flame retardant. ACS Nano 2014, 8, 2820–2825. [Google Scholar] [CrossRef]
- Mao, D.; Li, M.; Cui, X.; Zhang, W.; Lu, H.; Song, K.; Zhao, J. Stable high-power saturable absorber based on polymer-black-phosphorus films. Optic. Commun. 2018, 406, 254–259. [Google Scholar] [CrossRef]
- Sun, J.; Lee, H.W.; Pasta, M.; Yuan, H.T.; Zheng, G.Y.; Sun, Y.M.; Li, Y.Z.; Cui, Y. A phosphorene-graphene hybrid material as a high-capacity anode for sodium-ion batteries. Nat. Nanotechnol. 2015, 10, 980–985. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, H.; Huang, H.; Xiao, Q.; Xu, Y.; Guo, Z.; Xie, H.; Shao, J.; Sun, Z.; Han, W.; et al. Surface coordination of black phosphorus for robust Air and water stability. Angew. Chem. 2016, 55, 5003–5007. [Google Scholar] [CrossRef]
- Zhang, Z.; Xin, X.; Yan, Q.; Li, Q.; Yang, Y.; Ren, T.L. Two-step heating synthesis of sub-3 millimeter-sized orthorhombic black phosphorus single crystal by chemical vapor transport reaction method. Sci. China Mater. 2016, 59, 122–134. [Google Scholar] [CrossRef]
- Feng, J.J.; Ge, Z.; Chai, C.P.; Wang, S.P.; Yu, D.H.; Wu, G.; Luo, Y.J. Flame retardant modification of waterborne polyurethane fabric coating agent with high hydrostatic pressure resistance. Prog. Org. Coat. 2016, 97, 91–98. [Google Scholar] [CrossRef]
- Cao, Z.J.; Dong, X.; Fu, T.; Deng, S.B.; Liao, W.; Wang, Y.Z. Coated vs. naked red phosphorus: A comparative study on their fire retardancy and smoke suppression for rigid polyurethane foams. Polym. Degrad. Stabil. 2017, 136, 103–111. [Google Scholar] [CrossRef]
- Pecht, M.; Deng, Y.L. Electronic device encapsulation using red phosphorus flame retardants. Microelectron. Reliab. 2006, 46, 53–62. [Google Scholar] [CrossRef]
- Kuo, P.L.; Chang, J.M.; Wang, T.L. Flame-retarding materials-I. Syntheses and flame-retarding property of alkylphosphate-type polyols and corresponding polyurethanes. J. Appl. Polym. Sci. 1998, 69, 1635–1643. [Google Scholar] [CrossRef]
- Horold, S. Phosphorus flame retardants in thermoset resins. Polym. Degrad. Stabil. 1999, 64, 427–431. [Google Scholar] [CrossRef]
- Young, R.J.; Kinloch, I.A.; Gong, L.; Novoselov, K.S. The mechanics of graphene nanocomposites: A review. Compos. Sci. Technol. 2012, 72, 1459–1476. [Google Scholar] [CrossRef]
- Moreno-Navarro, F.; Sol-Sanchez, M.; Gamiz, F.; Rubio-Gamez, M.C. Mechanical and thermal properties of graphene modified asphalt binders. Constr. Build. Mater. 2018, 180, 265–274. [Google Scholar] [CrossRef]
- Bandla, S.; Hanan, J.C. Microstructure and elastic tensile behavior of polyethylene terephthalate-exfoliated graphene nanocomposites. J. Mater. Sci. 2012, 47, 876–882. [Google Scholar] [CrossRef]



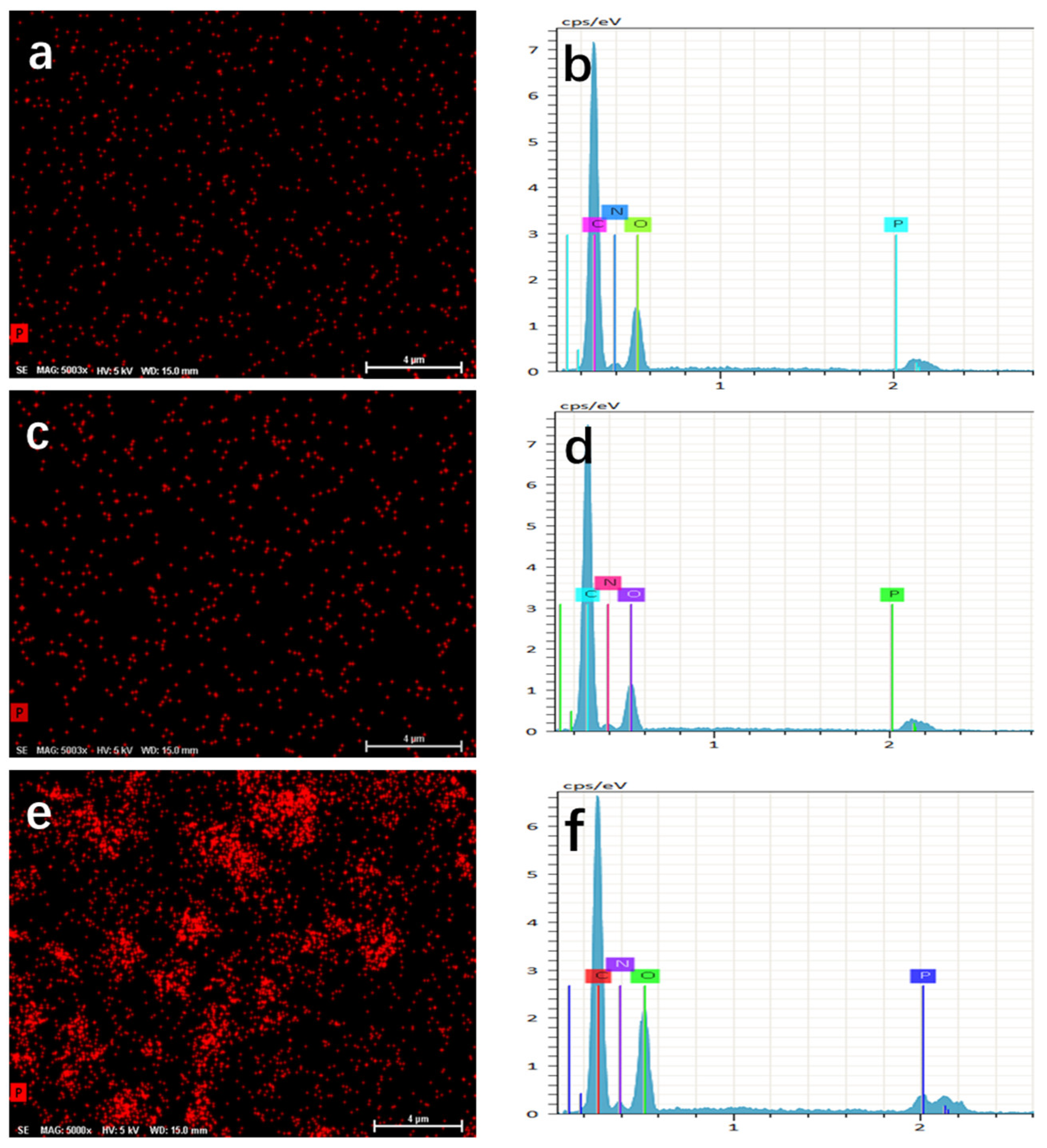



| Items | Initial Ratio of P/C | Ratio of P/C in BP/G Composite Material | Content of P in BP/G/WPU Membrane (%) | Additive Amount of BP/G in BP/G/WPU Membrane (%) |
|---|---|---|---|---|
| Value | 1 | 1.38 | 2.06 | 3.55 |
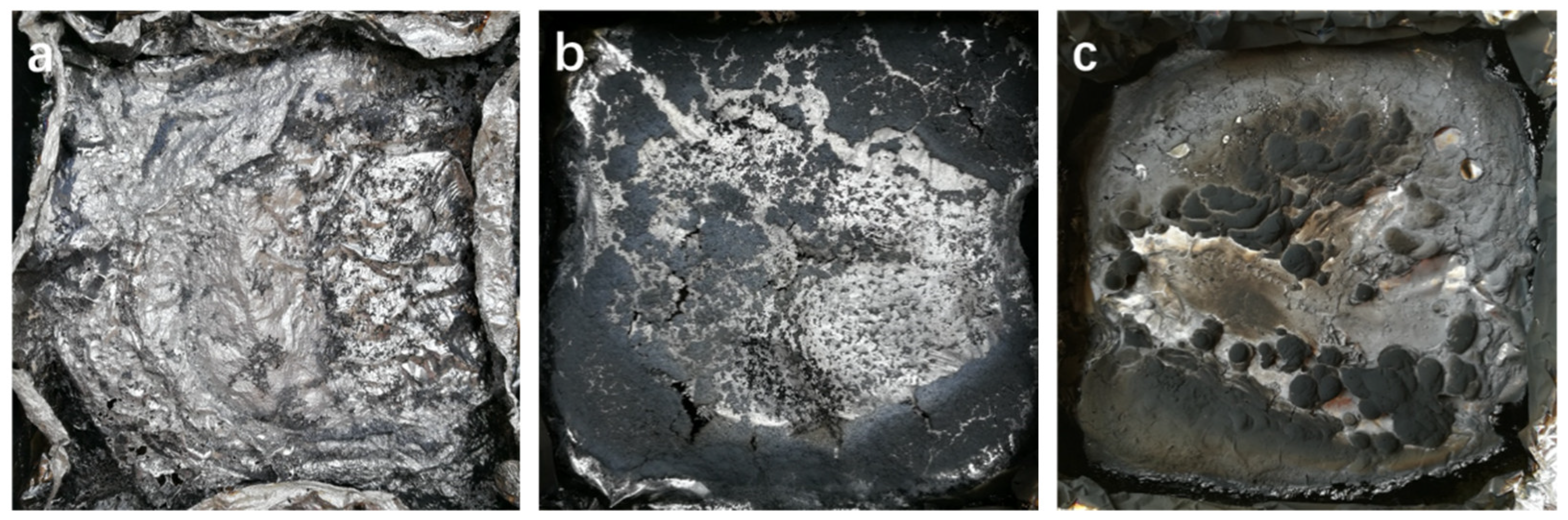
| Sample | TTI (s) | PHRR (kW/m2) | T-PHRR (s) | THR (MJ/m2) | Residue (%) |
|---|---|---|---|---|---|
| WPU | 65 | 454.3 | 297 | 84.21 | 0 |
| G/WPU | 54 | 358.0 | 257 | 78.30 | 4.0 |
| BP/G/WPU | 101 | 235.4 | 259 | 51.68 | 12.5 |
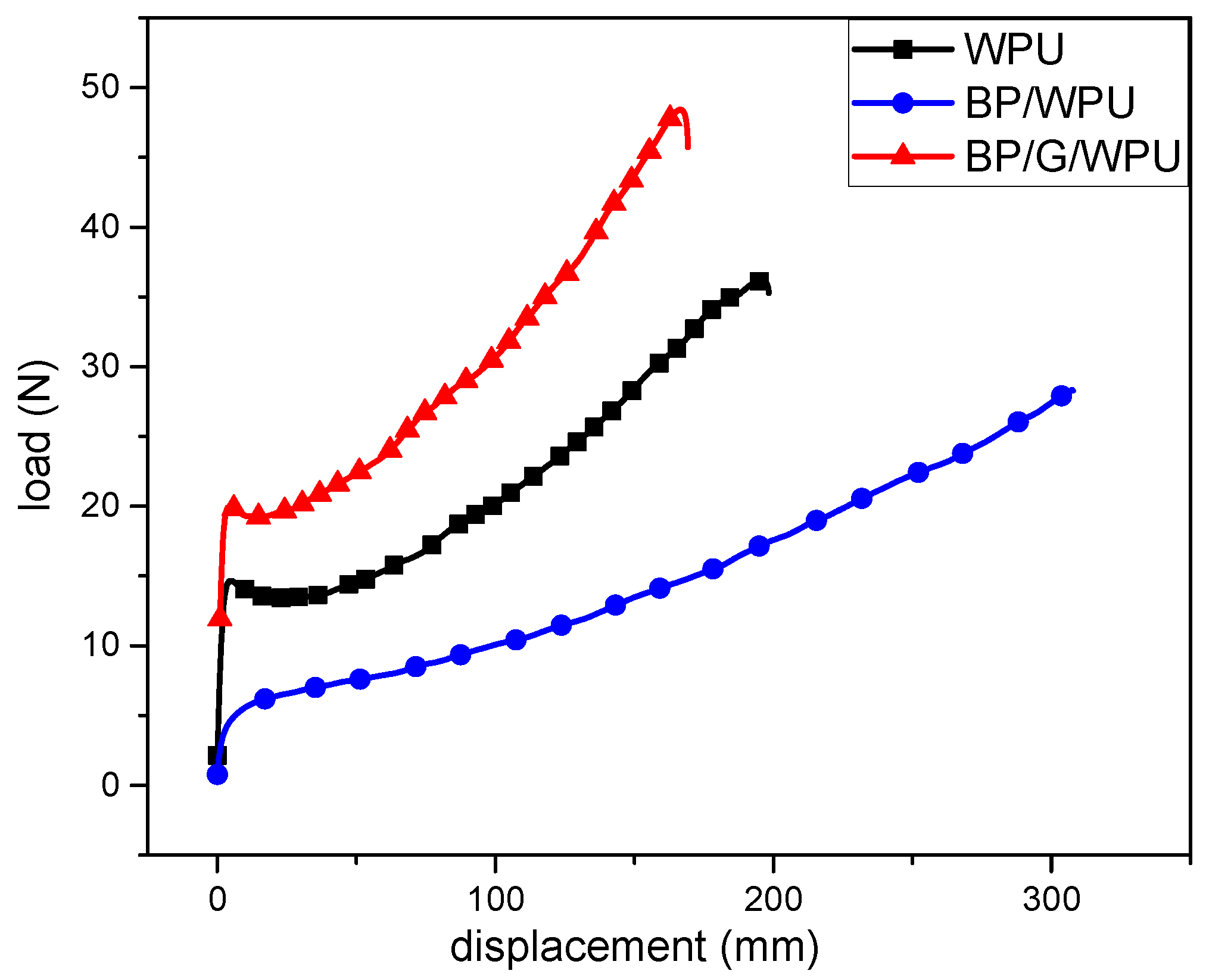
| Sample | Young’s Modulus (MPa) | Elongation at Break (%) | Ultimate Strength (MPa) |
|---|---|---|---|
| WPU | 76.24 | 793.45 | 14.7 |
| BP/WPU | 6.18 | 1231.51 | 10.82 |
| BP/G/WPU | 48.44 | 677.09 | 11.72 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, X.; Mei, Y.; Lian, P.; Xie, D.; Deng, W.; Wen, Y.; Luo, Y. Fabrication and Application of Black Phosphorene/Graphene Composite Material as a Flame Retardant. Polymers 2019, 11, 193. https://doi.org/10.3390/polym11020193
Ren X, Mei Y, Lian P, Xie D, Deng W, Wen Y, Luo Y. Fabrication and Application of Black Phosphorene/Graphene Composite Material as a Flame Retardant. Polymers. 2019; 11(2):193. https://doi.org/10.3390/polym11020193
Chicago/Turabian StyleRen, Xinlin, Yi Mei, Peichao Lian, Delong Xie, Weibin Deng, Yaling Wen, and Yong Luo. 2019. "Fabrication and Application of Black Phosphorene/Graphene Composite Material as a Flame Retardant" Polymers 11, no. 2: 193. https://doi.org/10.3390/polym11020193