Modeling of Miniemulsion Polymerization of Styrene with Macro-RAFT Agents to Theoretically Compare Slow Fragmentation, Ideal Exchange and Cross-Termination Cases
Abstract
:1. Introduction
2. Modeling Methodology
2.1. Reactions, Rate Coefficients, and Mass Transfer Parameters
2.2. Compartmentalization Model: Smith-Ewart and Moment Equations
3. Results and Discussion
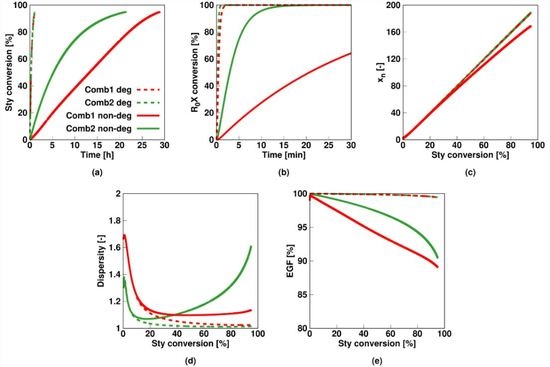
3.1. Results Considering a Non-Degenerative Mechanism at an Average Particle Size of 100 nm
3.2. Relevance of Description with an Explicit Calculation of the RAFT Intermediate
3.3. Relevance of the (Average) Particle Size in View of Future Experimental Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thickett, S.C.; Gilbert, R.G. Emulsion polymerization: State of the art in kinetics and mechanisms. Polymer (Guildf.) 2007, 48, 6965–6991. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, M.F. Controlled/living radical polymerization in aqueous dispersed systems. Prog. Polym. Sci. 2008, 33, 365–398. [Google Scholar] [CrossRef]
- Destarac, M. Controlled radical polymerization: Industrial stakes, obstacles and achievements. Macromol. React. Eng. 2010, 4, 165–179. [Google Scholar] [CrossRef]
- Zetterlund, P.B.; Thickett, S.C.; Perrier, S.; Bourgeat-Lami, E.; Lansalot, M. Controlled/Living Radical Polymerization in Dispersed Systems: An Update. Chem. Rev. 2015, 115, 9745–9800. [Google Scholar] [CrossRef] [PubMed]
- Matyjaszewski, K.; Spanswick, J. Controlled/living radical polymerization. Materialstoday 2005, 8, 26–33. [Google Scholar] [CrossRef]
- Grishin, D.F.; Grishin, I.D. Controlled radical polymerization: Prospects for application for industrial synthesis of polymers (Review). Russ. J. Appl. Chem. 2011, 84, 2021–2028. [Google Scholar] [CrossRef]
- Zetterlund, P.B.; Kagawa, Y.; Okubo, M. Controlled/living radical polymerization in dispersed systems. Chem. Rev. 2008, 108, 3747–3794. [Google Scholar] [CrossRef]
- Matyjaszewski, K. Controlled radical polymerization: State-of-the-art in 2011. ACS Symp. Ser. 2012, 1100, 1–13. [Google Scholar]
- Save, M.; Guillaneuf, Y.; Gilbert, R.G. Controlled radical polymerization in aqueous dispersed media. Aust. J. Chem. 2006, 59, 693–711. [Google Scholar] [CrossRef]
- Asua, J.M. Polymer Reaction Engineering; Blackwel Publishing Ltd.: Oxford, UK, 2007. [Google Scholar]
- Matyjaszewski, K. Controlled Radical Polymerization: Mechanisms. Curr. Opin. Solid State Mater. Sci. 1996, 1, 769–776. [Google Scholar] [CrossRef]
- Matyjaszewski, K. Advances in Controlled/Living Radical Polymerization; American Chemical Society: Washington, DC, USA, 2003. [Google Scholar]
- Barner, L.; Davis, T.P.; Stenzel, M.H.; Barner-Kowollik, C. Complex macromolecular architectures by reversible addition fragmentation chain transfer chemistry: Theory and practice. Macromol. Rapid Commun. 2007, 28, 539–559. [Google Scholar] [CrossRef]
- Fierens, S.; D’hooge, D.; Van Steenberge, P.; Reyniers, M.-F.; Marin, G. Exploring the Full Potential of Reversible Deactivation Radical Polymerization Using Pareto-Optimal Fronts. Polymers (Basel) 2015, 7, 655–679. [Google Scholar] [CrossRef] [Green Version]
- D’Hooge, D.R.; Van Steenberge, P.H.M.; Reyniers, M.F.; Marin, G.B. Fed-batch control and visualization of monomer sequences of individual ICAR ATRP gradient copolymer chains. Polymers (Basel) 2014, 6, 1074–1095. [Google Scholar] [CrossRef]
- Brandl, F.; Drache, M.; Beuermann, S. Kinetic Monte Carlo Simulation Based Detailed Understanding of the Transfer Processes in Semi-Batch Iodine Transfer Emulsion Polymerizations of Vinylidene Fluoride. Polymers (Basel) 2018, 10, 1008. [Google Scholar] [CrossRef]
- Barner-Kowollik, C. Handbook of RAFT Polymerization; Wiley-VCH: Baden-Württemberg, Germany, 2008. [Google Scholar]
- Moad, G.; Chiefari, J.; Chong, Y.K.; Krstina, J.; Mayadunne, R.T.A.; Postma, A.; Rizzardo, E.; Thang, S.H. Living free radical polymerization with reversible addition–fragmentation chain transfer (the life of RAFT). Polym. Int. 2000, 49, 993–1001. [Google Scholar] [CrossRef]
- De Rybel, N.; Van Steenberge, P.H.M.; Reyniers, M.-F.; D’hooge, D.R.; Marin, G.B. How chain length dependencies interfere with the bulk RAFT polymerization rate and microstructural control. Chem. Eng. Sci. 2018, 177, 163–179. [Google Scholar] [CrossRef]
- Gilbert, R.G. Emulsion Polymerization: A Mechanistic Approach; Academic Press Inc.: London, UK, 1995. [Google Scholar]
- Charleux, B.; Monteiro, M.J.; Heuts, H. Living Radical Polymerisation in Emulsion and Miniemulsion. In Chemistry and Technology of Emulsion Polymerisation; John Wiley & Sons: New York, NY, USA, 2013; pp. 105–143. [Google Scholar]
- Rawlston, J.A. Multiscale Modeling of Free-Radical Polymerization Kinetics. Ph.D. Thesis, School of Chemical and Biomolecular Engineering, Georgia Institute of Technology, Atlanta, GA, USA, May 2010. [Google Scholar]
- Prescott, S.W.; Ballard, M.J.; Rizzardo, E.; Gilbert, R.G. Rate optimization in controlled radical emulsion polymerization using RAFT. Macromol. Theory Simul. 2006, 15, 70–86. [Google Scholar] [CrossRef]
- Asua, J.M. Challenges for industrialization of miniemulsion polymerization. Prog. Polym. Sci. 2014, 39, 1797–1826. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Davies, T.P. Handbook of Radical Polymerisation; John Wiley & Sons: Hoboken, NJ, USA, 2002. [Google Scholar]
- Mastan, E.; Li, X.; Zhu, S. Modeling and theoretical development in controlled radical polymerization. Prog. Polym. Sci. 2015, 45, 71–101. [Google Scholar] [CrossRef]
- D’hooge, D.R.; Van Steenberge, P.H.M.; Reyniers, M.-F.; Marin, G.B. The strength of multi-scale modeling to unveil the complexity of radical polymerization. Prog. Polym. Sci. 2016, 58, 59–89. [Google Scholar] [CrossRef]
- Zetterlund, P.B. Controlled/living radical polymerization in nanoreactors: Compartmentalization effects. Polym. Chem. 2011, 2, 534–549. [Google Scholar] [CrossRef]
- Zetterlund, P.B.; Okubo, M. Compartmentalization in nitroxide-mediated radical polymerization in dispersed systems. Macromolecules 2006, 39, 8959–8967. [Google Scholar] [CrossRef]
- Cano-Valdez, A.; Saldívar-Guerra, E.; González-Blanco, R.; Cunningham, M.F.; Herrera-Ordóñez, J. Nitroxide Mediated Radical Emulsion Polymerization: Mathematical Modeling. Macromol. Symp. 2017, 374, 1–11. [Google Scholar] [CrossRef]
- Tobita, H. Threshold particle diameters in miniemulsion reversible-deactivation radical polymerization. Polymers (Basel) 2011, 3, 1944–1971. [Google Scholar] [CrossRef]
- Prescott, S.W.; Ballard, M.J.; Rizzardo, E.; Gilbert, R.G. Radical Loss in RAFT-mediated emulsion polymerizations. Macromolecules 2005, 38, 4901–4912. [Google Scholar] [CrossRef]
- Liu, S.; Hermanson, K.D.; Kaler, E.W. Reversible Addition−Fragmentation Chain Transfer Polymerization in Microemulsion. Macromolecules 2006, 39, 4345–4350. [Google Scholar] [CrossRef]
- Qiu, J.; Charleux, B.; Matyjaszewski, K. Controlled/living radical polymerization in aqueous media: Homogeneous and heterogeneous systems. Prog. Polym. Sci. 2001, 26, 2083–2134. [Google Scholar] [CrossRef]
- Monteiro, M.J.; Cunningham, M.F. Polymer Nanoparticles via Living Radical Polymerization in Aqueous Dispersions: Design and Applications. Macromolecules 2012, 45, 4939–4957. [Google Scholar] [CrossRef]
- Monteiro, M.J.; Hodgson, M.; De Brouwer, H. Influence of RAFT on the rates and molecular weight distributions of styrene in seeded emulsion polymerizations. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 3864–3874. [Google Scholar] [CrossRef]
- Landfester, K.; Willert, M.; Antonietti, M. Preparation of polymer particles in nonaqueous direct and inverse miniemulsions. Macromolecules 2000, 33, 2370–2376. [Google Scholar] [CrossRef]
- Ting, S.R.S.; Min, E.H.; Zetterlund, P.B. Reversible AdditionFragmentation Chain Transfer (RAFT) polymerization in miniemulsion based on in situ surfactant generation. Aust. J. Chem. 2011, 64, 1033–1040. [Google Scholar] [CrossRef]
- Jansen, T.G.T.; Meuldijk, J.; Lovell, P.A.; van Herk, A.M. On the Reaction Characteristics of Miniemulsion Polymerization with Aqueous Phase Initiation - Experiments and Modeling. Macromol. React. Eng. 2015, 9, 19–31. [Google Scholar] [CrossRef]
- Perrier, S.; Takolpuckdee, P. Macromolecular design via reversible addition-fragmentation chain transfer (RAFT)/xanthates (MADIX) polymerization. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 5347–5393. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Luo, Y.; Li, B. The Influence of Surfactant Coverage of the Minidroplets on RAFT Miniemulsion Polymerization. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 2293–2306. [Google Scholar] [CrossRef]
- Derboven, P.; Van Steenberge, P.H.M.; Reyniers, M.; Barner-kowollik, C.; Dagmar, R.D.; Marin, G.B.; D’hooge, D.R.; Marin, G.B.; Dagmar, R.D.; Marin, G.B. Chain transfer in degenerative RAFT polymerization revisited: A comparative study. Macromol. Theory Simul. 2016, 25, 104–115. [Google Scholar] [CrossRef]
- Devlaminck, D.J.G.; Van Steenberge, P.H.M.; De Keer, L.; Reyniers, M.-F.; D’hooge, D.R. A detailed mechanistic study of bulk MADIX of styrene and its chain extension. Polym. Chem. 2017, 8, 6948–6963. [Google Scholar] [CrossRef] [Green Version]
- Kubo, K.; Goto, A.; Sato, K.; Kwak, Y.; Fukuda, T. Kinetic study on reversible addition-fragmentation chain transfer (RAFT) process for block and random copolymerizations of styrene and methyl methacrylate. Polymer (Guildf.) 2005, 46, 9762–9768. [Google Scholar] [CrossRef]
- Moad, G.; Flagship, C.M.; Ave, B. Controlled Radical Polymerization: Mechanisms; American Chemical Society: Washington, DC, USA, 2015. [Google Scholar]
- Haven, J.J.; Junkers, T. Mapping dithiobenzoate-mediated RAFT polymerization products via online microreactor/mass spectrometry monitoring. Polymers (Basel) 2018, 10, 1228. [Google Scholar] [CrossRef]
- Derboven, P.; Van Steenberge, P.; Reyniers, M.-F.; Barner-Kowollik, C.; D’hooge, D.R.; Marin, G.B. A novel method for the measurement of degenerative chain transfer coefficients: Proof of concept and experimental validation. Polym. Chem. 2016, 7, 3334–3349. [Google Scholar] [CrossRef]
- De Rybel, N.; Van Steenberge, P.H.M.; Reyniers, M.-F.; Barner-Kowollik, C.; D’hooge, D.R.; Marin, G.B. An Update on the Pivotal Role of Kinetic Modeling for the Mechanistic Understanding and Design of Bulk and Solution RAFT Polymerization. Macromol. Theory Simul. 2017, 26. [Google Scholar] [CrossRef]
- Wang, A.R.; Zhu, S. Effects of Diffusion-Controlled Radical Reactions on RAFT Polymerization. Macromol. Theory Simul. 2003, 12, 196–208. [Google Scholar] [CrossRef]
- D’hooge, D.R.; Reyniers, M.F.; Marin, G.B. The crucial role of diffusional limitations in controlled radical polymerization. Macromol. React. Eng. 2013, 7, 362–379. [Google Scholar] [CrossRef]
- Devlaminck, D.J.G.; Van Steenberge, P.H.M.M.; Reyniers, M.-F.M.-F.; D’hooge, D.R.D.R. Deterministic modeling of RAFT miniemulsion conversion and average chain length characteristics: Invalidity of zero-one nature at higher monomer conversions. Macromolecules 2018. [Google Scholar] [CrossRef]
- Peklak, A.D.; Butté, A. Modeling of diffusion limitations in bulk RAFT polymerization. Macromol. Theory Simul. 2006, 15, 546–562. [Google Scholar] [CrossRef]
- Achilias, D.S. A review of modeling of diffusion controlled polymerization reactions. Macromol. Theory Simul. 2007, 16, 319–347. [Google Scholar] [CrossRef]
- Barner-Kowollik, C.; Russell, G.T. Chain-length-dependent termination in radical polymerization: Subtle revolution in tackling a long-standing challenge. Prog. Polym. Sci. 2009, 34, 1211–1259. [Google Scholar] [CrossRef]
- O’Driscoll, K.F. Comprehensive Polymer Science; Pergamon Press: London, UK, 1989. [Google Scholar]
- Russell, G.T. The kinetics of free-radical polymerization: Fundamental aspects. Aust. J. Chem. 2002, 55, 399–414. [Google Scholar] [CrossRef]
- McLeary, J.B.; Klumperman, B. RAFT mediated polymerisation in heterogeneous media. Soft Matter 2006, 2, 45–53. [Google Scholar] [CrossRef]
- Barner-Kowollik, C.; Quinn, J.F.; Morsley, D.R.; Davis, T.P. Modeling the reversible addition-fragmentation chain transfer process in cumyl dithiobenzoate-mediated styrene homopolymerizations: Assessing rate coefficients for the addition-fragmentation equilibrium. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 1353–1365. [Google Scholar] [CrossRef]
- Monteiro, M.J.; De Brouwer, H. Intermediate radical termination as the mechanism for retardation in reversible addition-fragmentation chain transfer polymerization. Macromolecules 2001, 34, 349–352. [Google Scholar] [CrossRef]
- Moad, G. Mechanism and Kinetics of Dithiobenzoate-Mediated RAFT Polymerization: Status of dilemma. Macromol. Chem. Phys. 2014, 215, 9–26. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E.; Thang, S.H. Living Radical Polymerization by the RAFT Process—A Third Update. Aust. J. Chem. 2012, 65, 985–1076. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E.; Thang, S.H. Living radical polymerization by the RAFT process A second update. Aust. J. Chem. 2009, 62, 1402–1472. [Google Scholar] [CrossRef]
- Barner-Kowollik, C.; Buback, M.; Charleux, B.; Coote, M.L.; Drache, M.; Fukuda, T.; Goto, A.; Klumperman, B.; Lowe, A.B.; Mcleary, J.B.; et al. Mechanism and Kinetics of Dithiobenzoate-Mediated RAFT Polymerization. I. The Current Situation. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 5809–5831. [Google Scholar] [CrossRef]
- Konkolewicz, D.; Hawkett, B.S.; Gray-Weale, A.; Perrier, S. RAFT Polymerization Kinetics: Combination of Apparently Conflicting Models. Macromolecules 2008, 41, 6400–6412. [Google Scholar] [CrossRef]
- Kwak, Y.; Goto, A.; Tsujii, Y.; Murata, Y.; Komatsu, K.; Fukuda, T. A Kinetic Study on the Rate Retardation in Radical Polymerization of Styrene with Addition−Fragmentation Chain Transfer. Macromolecules 2002, 35, 3026–3029. [Google Scholar] [CrossRef]
- Goto, A.; Sato, K.; Tsujii, Y.; Fukuda, T.; Moad, G.; Rizzardo, E.; Thang, S.H. Mechanism and Kinetics of RAFT-Based Living Radical Polymerizations of Styrene and Methyl Methacrylate. Macromolecules 2001, 34, 402–408. [Google Scholar] [CrossRef]
- McLeary, J.B.; Calitz, F.M.; McKenzie, J.M.; Tonge, M.P.; Sanderson, R.D.; Klumperman, B. Beyond Inhibition: A 1H NMR Investigation of the Early Kinetics of RAFT-Mediated Polymerization with the Same Initiating and Leaving Groups. Macromolecules 2004, 37, 2383–2394. [Google Scholar] [CrossRef]
- Calitz, F.M.; Tonge, M.P.; Sanderson, R.D.; Step, D. Kinetic and Electron Spin Resonance Analysis of RAFT Polymerization of Styrene. Macromolecules 2006, 36, 5–8. [Google Scholar] [CrossRef]
- Geelen, P.; Klumperman, B. Intermediate radical termination in reversible addition-fragmentation chain transfer-mediated polymerization: Identification of termination products. Macromolecules 2007, 40, 3914–3920. [Google Scholar] [CrossRef]
- Ranieri, K.; Delaittre, G.; Barner-Kowollik, C.; Junkers, T. Direct access to dithiobenzonate RAFT agent fragmenting rate coefficients by ESR spin-trapping. Macromol. Rapid Commun. 2014, 35, 2023–2028. [Google Scholar] [CrossRef] [PubMed]
- Kwak, Y.; Goto, A.; Fukuda, T. Rate Retardation in Reversible Addition-Fragmentation Chain Transfer (RAFT) Polymerization: Further Evidence for Cross-Termination Producing 3-Arm Star Chain. Macromolecules 2004, 37, 1219–1225. [Google Scholar] [CrossRef]
- Meiser, W.; Buback, M. Assessing the RAFT equilibrium constant via model systems: An EPR study-response to a comment. Macromol. Rapid Commun. 2011, 32, 1490–1494. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; He, J.; Liu, Y.; Zhou, Y.; Yang, Y. Probing the RAFT process using a model reaction between alkoxyamine and dithioester. Aust. J. Chem. 2012, 65, 1077–1089. [Google Scholar] [CrossRef]
- Konkolewicz, D.; Siauw, M.; Gray-Weale, A.; Hawkett, B.S.; Perrier, S. Obtaining kinetic information from the chain-length distribution of polymers produced by RAFT. J. Phys. Chem. B 2009, 113, 7086–7094. [Google Scholar] [CrossRef]
- Ting, S.R.S.; Davis, T.P.; Zetterlund, P.B. Retardation in RAFT polymerization: Does cross-termination occur with short radicals only? Macromolecules 2011, 44, 4187–4193. [Google Scholar] [CrossRef]
- Junkers, T.; Delaittre, G.; Chapman, R.; Günzler, F.; Chernikova, E.; Barner-Kowollik, C. Thioketone-mediated polymerization with dithiobenzoates: Proof for the existence of stable radical intermediates in RAFT polymerization. Macromol. Rapid Commun. 2012, 33, 984–990. [Google Scholar] [CrossRef]
- Klumperman, B.; Van Den Dungen, E.T.A.; Heuts, J.P.A.; Monteiro, M.J. RAFT-mediated polymerization-A story of incompatible data? Macromol. Rapid Commun. 2010, 31, 1846–1862. [Google Scholar] [CrossRef]
- Buback, M.; Vana, P. Mechanism of Dithiobenzoate-Mediated RAFT Polymerization: A Missing Reaction Step. Macromol. Rapid Commun. 2006, 27, 1299–1305. [Google Scholar] [CrossRef]
- Barner-Kowollik, C.; Coote, M.L.; Davis, T.P.; Radom, L.; Vana, P. The reversible addition-fragmentation chain transfer process and the strength and limitations of modeling: Comment on “The magnitude of the fragmentation rate coefficient”. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 2828–2832. [Google Scholar] [CrossRef]
- Wang, A.R.; Zhu, S.; Kwak, Y.; Goto, A.; Fukuda, T.; Monteiro, M.S. A difference of six orders of magnitude: A reply to “The magnitude of the fragmentation rate coefficient”. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 2833–2839. [Google Scholar] [CrossRef]
- Vana, P.; Davis, T.P.; Barner-Kowollik, C. Kinetic analysis of reversible addition fragmentation chain transfer (RAFT) polymerizations: Conditions for inhibition, retardation, and optimum living polymerization. Macromol. Theory Simul. 2002, 11, 823–835. [Google Scholar] [CrossRef]
- Zhang, M.; Ray, W.H. Modeling of “living” free-radical polymerization with RAFT chemistry. Ind. Eng. Chem. Res. 2001, 40, 4336–4352. [Google Scholar] [CrossRef]
- Monteiro, M.J. Modeling the molecular weight distribution of block copolymer formation in a reversible addition-fragmentation chain transfer mediated living radical polymerization. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 5643–5651. [Google Scholar] [CrossRef]
- McLeary, J.B.; Tonge, M.P.; Klumperman, B. A mechanistic interpretation of initialization processes in RAFT-mediated polymerization. Macromol. Rapid Commun. 2006, 27, 1233–1240. [Google Scholar] [CrossRef]
- Drache, M.; Schmidt-Naake, G.; Buback, M.; Vana, P. Modeling RAFT polymerization kinetics via Monte Carlo methods: Cumyl dithiobenzoate mediated methyl acrylate polymerization. Polymer (Guildf.) 2005, 46, 8483–8493. [Google Scholar] [CrossRef]
- Coote, M.L.; Radom, L. Ab initio evidence for slow fragmentation in RAFT polymerization. J. Am. Chem. Soc. 2003, 125, 1490–1491. [Google Scholar] [CrossRef]
- Coote, M.L. Ab initio study of the addition-fragmentation equilibrium in RAFT polymerization: When is polymerization retarded? Macromolecules 2004, 37, 5023–5031. [Google Scholar] [CrossRef]
- Junkers, T.; Barner-Kowollik, C.; Coote, M.L. Revealing model dependencies in “assessing the RAFT equilibrium constant via model systems: An EPR study”. Macromol. Rapid Commun. 2011, 32, 1891–1898. [Google Scholar] [CrossRef]
- Junkers, T. RAFT kinetics revisited: Revival of the RAFT debate. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 4154–4163. [Google Scholar] [CrossRef]
- Buback, M.; Meiser, W.; Vana, P. Mechanism of CPDB-mediated RAFT polymerization of methyl methacrylate: Influence of pressure and RAFT agent concentration. Aust. J. Chem. 2009, 62, 1484–1487. [Google Scholar] [CrossRef]
- Prescott, S.W. Chain-length dependence in living/controlled free-radical polymerizations: Physical manifestation and Monte Carlo simulation of reversible transfer agents. Macromolecules 2003, 36, 9608–9621. [Google Scholar] [CrossRef]
- Tobita, H. On the discrimination of RAFT models using miniemulsion polymerization. Macromol. Theory Simul. 2013, 22, 399–409. [Google Scholar] [CrossRef]
- Tobita, H. Effects of retardation and variation of monomer concentration in RAFT miniemulsion polymerization. Macromol. Theory Simul. 2011, 20, 709–720. [Google Scholar] [CrossRef]
- Tobita, H.; Yanase, F. Monte Carlo simulation of controlled/living radical polymerization in emulsified systems. Macromol. Theory Simul. 2007, 16, 476–488. [Google Scholar] [CrossRef]
- Lansalot, M.; Davis, T.P.; Heuts, J.P.A. RAFT miniemulsion polymerization: Influence of the structure of the RAFT agent. Macromolecules 2002, 35, 7582–7591. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, B.; Wang, Z.; Gao, J.; Li, B. Butyl Acrylate RAFT Polymerization in Miniemulsion. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 2304–2315. [Google Scholar] [CrossRef]
- Tsavalas, J.G.; Schork, F.J.; De Brouwer, H.; Monteiro, M.J. Living Radical Polymerization by reversible addition-fragmentation chain transfer in ionically stabilized miniemulsions. Macromolecules 2001, 34, 3938–3946. [Google Scholar] [CrossRef]
- Devlaminck, D.J.G.; Van Steenberge, P.H.M.; Reyniers, M.-F.; D’hooge, D.R. Deterministic Modeling of Degenerative RAFT Miniemulsion Polymerization Rate and Average Polymer Characteristics: Invalidity of Zero–One Nature at Higher Monomer Conversions. Macromolecules 2018, 51, 9442–9461. [Google Scholar] [CrossRef]
- Suzuki, K.; Kanematsu, Y.; Miura, T.; Minami, M.; Satoh, S.; Tobita, H. Experimental method to discriminate RAFT models between intermediate termination and slow fragmentation via comparison of rates of miniemulsion and bulk polymerization. Macromol. Theory Simul. 2014, 23, 136–146. [Google Scholar] [CrossRef]
- Tobita, H. Fundamentals of RAFT miniemulsion polymerization kinetics. Macromol. Symp. 2010, 288, 16–24. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Q.; Zhan, X.; Chen, F.; Rao, G.; Xiong, J. Preparation, kinetics and microstructures of well-defined PS-b-PS/Bd diblock copolymers via RAFT miniemulsion polymerization. J. Polym. Res. 2013, 20, 288–301. [Google Scholar] [CrossRef]
- Suzuki, K.; Nishimura, Y.; Kanematsu, Y.; Masuda, Y.; Satoh, S.; Tobita, H. Experimental Validation of Intermediate Termination in RAFT Polymerization with Dithiobenzoate via Comparison of Miniemulsion and Bulk Polymerization Rates. Macromol. React. Eng. 2012, 6, 17–23. [Google Scholar] [CrossRef]
- Altarawneh, I.S.; Gomes, V.G.; Srour, M.H. Polymer Chain Extension in Semibatch Emulsion Polymerization with RAFT-Based Transfer Agent: The influence of Reaction Conditions on Polymerization Rate and Product Properties. J. Appl. Polym. Sci. 2009, 114, 2356–2372. [Google Scholar] [CrossRef]
- Altarawneh, I.S.; Gomes, V.G.; Srour, M.S. The Influence of Xanthate-Based Transfer Agents on Styrene Emulsion Polymerization: Mathematical Modeling and Model Validation. Macromol. React. Eng. 2008, 2, 58–79. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, R.; Yang, L.; Yu, B.; Li, B.; Zhu, S. Effect of Reversible Addition-Fragmentation Transfer (RAFT) reactions on (mini)emulsion polymerization kinetics and estimate of RAFT equilibrium constant. Macromolecules 2006, 39, 1328–1337. [Google Scholar] [CrossRef]
- Li, X.; Wang, W.J.; Weng, F.; Li, B.G.; Zhu, S. Targeting copolymer composition distribution via model-based monomer feeding policy in semibatch RAFT mini-emulsion copolymerization of styrene and butyl acrylate. Ind. Eng. Chem. Res. 2014, 53, 7321–7332. [Google Scholar] [CrossRef]
- Jung, S.M.; Gomes, V.G. Miniemulsion polymerisation via reversible addition fragmentation chain transfer in pseudo-bulk regime. Macromol. React. Eng. 2011, 5, 303–315. [Google Scholar] [CrossRef]
- Peklak, A.D.; Butte, A. Kinetic Model of Reversible Addition Fragmentation Chain Transfer Polymerization of Styrene in Seeded Emulsion. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 6114–6135. [Google Scholar] [CrossRef]
- Smulders, W.; Gilbert, R.G.; Monteiro, M.J. A kinetic investigation of seeded emulsion polymerization of styrene using reversible addition-fragmentation chain transfer (RAFT) agents with a low transfer constant. Macromolecules 2003, 36, 4309–4318. [Google Scholar] [CrossRef]
- Pepels, M.P.F.; Holdsworth, C.I.; Pascual, S.; Monteiro, M.J. RAFT-Mediated emulsion polymerization of styrene with low reactive xanthate agents: Microemulsion-like behavior. Macromolecules 2010, 43, 7565–7576. [Google Scholar] [CrossRef]
- Butté, A.; Peklak, A.D.; Storti, G.; Morbidelli, M. RAFT Polymerization in Bulk and Emulsion. Radic. Polym. Kinet. Mech. 2007, 246, 168–181. [Google Scholar] [CrossRef]
- Luo, Y.; Yu, B. Monte Carlo Simulation of Droplet Nucleation in RAFT Free Radical Miniemulsion Polymerization. Polym. Plast. Technol. Eng. 2005, 43, 1299–1321. [Google Scholar] [CrossRef]
- Dossi, M.; Storti, G.; Moscatelli, D. Initiation Kinetics in Free-Radical Polymerization: Prediction of Thermodynamic and Kinetic Parameters Based on ab initio Calculations. Macromol. Theory Simul. 2010, 19, 170–178. [Google Scholar] [CrossRef]
- Buback, M.; Gilbert, R.G.; Hutchinson, R.A.; Klumberman, B.; Kuchta, F.-D.; Manders, B.G.; O’Driscoll, K.F.; Russell, G.T.; Schweer, J. Critically evaluated rate coefficients for free-radical Propagation rate coefficient for styrene. Macromol. Chem. Phys. 1995, 196, 3267–3280. [Google Scholar] [CrossRef]
- Johnston-Hall, G.; Monteiro, M.J. Bimolecular Radical Termination: New Perspectives and Insights. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 3155–3176. [Google Scholar] [CrossRef]
- Derboven, P.; D’hooge, D.R.; Reyniers, M.-F.; Marin, G.B.; Barner-Kowollik, C. The Long and the Short of Radical Polymerization. Macromolecules 2015, 48, 492–501. [Google Scholar] [CrossRef]
- Moad, G.; Solomon, D.H. The Chemistry of Radical Polymerization; Elsevier: Oxford, UK, 2006. [Google Scholar]
- Wang, A.R.; Zhu, S. Modeling the Reversible Addition–Fragmentation Transfer Polymerization Process. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 1553–1566. [Google Scholar] [CrossRef]
- Houshyar, S.; Keddie, D.J.; Moad, G.; Mulder, R.J.; Saubern, S.; Tsanaktsidis, J. The scope for synthesis of macro-RAFT agents by sequential insertion of single monomer units. Polym. Chem. 2012, 3, 1879. [Google Scholar] [CrossRef]
- Van Steenberge, P.H.M.; D’hooge, D.R.; Reyniers, M.F.; Marin, G.B.; Cunningham, M.F. 4-Dimensional modeling strategy for an improved understanding of miniemulsion NMP of acrylates initiated by SG1-macroinitiator. Macromolecules 2014, 47, 7732–7741. [Google Scholar] [CrossRef]
- Hui, A.W.; Hamielec, A.E. Thermal Polymerization of Styrene at High Conversion and Temperatures. An Experimental Study. J. Appl. Polym. Sci. 1972, 16, 749–769. [Google Scholar] [CrossRef]
- Van Steenberge, P.H.M.; D’hooge, D.R.; Wang, Y.; Zhong, M.; Reyniers, M.-F.; Konkolewicz, D.; Matyjaszewski, K.; Marin, G.B. Linear Gradient Quality of ATRP Copolymers. Macromolecules 2012, 45, 8519–8531. [Google Scholar] [CrossRef]
- Bevington, J.C.; Melville, H.W.; Taylor, R.P. The termination reaction in radical polymerizations. II. Polymerizations of styrene at 60° and of methyl methacrylate at 0 and 60°, and the copolymerization of these monomers at 60°. J. Polym. Sci. 1954, 14, 463–476. [Google Scholar] [CrossRef]
- Fierens, S.K.; D’hooge, D.R.; Van Steenberge, P.H.M.; Reyniers, M.-F.; Marin, G.B. MAMA-SG1 initiated nitroxide mediated polymerization of styrene: From Arrhenius parameters to model-based design. Chem. Eng. J. 2014, 278, 407–420. [Google Scholar] [CrossRef]
- Khuong, K.S.; Jones, W.H.; Pryor, W.A.; Houk, K.N. The mechanism of the self-initiated thermal polymerization of styrene. Theoretical solution of a classic problem. J. Am. Chem. Soc. 2005, 127, 1265–1277. [Google Scholar] [CrossRef]
- Kotoulas, C.; Krallis, A.; Pladis, P.; Kiparissides, C. A comprehensive kinetic model for the combined chemical and thermal polymerization of styrene up to high conversions. Macromol. Chem. Phys. 2003, 204, 1305–1314. [Google Scholar] [CrossRef]
- Buback, M.; Barner-kowollik, C.; Kurz, C.; Wahl, A. Termination kinetics of styrene free-radical polymerization studied by time-resolved pulsed laser experiments. Macromol. Chem. Phys. 2000, 201, 464–469. [Google Scholar] [CrossRef]
- Vivaldo-Lima, E.; Mendoza-Fuentes, A.D.J. Development of a kinetic model for INIFERTER controlled/“living” free-radical polymerization considering diffusion-controlled effects. Polym. React. Eng. 2002, 10, 193–226. [Google Scholar] [CrossRef]
- Peklak, A.D.; Butté, A.; Storti, G.; Morbidelli, M. Gel effect in the bulk reversible addition-fragmentation chain transfer polymerization of methyl methacrylate: Modeling and experiments. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 1071–1085. [Google Scholar] [CrossRef]
- Garg, D.K.; Serra, C.A.; Hoarau, Y.; Parida, D.; Bouquey, M.; Muller, R. Analytical solution of free radical polymerization: Applications-implementing gel effect using AK model. Macromolecules 2014, 47, 7370–7377. [Google Scholar] [CrossRef]
- Tefera, N.; Weickert, G.; Westerterp, K.R. Modeling of Free Radical Polymerization up to High Conversion. I. A Method for the Selection of Models by Simultaneous Parameter Estimation. J. Appl. Polym. Sci. 1996, 63, 1649–1661. [Google Scholar] [CrossRef]
- Carswell, T.G.; Hill, D.J.T.; Londero, D.I.; O’Donnell, J.H.; Pomery, P.J.; Winzor, C.L. Kinetic parameters for polymerization of methyl methacrylate at 60 °C. Polymer (Guildf.) 1992, 33, 137–140. [Google Scholar] [CrossRef]
- Mastan, E.; Zhu, S. Method of moments: A versatile tool for deterministic modeling of polymerization kinetics. Eur. Polym. J. 2015, 68, 139–160. [Google Scholar] [CrossRef]
- D’hooge, D.R.; Reyniers, M.-F.; Marin, G.B. Methodology for Kinetic Modeling of Atom Transfer Radical Polymerization. Macromol. React. Eng. 2009, 3, 185–209. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, Y.; Shi, L.; Luo, Z.-H. Modeling of the Atom Transfer Radical Copolymerization Processes of Methyl Methacrylate and 2-(Trimethylsilyl) Ethyl Methacrylate under Batch, Semibatch, and Continuous Feeding: A Chemical Reactor Engineering Viewpoint. Ind. Eng. Chem. Res. 2014, 53, 11873–11883. [Google Scholar] [CrossRef]
- Zhou, Y.-N.; Luo, Z.-H. State-of-the-Art and Progress in Method of Moments for the Model-Based Reversible-Deactivation Radical Polymerization. Macromol. React. Eng. 2016, 10, 516–534. [Google Scholar] [CrossRef]
- Smith, W.V.; Ewart, R.H. Kinetics of emulsion polymerization. J. Chem. Phys. 1948, 16, 592–599. [Google Scholar] [CrossRef]
- Zetterlund, P.B.; Okubo, M. Compartmentalization in NMP in dispersed systems: Relative contributions of confined space effect and segregation effect depending on nitroxide type. Macromol. Theory Simul. 2009, 18, 277–286. [Google Scholar] [CrossRef]
- Prescott, S.W.; Ballard, M.J.; Gilbert, R.G. Average termination rate coefficients in emulsion polymerization: Effect of compartmentalization on free-radical lifetimes. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 1076–1089. [Google Scholar] [CrossRef]
- Jia, Z.; Monteiro, M.J. Kinetic simulations of RAFT-mediated microemulsion polymerizations of styrene. ACS Symp. Ser. 2012, 1100, 293–304. [Google Scholar]
- Zetterlund, P.B. Nitroxide-Mediated Radical Polymerization in Dispersed Systems: Compartmentalization and Nitroxide Partitioning. Macromol. Theory Simul. 2009, 19, 11–23. [Google Scholar] [CrossRef]














| Reaction | DM | NDM | Equation | Comb 1 | Comb 2 | Comb 3 | Ref |
|---|---|---|---|---|---|---|---|
| Aqueous phase reactions | |||||||
| Diss.(a),(b) of I2 | ∨ | ∨ | [105] | ||||
| Chain ini I | ∨ | ∨ | [113] | ||||
| Propagation (c),(d) | ∨ | ∨ | [114] | ||||
| Organic phase reactions | |||||||
| (e) | ∨ | ∨ | [114] | ||||
| Propagation | ∨ | ∨ | [114] | ||||
| Termination (f) | ∨ | ∨ | [115,116] | ||||
| (f) | ∨ | ∨ | [115,116] | ||||
| (f) | ∨ | ∨ | [115,116] | ||||
| RAFT add/frag (i) | ∨ | (j) | |||||
| (i) | ∨ | (j) | |||||
| (i) | ∨ | (j) | |||||
| (i) | ∨ | (j) | |||||
| (a),(i) | ∨ | (j) | |||||
| (a),(i) | ∨ | (j) | |||||
| (a),(i) | ∨ | (j) | |||||
| (a),(i) | ∨ | (j) | |||||
| RAFT cross-t(i) | ∨ | (k) | (j) | ||||
| (i) | ∨ | (j) | |||||
| (i) | ∨ | (j) | |||||
| (i) | ∨ | (j) | |||||
| (i) | ∨ | (j) | |||||
| (i) | ∨ | (j) | |||||
| RAFT exchange | ∨ | (g) | |||||
| ∨ | (g) | ||||||
| ∨ | (g) | ||||||
| interphase mass transport | |||||||
| Entry of | ∨ | ∨ | (d),(h) | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Devlaminck, D.J.G.; Van Steenberge, P.H.M.; Reyniers, M.-F.; D’hooge, D.R. Modeling of Miniemulsion Polymerization of Styrene with Macro-RAFT Agents to Theoretically Compare Slow Fragmentation, Ideal Exchange and Cross-Termination Cases. Polymers 2019, 11, 320. https://doi.org/10.3390/polym11020320
Devlaminck DJG, Van Steenberge PHM, Reyniers M-F, D’hooge DR. Modeling of Miniemulsion Polymerization of Styrene with Macro-RAFT Agents to Theoretically Compare Slow Fragmentation, Ideal Exchange and Cross-Termination Cases. Polymers. 2019; 11(2):320. https://doi.org/10.3390/polym11020320
Chicago/Turabian StyleDevlaminck, Dries J.G., Paul H.M. Van Steenberge, Marie-Françoise Reyniers, and Dagmar R. D’hooge. 2019. "Modeling of Miniemulsion Polymerization of Styrene with Macro-RAFT Agents to Theoretically Compare Slow Fragmentation, Ideal Exchange and Cross-Termination Cases" Polymers 11, no. 2: 320. https://doi.org/10.3390/polym11020320