Tuning Physical Crosslinks in Hybrid Hydrogels for Network Structure Analysis and Mechanical Reinforcement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
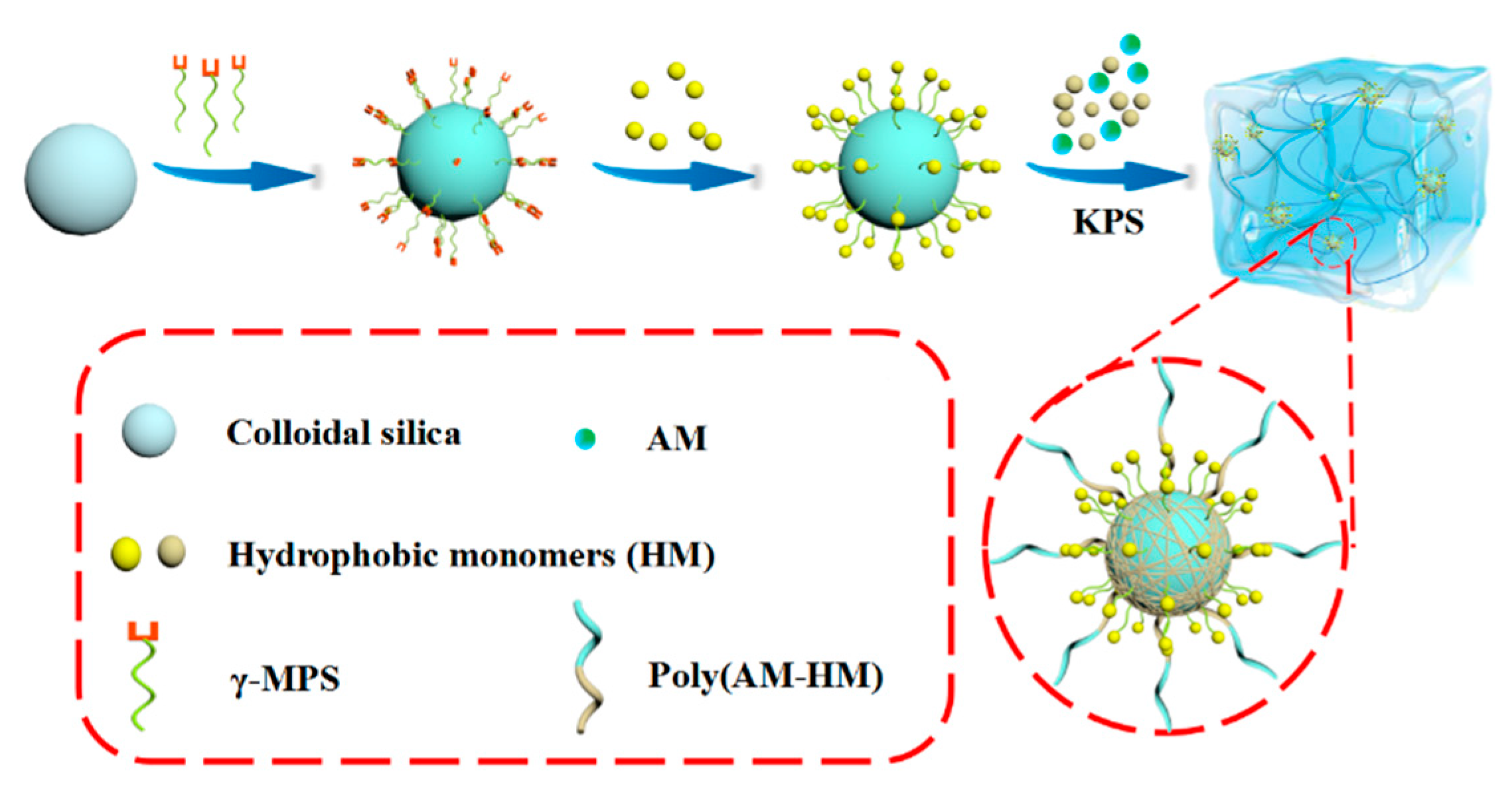
2.2. Preparation of Core-Shell Hybrid Latex Particles
2.3. Synthesis of Hydrogels
2.4. Characterization
2.4.1. IR Test
2.4.2. TGA Test
2.4.3. DLS Measurement
2.4.4. Mechanical Measurement
2.4.5. Swelling Measurement
2.4.6. Rheological Measurement
3. Results
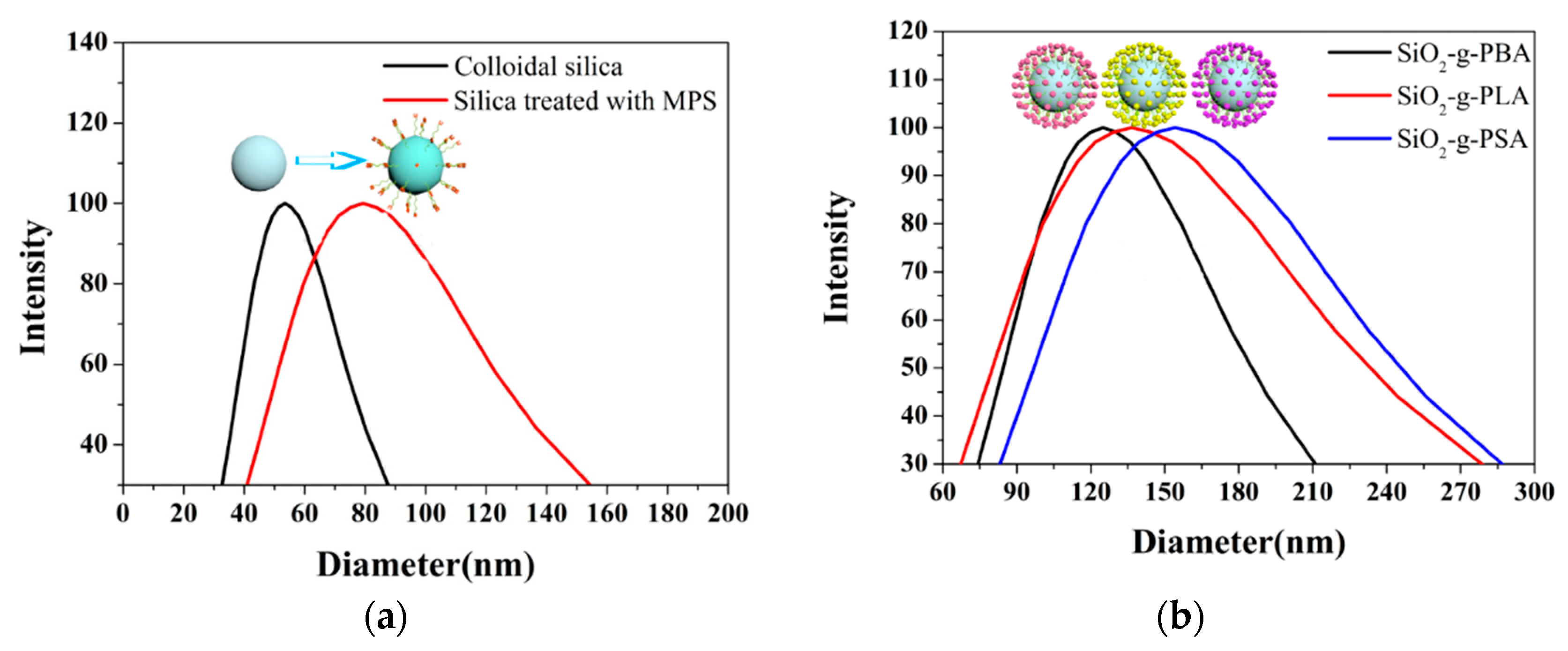
3.1. Characterization of Hybrid Latexes Particles
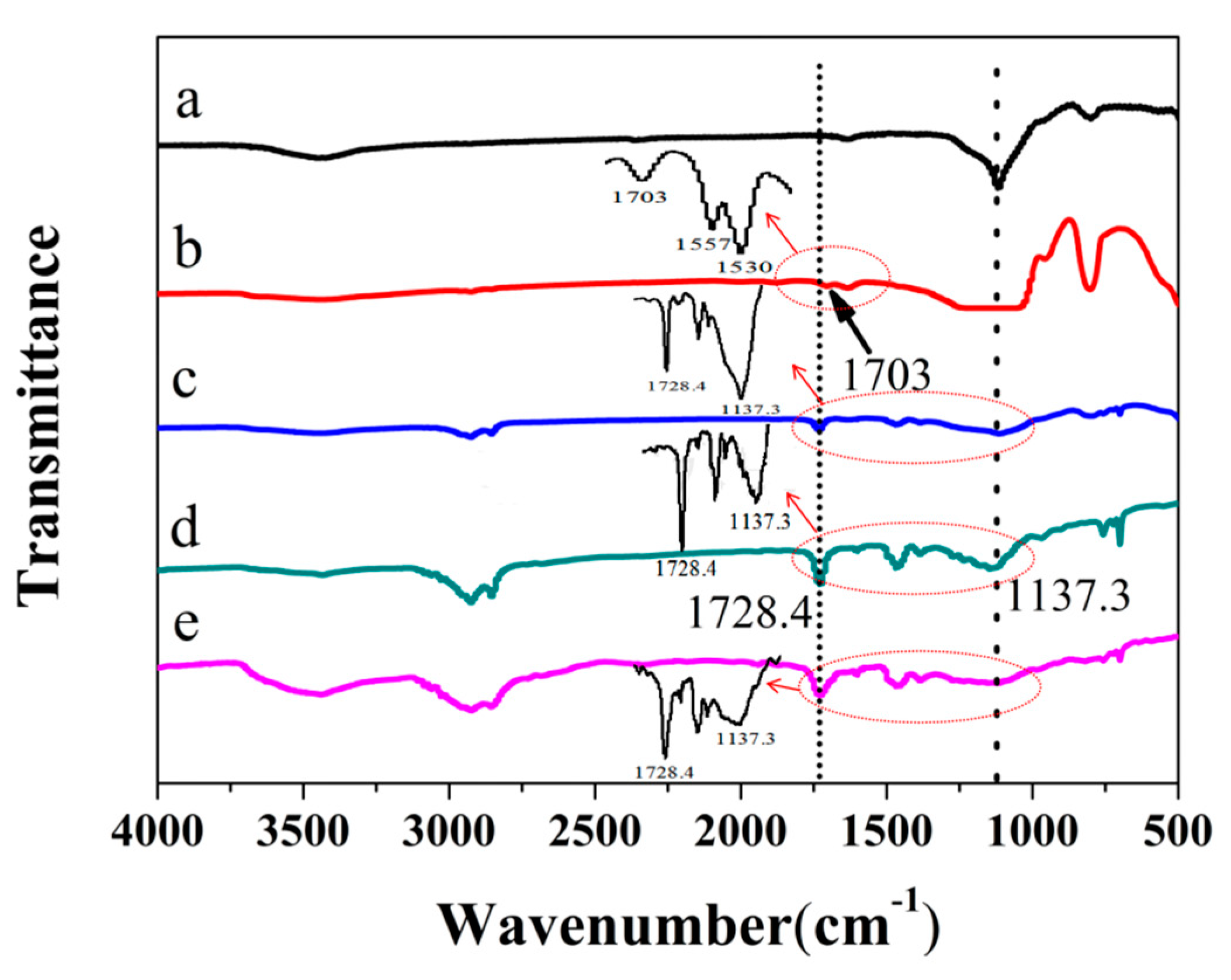
3.2. FTIR Analysis
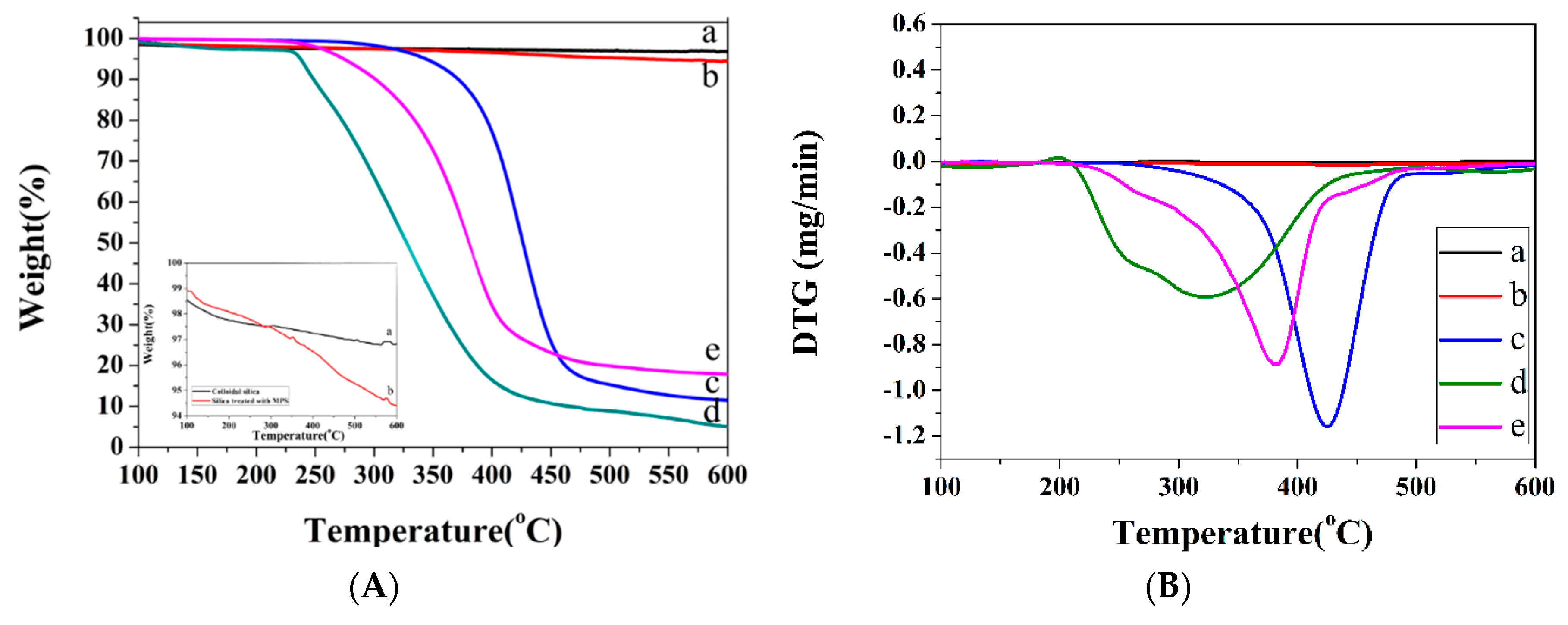
3.3. TGA Analysis
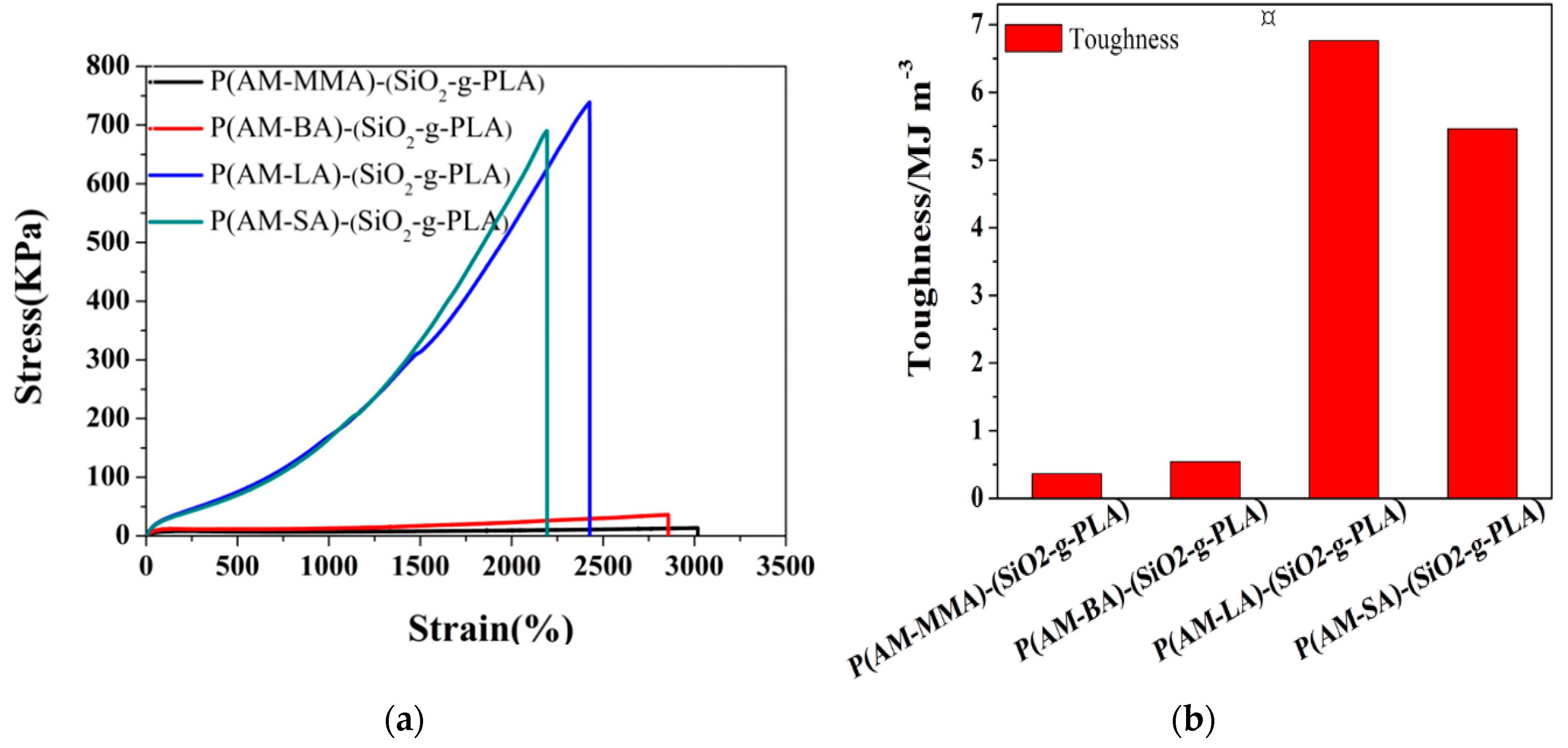
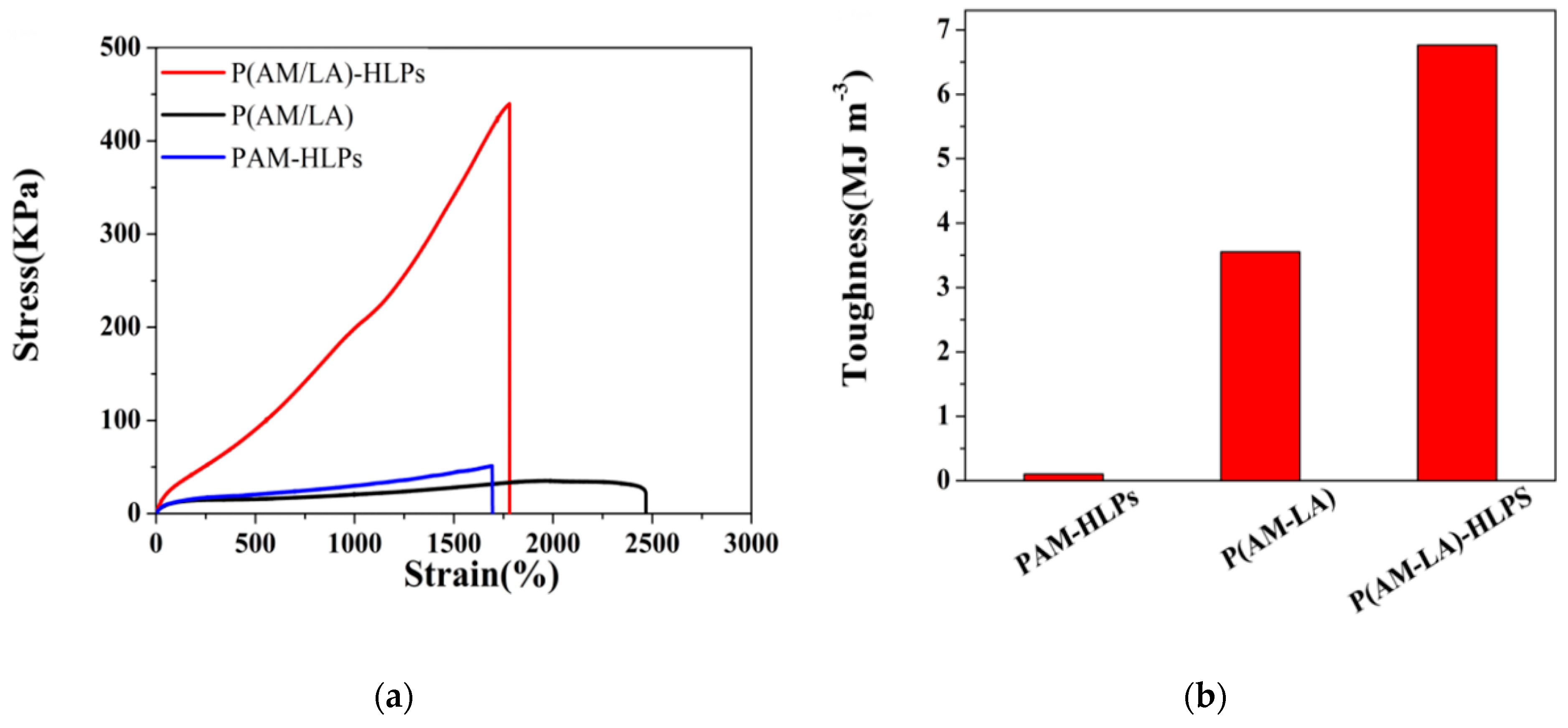
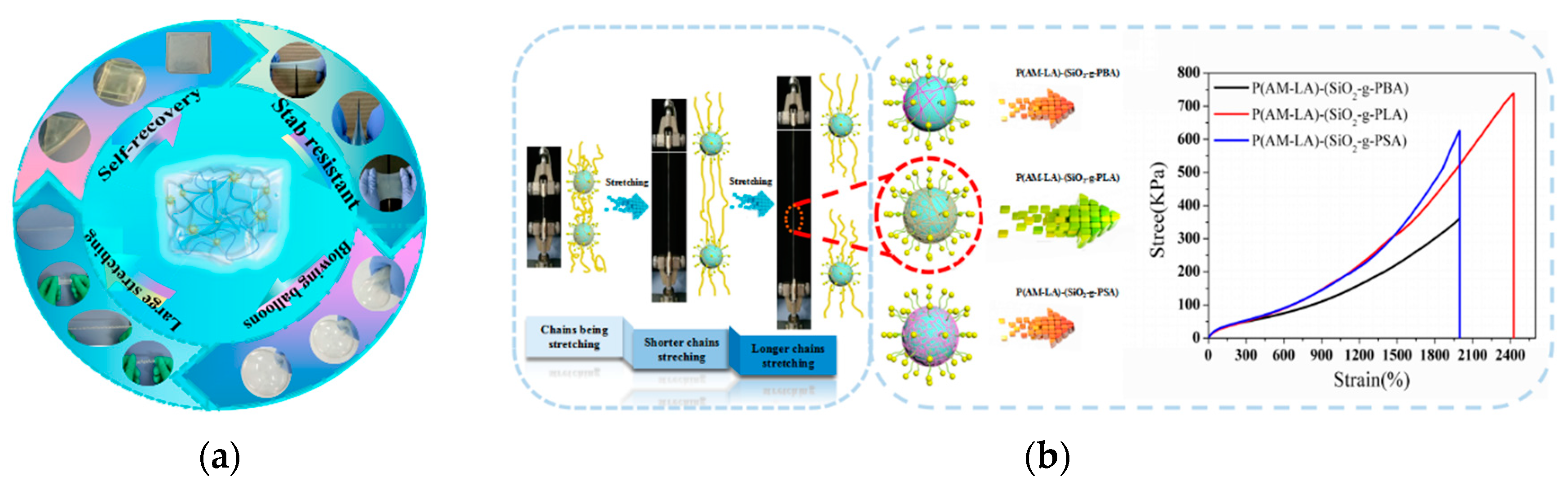
3.4. Mechanical Properties of Hydrogels
3.5. Formation Mechanism of Hydrogels
3.6. Rheology Properties of Hydrogels
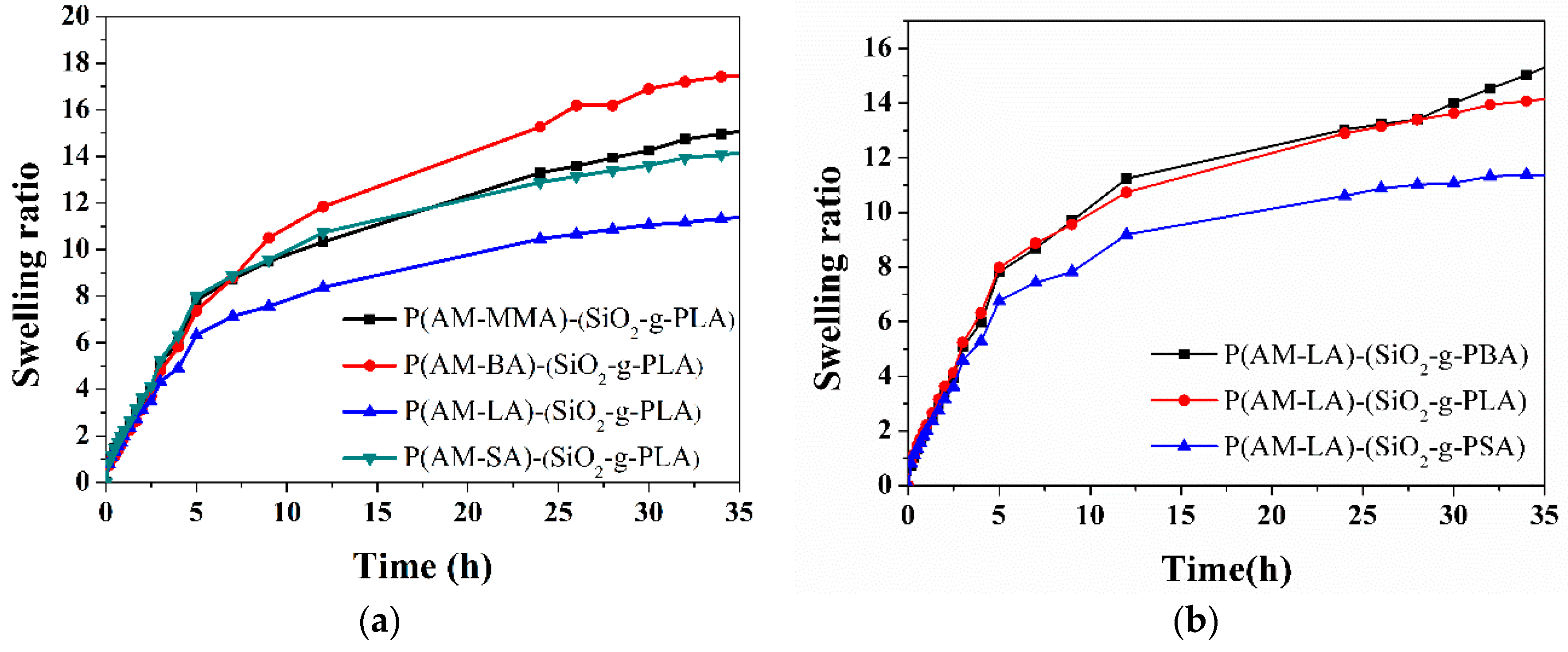
3.7. Swelling Properties of Hydrogels
3.8. Parameters of the Network Structure of the Hydrogels
3.9. Self-Recovery of HA-Gels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Duan, J.; Liang, X.; Cao, Y.; Wang, S.; Zhang, L. High strength chitosan hydrogels with biocompatibility via new avenue based on constructing nanofibrous architecture. Macromolecules 2015, 48, 2706–2714. [Google Scholar] [CrossRef]
- Chen, Q.; Yan, X.; Zhu, L.; Chen, H.; Jiang, B.; Wei, D.; Huang, L.; Yang, J.; Liu, B.; Zheng, J. Improvement of mechanical strength and fatigue resistance of double network hydrogels by ionic coordination interactions. Chem. Mater. 2016, 28, 5710–5720. [Google Scholar] [CrossRef]
- Lv, X.; Sun, S.; Yang, H.; Gao, G.; Liu, F. Effect of the sodium dodecyl sulfate/monomer ratio on the network structure of hydrophobic association hydrogels with adjustable mechanical properties. J. Appl. Polym. Sci. 2017, 134, 45196. [Google Scholar] [CrossRef]
- Korogiannaki, M.; Zhang, J.; Sheardown, H. Surface modification of model hydrogel contact lenses with hyaluronic acid via thiol-ene “click” chemistry for enhancing surface characteristics. J. Biomater. Appl. 2017, 32, 446–462. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Wang, J.; Wang, K.; Zhu, J. Highly sensitive mechanochromic photonic hydrogels with fast reversibility and mechanical stability. Langmuir 2015, 31, 8732–8737. [Google Scholar] [CrossRef] [PubMed]
- Mu, Z.; Zhao, X.; Huang, Y.; Lu, M.; Gu, Z. Photonic crystal hydrogel enhanced plasmonic staining for multiplexed protein analysis. Small 2015, 11, 6036–6043. [Google Scholar] [CrossRef]
- Liu, Z.; Fan, L.; Xiao, H.; Cao, C. A multiple covalent crosslinked soft hydrogel for bioseparation. Chem. Commun. 2016, 52, 3247–3250. [Google Scholar] [CrossRef]
- Chen, L.; An, H.Z.; Haghgooie, R.; Shank, A.T.; Martel, J.M.; Toner, M.; Doyle, P.S. Flexible Octopus-Shaped Hydrogel Particles for Specific Cell Capture. Small 2016, 12, 2001–2008. [Google Scholar] [CrossRef] [Green Version]
- Mauri, E.; Negri, A.; Rebellato, E.; Masi, M.; Perale, G.; Rossi, F. Hydrogel-Nanoparticles Composite System for Controlled Drug Delivery. Gels 2018, 4, 74. [Google Scholar] [CrossRef]
- Li, J.; Hu, W.; Zhang, Y.; Tan, H.; Yan, X.; Zhao, L.; Liang, H. pH and glucose dually responsive injectable hydrogel prepared by in situ crosslinking of phenylboronic modified chitosan and oxidized dextran. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 1235–1244. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Urbanska, A.M.; Gholizadeh, S.S.; Goodarzi, V.; Saeb, M.R.; Mozafari, M. A facile route to the synthesis of anilinic electroactive colloidal hydrogels for neural tissue engineering applications. J. Colloid Interface Sci. 2018, 516, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sui, Y.; Liu, C.; Liu, C.; Wu, M.; Li, B.; Li, Y. A physically crosslinked polydopamine/nanocellulose hydrogel as potential versatile vehicles for drug delivery and wound healing. Carbohydr. Polym. 2018, 188, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Meng, X.; Duan, H.; Ding, Z.; Liu, Y.; Lucia, L. Super Stable and Tough Hydrogel Containing Covalent, Crystalline, and Ionic Cross-Links. Macromol. Chem. Phys. 2016, 217, 32–38. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, S.; Hu, J.; Yu, J.; Feng, G.; Yang, B.; Li, C.; Zhao, N.; Zhu, C.; Xu, J. Microgel-Enhanced Double Network Hydrogel Electrode with High Conductivity and Stability for Intrinsically Stretchable and Flexible All-Gel-State Supercapacitor. ACS Appl. Mater. Interfaces 2018, 10, 19323–19330. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Liang, X.; Li, P.; Deng, Y.; Pei, X.; Tan, Y.; Zhai, K.; Wang, P. Tough, stretchable chemically cross-linked hydrogel using core–shell polymer microspheres as cross-linking junctions. Polymer 2017, 118, 58–67. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, F.; Tsang, W.; Wan, C.; Wu, C. Fabrication of injectable high strength hydrogel based on 4-arm star PEG for cartilage tissue engineering. Biomaterials 2017, 120, 11–21. [Google Scholar] [CrossRef]
- Liu, Y.; Zahedmanesh, H.; Lally, C.; Cahill, P.A.; McGuinness, G.B. Compliance properties of a composite electrospun fibre—Hydrogel blood vessel scaffold. Mater. Lett. 2016, 178, 296–299. [Google Scholar] [CrossRef]
- Deng, J.; Cheng, C.; Teng, Y.; Nie, C.; Zhao, C. Mussel-inspired post-heparinization of a stretchable hollow hydrogel tube and its potential application as an artificial blood vessel. Polym. Chem. 2017, 8, 2266–2275. [Google Scholar] [CrossRef]
- Liu, B.; Fu, Z.; Meng, W.; Chen, M.; Wu, G.; Zhang, M.; Zhang, H. New insights on in situ charge neutralization governing particle size distribution in macroemulsion polymerization. Colloids Surf. A Physicochem. Eng. Asp. 2018, 540, 242–248. [Google Scholar] [CrossRef]
- Chen, J.; Su, Q.; Guo, R.; Zhang, J.; Dong, A.; Lin, C.; Zhang, J. A Multitasking Hydrogel Based on Double Dynamic Network with Quadruple-Stimuli Sensitiveness, Autonomic Self-Healing Property, and Biomimetic Adhesion Ability. Macromol. Chem. Phys. 2017, 218, 1700166. [Google Scholar] [CrossRef]
- Murakami, T.; Schmidt, B.V.; Brown, H.R.; Hawker, C.J. Structural Versatility in Slide-Ring Gels: Influence of Co-Threaded Cyclodextrin Spacers. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 1156–1165. [Google Scholar] [CrossRef]
- Wei, Z.; Yang, J.H.; Liu, Z.Q.; Xu, F.; Zhou, J.X.; Zrínyi, M.; Osada, Y.; Chen, Y.M. Novel biocompatible polysaccharide-based self-healing hydrogel. Adv. Funct. Mater. 2015, 25, 1352–1359. [Google Scholar] [CrossRef]
- Hauck, N.; Seixas, N.; Centeno, S.; Schlüßler, R.; Cojoc, G.; Müller, P.; Guck, J.; Wöll, D.; Wessjohann, L.; Thiele, J. Droplet-Assisted Microfluidic Fabrication and Characterization of Multifunctional Polysaccharide Microgels Formed by Multicomponent Reactions. Polymers 2018, 10, 1055. [Google Scholar] [CrossRef]
- Murphy, R.; Zhu, L.; Narsimhan, G.; Jones, O. Impacts of Size and Deformability of β-Lactoglobulin Microgels on the Colloidal Stability and Volatile Flavor Release of Microgel-Stabilized Emulsions. Gels 2018, 4, 79. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Duan, L.; Guan, S.; Gao, G.; Cheng, Y.; Ren, X.; Wang, Y. The effect of hydrophobic alkyl chain length on the mechanical properties of latex particle hydrogels. RSC Adv. 2017, 7, 44673–44679. [Google Scholar] [CrossRef] [Green Version]
- Mangadlao, J.D.; Huang, R.; Foster, E.L.; Pangilinan, K.D.; Danda, C.; Advincula, A.; Maia, J.M.; Advincula, R.C. Graphene Oxide–Poly (ethylene glycol) methyl ether methacrylate nanocomposite Hydrogels. Macromol. Chem. Phys. 2016, 217, 101–107. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Niu, N.; Zou, J.; Liu, F. A simple coordination strategy for preparing a complex hydrophobic association hydrogel. J. Appl. Polym. Sci. 2018, 135, 46400. [Google Scholar] [CrossRef]
- Du, G.; Cong, Y.; Chen, L.; Chen, J.; Fu, J. Tough and Multi-responsive Hydrogels Based on Core-Shell Structured Macro-crosslinkers. Chin. J. Polym. Sci. 2017, 35, 1286–1296. [Google Scholar] [CrossRef]
- Gu, S.; Cheng, G.; Yang, T.; Ren, X.; Gao, G. Mechanical and Rheological Behavior of Hybrid Cross-Linked Polyacrylamide/Cationic Micelle Hydrogels. Macromol. Mater. Eng. 2017, 302, 1700402. [Google Scholar] [CrossRef]
- Hu, J.; Kurokawa, T.; Nakajima, T.; Wu, Z.L.; Liang, S.M.; Gong, J.P. Fracture process of microgel-reinforced hydrogels under uniaxial tension. Macromolecules 2014, 47, 3587–3594. [Google Scholar] [CrossRef]
- Zhao, W.; Duan, L.; Zhang, B.; Ren, X.; Gao, G.H. Tough and ultrastretchable hydrogels reinforced by poly (butyl acrylate-co-acrylonitrile) latex microspheres as crosslinking centers for hydrophobic association. Polymer 2017, 112, 333–341. [Google Scholar] [CrossRef]
- Ren, X.; Huang, C.; Duan, L.; Liu, B.; Bu, L.; Guan, S.; Hou, J.; Zhang, H.; Gao, G. Super-tough, ultra-stretchable and strongly compressive hydrogels with core–shell latex particles inducing efficient aggregation of hydrophobic chains. Soft Matter 2017, 13, 3352–3358. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Ren, X.; Guan, S.; Duan, L.; Gao, G.H.; Kuai, Y.; Zhang, H. Rapidly recoverable, anti-fatigue, super-tough double-network hydrogels reinforced by macromolecular microspheres. Soft Matter 2017, 13, 1357–1363. [Google Scholar] [CrossRef]
- Ren, X.Y.; Yu, Z.; Liu, B.; Liu, X.J.; Wang, Y.J.; Su, Q.; Gao, G.H. Highly tough and puncture resistant hydrogels driven by macromolecular microspheres. RSC Adv. 2016, 6, 8956–8963. [Google Scholar] [CrossRef]
- Abdolbaghi, S.; Pourmahdian, S.; Saadat, Y. Preparation of poly (acrylamide)/nanoclay organic-inorganic hybrid nanoparticles with average size of ~250 nm via inverse Pickering emulsion polymerization. Colloid Polym. Sci. 2014, 292, 1091–1097. [Google Scholar] [CrossRef]
- Yao, C.; Liu, Z.; Yang, C.; Wang, W.; Ju, X.J.; Xie, R.; Chu, L.Y. Poly (N-isopropylacrylamide)-Clay nanocomposite Hydrogels with Responsive Bending Property as Temperature-Controlled Manipulators. Adv. Funct. Mater. 2015, 25, 2980–2991. [Google Scholar] [CrossRef]
- Xia, M.; Wu, W.; Liu, F.; Theato, P.; Zhu, M. Swelling behavior of thermosensitive nanocomposite hydrogels composed of oligo (ethylene glycol) methacrylates and clay. Eur. Polym. J. 2015, 69, 472–482. [Google Scholar] [CrossRef]
- De, B.; Kuila, T.; Kim, N.H.; Lee, J.H. Carbon dot stabilized copper sulphide nanoparticles decorated graphene oxide hydrogel for high performance asymmetric supercapacitor. Carbon 2017, 122, 247–257. [Google Scholar] [CrossRef]
- Hu, M.; Gu, X.; Hu, Y.; Deng, Y.; Wang, C. PVA/Carbon Dot Nanocomposites Hydrogels for Simple Introduction of Ag Nanoparticles with Enhanced Antibacterial Activity. Macromol. Mater. Eng. 2016, 301, 1352–1362. [Google Scholar] [CrossRef]
- Hou, K.; Li, Y.; Liu, Y.; Zhang, R.; Hsiao, B.S.; Zhu, M. Continuous fabrication of cellulose nanocrystal/poly (ethylene glycol) diacrylate hydrogel fiber from nanocomposites dispersion: Rheology, preparation and characterization. Polymer 2017, 123, 55–64. [Google Scholar] [CrossRef]
- Liu, S.; Jin, M.; Chen, Y.; Gao, H.; Shi, X.; Cheng, W.; Ren, L.; Wang, Y. High internal phase emulsions stabilised by supramolecular cellulose nanocrystals and their application as cell-adhesive macroporous hydrogel monoliths. J. Mater. Chem. B 2017, 5, 2671–2678. [Google Scholar] [CrossRef]
- Yang, J.; Han, C.; Duan, J.; Ma, M.; Zhang, X.; Xu, F.; Sun, R. Synthesis and characterization of mechanically flexible and tough cellulose nanocrystals–polyacrylamide nanocomposite hydrogels. Cellulose 2013, 20, 227–237. [Google Scholar] [CrossRef]
- Hu, Y.; Han, W.; Huang, G.; Zhou, W.; Yang, Z.; Wang, C. Highly Stretchable, Mechanically Strong, Tough, and Self-Recoverable nanocomposite Hydrogels by Introducing Strong Ionic Coordination Interactions. Macromol. Chem. Phys. 2016, 217, 2717–2725. [Google Scholar] [CrossRef]
- Shi, K.; Liu, Z.; Wei, Y.-Y.; Wang, W.; Ju, X.-J.; Xie, R.; Chu, L.-Y. Near-infrared light-responsive poly (N-isopropylacrylamide)/graphene oxide nanocomposite hydrogels with ultrahigh tensibility. ACS Appl. Mater. Interfaces 2015, 7, 27289–27298. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Huang, J.; Wang, T.; Sun, W.; Tong, Z. Multiple Shape Memory, Self-Healable, and Supertough PAA-GO-Fe3+ Hydrogel. Macromol. Mater. Eng. 2017, 302, 1600359. [Google Scholar] [CrossRef]
- Zhang, W.; Yao, R.; Tao, W.; He, H.; Shui, S. Preparation of monodisperse HPMC/PAA hybrid nanogels via surfactant-free seed polymerization. Colloid Polym. Sci. 2014, 292, 317–324. [Google Scholar] [CrossRef]
- Zhao, J.; Jiao, K.; Yang, J.; He, C.; Wang, H. Mechanically strong and thermosensitive macromolecular microsphere composite poly (N-isopropylacrylamide) hydrogels. Polymer 2013, 54, 1596–1602. [Google Scholar] [CrossRef]
- Xia, S.; Song, S.; Ren, X.; Gao, G. Highly tough, anti-fatigue and rapidly self-recoverable hydrogels reinforced with core–shell inorganic–organic hybrid latex particles. Soft Matter 2017, 13, 6059–6067. [Google Scholar] [CrossRef]
- Shao, C.; Chang, H.; Wang, M.; Xu, F.; Yang, J. High-strength, tough, and self-healing nanocomposite physical hydrogels based on the synergistic effects of dynamic hydrogen bond and dual coordination bonds. ACS Appl. Mater. Interfaces 2017, 9, 28305–28318. [Google Scholar] [CrossRef]
- Tissot, I.; Reymond, J.; Lefebvre, F.; Bourgeat-Lami, E. SiOH-functionalized polystyrene latexes. A step toward the synthesis of hollow silica nanoparticles. Chem. Mater. 2002, 14, 1325–1331. [Google Scholar] [CrossRef]
- Song, S.; Sun, S.; Zhang, H. Enhanced properties of poly (vinylidene fluoride) with low filler content SiO 2-g-(MMA-co-BA) core-shell nanoparticles. J. Polym. Res. 2016, 23, 119. [Google Scholar] [CrossRef]
- Xu, J.; Liu, X.; Ren, X.; Gao, G. The role of chemical and physical crosslinking in different deformation stages of hybrid hydrogels. Eur. Polym. J. 2018, 100, 86–95. [Google Scholar] [CrossRef]
- Mark, J.E.; Erman, B. Rubberlike Elasticity: A Molecular Primer; Cambridge University Press: New York, NY, USA, 2007. [Google Scholar]
- Gundogan, N.; Okay, O.; Oppermann, W. Swelling, Elasticity and Spatial Inhomogeneity of Poly (N, N-dimethylacrylamide) Hydrogels Formed at Various Polymer Concentrations. Macromol. Chem. Phys. 2004, 205, 814–823. [Google Scholar] [CrossRef]
- Song, G.; Zhao, Z.; Peng, X.; He, C.; Weiss, R.; Wang, H. Rheological behavior of tough PVP-in situ-PAAm hydrogels physically cross-linked by cooperative hydrogen bonding. Macromolecules 2016, 49, 8265–8273. [Google Scholar] [CrossRef]
- Li, S.; Dai, J.; Gao, G.; Ren, X.; Xia, S.; Gao, Y.; Wang, Q.; Duan, L. Mechanical Property of Hydrogels Regulated by Different Ratios of Latex Particles and Hydrophobic Segments. ChemistrySelect 2018, 3, 4562–4568. [Google Scholar] [CrossRef]
- Xue, W.; Hamley, I.W.; Castelletto, V.; Olmsted, P.D. Synthesis and characterization of hydrophobically modified polyacrylamides and some observations on rheological properties. Eur. Polym. J. 2004, 40, 47–56. [Google Scholar] [CrossRef]



| Sample | ρ (g/mL) | qF | υ2 | G’ (ω = 1 rad/s) | υe (mol/m3) | υ0 | MC |
|---|---|---|---|---|---|---|---|
| P(AM-MMA)-(SiO2-g-PLA) | 1.2923 | 4.066 | 0.2015 | 10180 | 22.06 | 8.38 | 11.08 |
| P(AM-BA)-(SiO2-g-PLA) | 1.2933 | 3.700 | 0.2226 | 13380 | 27.13 | 13.69 | 10.66 |
| P(AM-LA)-(SiO2-g-PLA) | 1.2959 | 3.466 | 0.2383 | 15070 | 29.19 | 32.95 | 8.88 |
| P(AM-SA)-(SiO2-g-PLA) | 1.2979 | 4.130 | 0.1975 | 12720 | 27.94 | 31.12 | 11.75 |
| Pure PAM | 1.1890 | 3.758 | 0.2337 | 6400 | 12.81 a | - | - |
| Sample | Mn |
|---|---|
| P(AM-LA)-(SiO2-g-PBA) | 1.15 × 1017 |
| P(AM-LA)-(SiO2-g-PLA) | 8.2 × 1016 |
| P(AM-LA)-(SiO2-g-PSA) | 4.9 × 1016 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, X.; Liu, C.; Shao, Z.; Sun, S. Tuning Physical Crosslinks in Hybrid Hydrogels for Network Structure Analysis and Mechanical Reinforcement. Polymers 2019, 11, 352. https://doi.org/10.3390/polym11020352
Lv X, Liu C, Shao Z, Sun S. Tuning Physical Crosslinks in Hybrid Hydrogels for Network Structure Analysis and Mechanical Reinforcement. Polymers. 2019; 11(2):352. https://doi.org/10.3390/polym11020352
Chicago/Turabian StyleLv, Xue, Chuang Liu, Zhubao Shao, and Shulin Sun. 2019. "Tuning Physical Crosslinks in Hybrid Hydrogels for Network Structure Analysis and Mechanical Reinforcement" Polymers 11, no. 2: 352. https://doi.org/10.3390/polym11020352