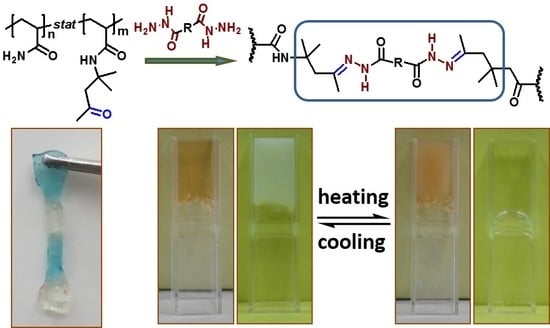
Self-Healing Hydrogels with both LCST and UCST through Cross-Linking Induced Thermo-Response
Abstract
:1. Introduction
2. Experimental section
2.1. Materials
2.2. Characterizations
2.3. Polymerization of AM and DAA Mediated by DDMAT
2.4. Synthesis of PEO23DNH
2.5. Preparation of Self-Healing Hydrogel Containing Ketone type Acylhydrazone Bonds
2.6. Thermo-Response of Hydrogels Regulated by Composition and Cross-linkers
2.7. Gel-Sol-Gel Transition of the Hydrogels Under a Variety of Triggers
2.8. Morphology Observation of the Self-Healing Hydrogels with Different Cross-linker and Transparency
3. Results and Discussion
3.1. Preparation of P(AM-stat-DAA) with Pendant Ketone Groups and Synthesis of PEO23 DNH
3.2. Preparation of Hydrogels from P(AM-stat-DAA) Cross-Linked by Various Diacylhydrazides
3.3. Rheological Properties of Self-Healing Hydrogels
3.4. Thermo-Responsive Property of Hydrazone Bond Containing Hydrogels
3.5. Self-Healing Property of the Hydrogels
3.6. Gel-Sol-Gel Transition of the Self-Healing Hydrogels Triggered by pH, Redox, and Group Ratios
3.7. Thermostability of the Copolymer and Hydrogels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yang, X.; Liu, G.; Peng, L.; Guo, J.; Tao, L.; Yuan, J.; Chang, C.; Wei, Y.; Zhang, L. Highly Efficient Self-Healing and Dual Responsive Cellulose-Based Hydrogels for Controlled Release and 3D Cell Culture. Adv. Funct. Mater. 2017, 27, 1703174. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, C.; Li, Y.; Wang, K.; Wang, X.; Wei, Y.; Tao, L. Synthesis of an injectable, self-healing and dual responsive hydrogel for drug delivery and 3D cell cultivation. Polym. Chem. 2017, 8, 537–544. [Google Scholar] [CrossRef]
- Li, G.; Wu, J.; Wang, B.; Yan, S.; Zhang, K.; Ding, J.; Yin, J. Self-Healing Supramolecular Self-Assembled Hydrogels Based on Poly(L-glutamic acid). Biomacromolecules 2015, 16, 3508–3518. [Google Scholar] [CrossRef] [PubMed]
- Park, H.I.; Park, S.Y. Smart Fluorescent Hydrogel Glucose BiosensingMicrodroplets with Dual-Mode Fluorescence Quenching and Size Reduction. ACS Appl. Mater. Interfaces 2018, 10, 30172–30179. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.K.; Taneja, S.; Singh, Y. Hydrazone-Linkage-Based Self-Healing and Injectable Xanthan-Poly(ethylene glycol) Hydrogels for Controlled Drug Release and 3D Cell Culture. ACS Appl. Mater. Interfaces 2018, 10, 30936–30945. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Tang, Q.; Zhou, Q.; Peng, K.; Yang, H.; Zhang, X. A photo-degradable injectable self-healing hydrogel based on star poly(ethylene glycol)-b-polypeptide as a potential pharmaceuticals delivery carrier. Soft Matter. 2018, 14, 7420–7428. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Zhao, X.; Liang, Y.; Zhang, T.; Ma, P.X.; Guo, B. Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials 2018, 183, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Huo, S.; Wang, T.; Sun, W.; Tong, Z. Self-healable tough supramolecular hydrogels crosslinked by polycyclodextrin through host-guest interaction. Carbohydr. Polym. 2018, 193, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Phadke, A.; Zhang, C.; Arman, B.; Hsu, C.-C.; Mashelkar, R.A.; Lele, A.K.; Tauber, M.J.; Arya, G.; Varghese, S. Rapid self-healing hydrogels. Proc. Natl. Acad. Sci. USA 2012, 109, 4383–4388. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Mynar, J.L.; Yoshida, M.; Lee, E.; Lee, M.; Okuro, K.; Kinbara, K.; Aida, T. High-water-content mouldable hydrogels by mixing clay and a dendritic molecular binder. Nature 2010, 463, 339–343. [Google Scholar] [CrossRef]
- Ghosh, B.; Urban, M.W. Self-Repairing Oxetane-Substituted Chitosan Polyurethane Networks. Science 2009, 323, 1458–1460. [Google Scholar] [CrossRef] [PubMed]
- Kakuta, T.; Takashima, Y.; Nakahata, M.; Otsubo, M.; Yamaguchi, H.; Harada, A. Preorganized Hydrogel: Self-Healing Properties of Supramolecular Hydrogels Formed by Polymerization of Host–Guest-Monomers that Contain Cyclodextrins and Hydrophobic Guest Groups. Adv. Mater. 2013, 25, 2849–2853. [Google Scholar] [CrossRef] [PubMed]
- Jeon, I.; Cui, J.; Illeperuma, W.R.K.; Aizenberg, J.; Vlassak, J.J. Extremely Stretchable and Fast Self-Healing Hydrogels. Adv. Mater. 2016, 28, 4678–4683. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.-M.; Roh, Y.-S.; Cho, S.-Y.; Kim, J.-G. Crack Healing in Polymeric Materials via Photochemical [2+2] Cycloaddition. Chem. Mater. 2004, 16, 3982–3984. [Google Scholar] [CrossRef]
- Kuhl, N.; Bode, S.; Bose, R.K.; Vitz, J.; Seifert, A.; Hoeppener, S.; Garcia, S.J.; Spange, S.; van der Zwaag, S.; Hager, M.D.; et al. Acylhydrazones as Reversible Covalent Crosslinkers for Self-Healing Polymers. Adv. Funct. Mater. 2015, 25, 3295–3301. [Google Scholar] [CrossRef]
- Ying, H.; Zhang, Y.; Cheng, J. Dynamic urea bond for the design of reversible and self-healing polymers. Nat. Commun. 2014, 5, 3218. [Google Scholar] [CrossRef]
- Chen, X.; Dam, M.A.; Ono, K.; Mal, A.; Shen, H.; Nutt, S.R.; Sheran, K.; Wudl, F. A Thermally Re-mendable Cross-Linked Polymeric Material. Science 2002, 295, 1698–1702. [Google Scholar] [CrossRef] [PubMed]
- Imato, K.; Ohishi, T.; Nishihara, M.; Takahara, A.; Otsuka, H. Network Reorganization of Dynamic Covalent Polymer Gels with Exchangeable Diarylbibenzofuranone at Ambient Temperature. J. Am. Chem. Soc. 2014, 136, 11839–11845. [Google Scholar] [CrossRef]
- Imato, K.; Nishihara, M.; Kanehara, T.; Amamoto, Y.; Takahara, A.; Otsuka, H. Self-Healing of Chemical Gels Cross-Linked by Diarylbibenzofuranone-Based Trigger-Free Dynamic Covalent Bonds at Room Temperature. Angew. Chem. Int. Ed. 2012, 51, 1138–1142. [Google Scholar] [CrossRef]
- Cash, J.J.; Kubo, T.; Bapat, A.P.; Sumerlin, B.S. Room-Temperature Self-Healing Polymers Based on Dynamic-Covalent Boronic Esters. Macromolecules 2015, 48, 2098–2106. [Google Scholar] [CrossRef]
- Cromwell, O.R.; Chung, J.; Guan, Z. Malleable and Self-Healing Covalent Polymer Networks through Tunable Dynamic Boronic Ester Bonds. J. Am. Chem. Soc. 2015, 137, 6492–6495. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Deng, F.; Peng, Y.; Chen, H.; Gao, Y.; Li, H. Redox- and pH-responsive polymer gels with reversible sol-gel transitions and self-healing properties. RSC Adv. 2014, 4, 47361–47367. [Google Scholar] [CrossRef]
- Li, H.; Bai, J.; Shi, Z.; Yin, J. Environmental friendly polymers based on schiff-base reaction with self-healing, remolding and degradable ability. Polymer 2016, 85, 106–113. [Google Scholar] [CrossRef]
- An, S.Y.; Noh, S.M.; Nam, J.H.; Oh, J.K. Dual Sulfide-Disulfide Crosslinked Networks with Rapid and Room Temperature Self-Healability. Macromol. Rapid Commun. 2015, 36, 1255–1260. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ding, X.; Urban, M.W. Chemical and physical aspects of self-healing materials. Prog. Polym. Sci. 2015, 49–50, 34–59. [Google Scholar] [CrossRef]
- Scheiner, M.; Dickens, T.J.; Okoli, O. Progress towards self-healing polymers for composite structural applications. Polymer 2016, 83, 260–282. [Google Scholar] [CrossRef]
- Wei, Z.; Yang, J.H.; Zhou, J.; Xu, F.; Zrinyi, M.; Dussault, P.H.; Osada, Y.; Chen, Y.M. Self-healing gels based on constitutional dynamic chemistry and their potential applications. Chem. Soc. Rev. 2014, 43, 8114–8131. [Google Scholar] [CrossRef] [PubMed]
- Amamoto, Y.; Kamada, J.; Otsuka, H.; Takahara, A.; Matyjaszewski, K. Repeatable Photoinduced Self-Healing of Covalently Cross-Linked Polymers through Reshuffling of Trithiocarbonate Units. Angew. Chem. Int. Ed. 2011, 50, 1660–1663. [Google Scholar] [CrossRef]
- Deng, C.C.; Brooks, W.L.A.; Abboud, K.A.; Sumerlin, B.S. Boronic Acid-Based Hydrogels Undergo Self-Healing at Neutral and Acidic pH. ACS Macro Lett. 2015, 4, 220–224. [Google Scholar] [CrossRef]
- Amaral, A.J.R.; Emamzadeh, M.; Pasparakis, G. Transiently malleable multi-healing hydrogel nanocomposites based on responsive boronic acid copolymers. Polym. Chem. 2018, 9, 525–537. [Google Scholar] [CrossRef]
- Guo, R.W.; Su, Q.; Zhang, J.W.; Dong, A.J.; Lin, C.G.; Zhang, J.H. Facile Access to Multisensitive and Self-Healing Hydrogels with Reversible and Dynamic Boronic Ester and Disulfide Linkages. Biomacromolecules 2017, 18, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Shan, M.; Gong, C.; Li, B.; Wu, G. A pH, glucose, and dopamine triple-responsive, self-healing adhesive hydrogel formed by phenylborate-catechol complexation. Polym. Chem. 2017, 8, 2997–3005. [Google Scholar] [CrossRef]
- Mukherjee, S.; Hill, M.R.; Sumerlin, B.S. Self-healing hydrogels containing reversible oxime crosslinks. Soft Matter 2015, 11, 6152–6161. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tao, L.; Li, S.; Wei, Y. Synthesis of Multiresponsive and Dynamic Chitosan-Based Hydrogels for Controlled Release of Bioactive Molecules. Biomacromolecules 2011, 12, 2894–2901. [Google Scholar] [CrossRef] [PubMed]
- Raza-Karimi, A.; Khodadadi, A. Mechanically Robust 3D Nanostructure Chitosan-Based Hydrogels with Autonomic Self-Healing Properties. ACS Appl. Mater. Interfaces 2016, 8, 27254–27263. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.H.; Li, F.Y.; Yu, H.X.; Liu, F.Y.; Liu, C.Y.; Sun, W.X.; Jiang, H.F.; Chen, Y.M. Dynamic Hydrogels with an Environmental Adaptive Self-Healing Ability and Dual Responsive Sol-Gel Transitions. ACS Macro Lett. 2012, 1, 275–279. [Google Scholar] [CrossRef]
- Chang, R.; Wang, X.; Li, X.; An, H.; Qin, J. Self-Activated Healing Hydrogels with Reversible Temperature Responsiveness. ACS Appl. Mater. Interfaces 2016, 8, 25544–25551. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Cao, X.; Du, J.; Wang, G.; Chen, X. Multifunctional Hydrogel with Good Structure Integrity, Self-Healing, and Tissue-Adhesive Property Formed by Combining Diels–Alder Click Reaction and Acylhydrazone Bond. ACS Appl. Mater. Interfaces 2015, 7, 24023–24031. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; An, H.; Li, X.; Zhou, R.; Qin, J.; Tian, Y.; Deng, K. Self-healing polymer gels with multi-responsiveness of gel-sol-gel transition and degradability. Polym. Chem. 2017, 8, 1263–1271. [Google Scholar] [CrossRef]
- Ma, C.; Lu, W.; Yang, X.; He, J.; Le, X.; Wang, L.; Zhang, J.; Serpe, M.J.; Huang, Y.; Chen, T. Bioinspired Anisotropic Hydrogel Actuators with On–Off Switchable and Color-Tunable Fluorescence Behaviors. Adv. Funct. Mater. 2018, 28, 1704568. [Google Scholar] [CrossRef]
- Fundueanu, G.; Constantin, M.; Bucatariu, S.; Ascenzi, P. pH/thermo-responsive poly(N-isopropylacrylamide-co-maleic acid) hydrogel with a sensor and an actuator for biomedical applications. Polymer 2017, 110, 177–186. [Google Scholar] [CrossRef]
- Guan, Y.; Zhang, Y. PNIPAM microgels for biomedical applications: From dispersed particles to 3D assemblies. Soft Matter 2011, 7, 6375–6384. [Google Scholar] [CrossRef]
- Wang, X.; Bian, G.; Zhang, M.; Chang, L.; Li, Z.; Li, X.; An, H.; Qin, J.; Chang, R.; Wang, H. Self-healing hydrogels with cross-linking induced thermo-responsiveness and multi-triggered gel-sol-gel transition. Polym. Chem. 2017, 8, 2872–2880. [Google Scholar] [CrossRef]
- Wang, X.; Chang, L.; Hu, J.; Lang, X.; Fu, X.; An, H.; Wang, Y.; Wang, H.; Qin, J. Self-healing hydrogels with crosslinking induced thermo-responsiveness and regulated properties from water soluble polymer. Polymer 2017, 131, 202–208. [Google Scholar] [CrossRef]
- Guo, Z.; Ma, W.; Gu, H.; Feng, Y.; He, Z.; Chen, Q.; Mao, X.; Zhang, J.; Zheng, L. pH-Switchable and self-healing hydrogels based on ketone type acylhydrazone dynamic covalent bonds. Soft Matter 2017, 13, 7371–7380. [Google Scholar] [CrossRef] [PubMed]
- Vatankhah-Varnoosfaderani, M.; Hashmi, S.; GhavamiNejad, A.; Stadler, F.J. Rapid self-healing and triple stimuli responsiveness of a supramolecular polymer gel based on boron-catechol interactions in a novel water-soluble mussel-inspired copolymer. Polym. Chem. 2014, 5, 512–523. [Google Scholar] [CrossRef]
- Lai, J.T.; Filla, D.; Shea, R. Functional Polymers from Novel Carboxyl-Terminated Trithiocarbonates as Highly Efficient RAFT Agents. Macromolecules 2002, 35, 6754–6756. [Google Scholar] [CrossRef]
- Kamada, J.; Koynov, K.; Corten, C.; Juhari, A.; Yoon, J.A.; Urban, M.W.; Balazs, A.C.; Matyjaszewski, K. Redox Responsive Behavior of Thiol/Disulfide-Functionalized Star Polymers Synthesized via Atom Transfer Radical Polymerization. Macromolecules 2010, 43, 4133–4139. [Google Scholar] [CrossRef]
- An, H.; Li, X.; Fu, X.; Hu, J.; Lang, X.; Liu, X.; Wang, Y.; Wang, H.; Chang, R.; Qin, J. Self-healing hydrogels with NaHCO3 degradability and a reversible gel–sol–gel transition from phenolic ester containing polymers. RSC Adv. 2017, 7, 31212–31220. [Google Scholar] [CrossRef]
- Shi, L.Y.; Wang, F.L.; Zhu, W.; Xu, Z.P.; Fuchs, S.; Hilborn, J.; Zhu, L.J.; Ma, Q.; Wang, Y.J.; Weng, X.S.; Ossipov, D.A. Self-Healing Silk Fibroin-Based Hydrogel for Bone Regeneration: Dynamic Metal-Ligand Self-Assembly Approach. Adv.Funct. Mater. 2017, 27, 1700591. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, P.; Zhao, C.; Wang, W.; Yang, J.; Liu, Q. Light-Switchable Self-Healing Hydrogel Based on Host-Guest Macro-Crosslinking. Macromol. Rapid Commun. 2017, 38, 1600741. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zheng, S.; Sun, W.; Liu, X.; Fu, S.; Tong, Z. Notch insensitive and self-healing PNIPAm-PAM-clay nanocomposite hydrogels. Soft Matter 2014, 10, 3506–3512. [Google Scholar] [CrossRef] [PubMed]
- Kunz, M.J.; Hayn, G.; Saf, R.; Binder, W.H. Hydrogen-bonded supramolecularpoly(ether ketone)s. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 661–674. [Google Scholar] [CrossRef]
- Owusu-Nkwantabisah, S.; Gillmor, J.; Switalski, S.; Mis, M.R.; Bennett, G.; Moody, R.; Antalek, B.; Gutierrez, R.; Slater, G. Synergistic Thermoresponsive Optical Properties of a Composite Self-Healing Hydrogel. Macromolecules 2017, 2017, 3671–3679. [Google Scholar] [CrossRef]













| Copolymer | Composition | DAA Molar Ratio a | Mn (kg mol−1) b | Tgc |
|---|---|---|---|---|
| P1 | P(AM77-stat-DAA8) | 9.4 | 6.8 | 188.8 |
| P2 | P(AM71-stat-DAA14) | 16.5 | 7.4 | 180.5 |
| P3 | P(AM65-stat-DAA20) | 23.2 | 8.0 | 174.6 |
| P4 | P(AM72-stat-DAA28) | 28.0 | 9.9 | 159.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; An, H.; Xi, B.; Yang, Y.; Qin, J.; Wang, Y.; He, Y.; Wang, X. Self-Healing Hydrogels with both LCST and UCST through Cross-Linking Induced Thermo-Response. Polymers 2019, 11, 490. https://doi.org/10.3390/polym11030490
Zhao H, An H, Xi B, Yang Y, Qin J, Wang Y, He Y, Wang X. Self-Healing Hydrogels with both LCST and UCST through Cross-Linking Induced Thermo-Response. Polymers. 2019; 11(3):490. https://doi.org/10.3390/polym11030490
Chicago/Turabian StyleZhao, Haifeng, Heng An, Baozhong Xi, Yan Yang, Jianglei Qin, Yong Wang, Yingna He, and Xinguo Wang. 2019. "Self-Healing Hydrogels with both LCST and UCST through Cross-Linking Induced Thermo-Response" Polymers 11, no. 3: 490. https://doi.org/10.3390/polym11030490