Figure 1.
0-PEG. Temperature dependence of the conservative and dissipative components of the dynamic modulus, E’ and E’’, respectively, and of the loss factor, tan(δ) = E’’/E’. Tg is defined as the temperature at maximum of tan(δ) curve.
Figure 1.
0-PEG. Temperature dependence of the conservative and dissipative components of the dynamic modulus, E’ and E’’, respectively, and of the loss factor, tan(δ) = E’’/E’. Tg is defined as the temperature at maximum of tan(δ) curve.
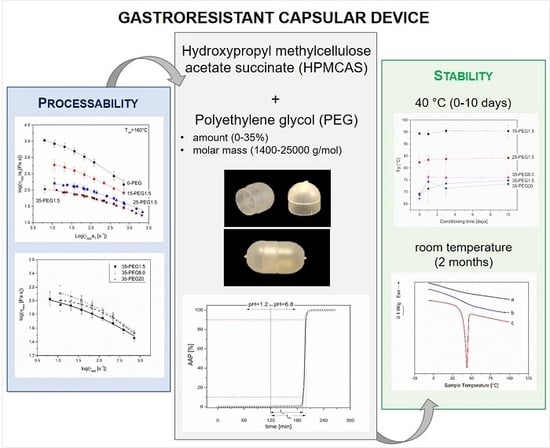
Figure 2.
Photographs of 25-PEG1.5-based body and cap (left) and assembled capsular device (right).
Figure 2.
Photographs of 25-PEG1.5-based body and cap (left) and assembled capsular device (right).
Figure 3.
Release profile of a 25-PEG1.5-based capsular device; calculated t10% and t90% − t10% were about 72 min and 7 min, respectively.
Figure 3.
Release profile of a 25-PEG1.5-based capsular device; calculated t10% and t90% − t10% were about 72 min and 7 min, respectively.
Figure 4.
Steady-state apparent shear viscosity, ηapp, versus apparent shear rate at die wall, app,w, for 0-PEG, 15-PEG1.5, 25-PEG1.5, 35-PEG1.5 from capillary rheometry tests at: 160 °C (■), 155 °C (●), 150 °C (▲), 140 °C (▼). The reference temperature for 25-PEG1.5 and 35-PEG1.5 master curves is 160 °C. Lines represent the fitting with Cross equation (Equation (2)).
Figure 4.
Steady-state apparent shear viscosity, ηapp, versus apparent shear rate at die wall, app,w, for 0-PEG, 15-PEG1.5, 25-PEG1.5, 35-PEG1.5 from capillary rheometry tests at: 160 °C (■), 155 °C (●), 150 °C (▲), 140 °C (▼). The reference temperature for 25-PEG1.5 and 35-PEG1.5 master curves is 160 °C. Lines represent the fitting with Cross equation (Equation (2)).
Figure 5.
Steady-state apparent shear viscosity, ηapp, versus apparent shear rate at die wall, , at 160 °C for 35-PEG1.5, 35-PEG8.0 and 35-PEG20. Lines represent the fitting with Cross equation (Equation (2)).
Figure 5.
Steady-state apparent shear viscosity, ηapp, versus apparent shear rate at die wall, , at 160 °C for 35-PEG1.5, 35-PEG8.0 and 35-PEG20. Lines represent the fitting with Cross equation (Equation (2)).
Figure 6.
Tg dependence on PEG mass fraction of HPMCAS-based formulations. Tests were performed immediately after sample production. The interpolation line uses the Fox equation (Equation (3)).
Figure 6.
Tg dependence on PEG mass fraction of HPMCAS-based formulations. Tests were performed immediately after sample production. The interpolation line uses the Fox equation (Equation (3)).
Figure 7.
E’ dependence on PEG mass fraction of HPMCAS-PEG blends. Tests were performed immediately after sample production. The value was measured at 40 °C, during a temperature ramp experiment.
Figure 7.
E’ dependence on PEG mass fraction of HPMCAS-PEG blends. Tests were performed immediately after sample production. The value was measured at 40 °C, during a temperature ramp experiment.
Figure 8.
Variation in Tg of plasticized HPMCAS samples after thermal treatment at 40 °C. Effect of concentration of PEG1.5 and of molar mass in the case of 35-PEG.
Figure 8.
Variation in Tg of plasticized HPMCAS samples after thermal treatment at 40 °C. Effect of concentration of PEG1.5 and of molar mass in the case of 35-PEG.
Figure 9.
Variation in E’ of plasticized HPMCAS samples after thermal treatment at 40 °C. Effect of concentration of PEG1.5 and of molar mass in the case of 35-PEG.
Figure 9.
Variation in E’ of plasticized HPMCAS samples after thermal treatment at 40 °C. Effect of concentration of PEG1.5 and of molar mass in the case of 35-PEG.
Figure 10.
Average thickness values, with relevant sd, resulting from measurements performed on thin and thick sections of caps (a,b) and bodies (c,d) obtained from15-PEG1.5, 25-PEG1.5 and 35-PEG1.5 formulations.
Figure 10.
Average thickness values, with relevant sd, resulting from measurements performed on thin and thick sections of caps (a,b) and bodies (c,d) obtained from15-PEG1.5, 25-PEG1.5 and 35-PEG1.5 formulations.
Figure 11.
25-PEG1.5 and 35-PEG1.5-based capsular devices filled with a dye-containing formulation at different time points during immersion in unstirred pH 6.8 buffer. Break-up was highlighted by the presence of blue coloration outside the shell after 260 min.
Figure 11.
25-PEG1.5 and 35-PEG1.5-based capsular devices filled with a dye-containing formulation at different time points during immersion in unstirred pH 6.8 buffer. Break-up was highlighted by the presence of blue coloration outside the shell after 260 min.
Figure 12.
Comparison between the dynamical mechanical responses of 25-PEG1.5 either maintained in an oven at 40 °C, indicated as “Transparent”, or at room temperature, indicated as “Translucent”. (a) Conservative component of the complex modulus, E’, versus temperature; (b) Loss factor, tan(δ) versus temperature.
Figure 12.
Comparison between the dynamical mechanical responses of 25-PEG1.5 either maintained in an oven at 40 °C, indicated as “Transparent”, or at room temperature, indicated as “Translucent”. (a) Conservative component of the complex modulus, E’, versus temperature; (b) Loss factor, tan(δ) versus temperature.
Figure 13.
Comparison between the DSC thermograms of dynamical mechanical responses of (a) a 35-PEG1.5 extruded bar immediately after extrusion; (b) the inner, transparent region of a 35-PEG1.5 bar maintained at room temperature for 2 months after extrusion; (c) the external thin opaque layer of a 35-PEG1.5 bar maintained at room temperature for 2 months after extrusion.
Figure 13.
Comparison between the DSC thermograms of dynamical mechanical responses of (a) a 35-PEG1.5 extruded bar immediately after extrusion; (b) the inner, transparent region of a 35-PEG1.5 bar maintained at room temperature for 2 months after extrusion; (c) the external thin opaque layer of a 35-PEG1.5 bar maintained at room temperature for 2 months after extrusion.
Table 1.
Composition of the formulations investigated and relevant codes.
Table 1.
Composition of the formulations investigated and relevant codes.
| | PEG | Formulation Code |
|---|
| Nominal Molar Mass | wt % |
|---|
| HPMCAS | - | 0 | 0-PEG |
| 1500 | 15 | 15-PEG1.5 |
| 25 | 25-PEG1.5 |
| 35 | 35-PEG1.5 |
| 8000 | 35 | 35-PEG8.0 |
| 20000 | 35 | 35-PEG20 |
Table 2.
IM operating conditions.
Table 2.
IM operating conditions.
| Formulation | T1 (°C) | T2 (°C) | T3 (°C) | T4 (°C) | C (mm) | P1 (bar) | r1 (%) | P2 (bar) | r2 (%) |
|---|
| 15-PEG1.5 | 130 | 135 | 155 | 165 | 6 | 30 | 0.4 | 20 | 0.3 |
| 25-PEG1.5 | 130 | 135 | 150 | 160 | 6 | 30 | 0.4 | 20 | 0.3 |
| 35-PEG1.5 | 130 | 135 | 150 | 160 | 7 | 30 | 0.4 | 20 | 0.3 |
| 35-PEG8.0 | 130 | 135 | 160 | 170 | 6 | 30 | 0.4 | 20 | 0.3 |
| 35-PEG20 | 130 | 135 | 160 | 170 | 6 | 30 | 0.4 | 20 | 0.3 |
Table 3.
Fitting parameters of the Cross equation (Equation (2)) for unplasticized HPMCAS and its blends with different PEGs.
Table 3.
Fitting parameters of the Cross equation (Equation (2)) for unplasticized HPMCAS and its blends with different PEGs.
| Formulation | η0 (Pa s) | λ (s) | m (–) | R2 |
|---|
| 0-PEG | 5037 ± 389 | 0.071 ± 0.014 | 0.84 ± 0.04 | 0.998 |
| 15-PEG1.5 | 1003 ± 55 | 0.050 ± 0.008 | 0.68 ± 0.01 | 0.994 |
| 25-PEG1.5 | 236 ± 9 | 0.021 ± 0.003 | 0.55 ± 0.04 | 0.996 |
| 35-PEG1.5 | 141 ± 17 | 0.019 ± 0.004 | 0.52 ± 0.02 | 0.995 |
| 35-PEG8.0 | 115 ± 2 | 0.005 ± 0.003 | 0.71 ± 0.02 | 0.999 |
| 35-PEG20 | 184 ± 14 | 0.019 ± 0.005 | 0.55 ± 0.04 | 0.998 |
Table 4.
Average weight values, with relevant sd, resulting from measurements performed on caps and bodies obtained from 15-PEG1.5, 25-PEG1.5 and 35-PEG1.5 formulations.
Table 4.
Average weight values, with relevant sd, resulting from measurements performed on caps and bodies obtained from 15-PEG1.5, 25-PEG1.5 and 35-PEG1.5 formulations.
| Formulation | Weight (mg) |
|---|
| Cap | Body |
|---|
| 15-PEG1.5 | 114.57 (1.02) | 125.79 (1.99) |
| 25-PEG1.5 | 115.46 (0.62) | 128.07 (0.28) |
| 35-PEG1.5 | 115.85 (0.31) | 127.51 (0.32) |
Table 5.
t10% and t90% − t10% (sd in brackets) relevant to capsular devices of different composition immediately after molding and after 30 days storage at 40 °C.
Table 5.
t10% and t90% − t10% (sd in brackets) relevant to capsular devices of different composition immediately after molding and after 30 days storage at 40 °C.
| Formulation | t0 (min) | t30days (min) |
|---|
| t10% | t90% − t10% | t10% | t90% − t10% |
|---|
| 25-PEG1.5 | 65.2 (6.1) | 19.7 (11.9) | 74.7 (6.4) | 17.9 (8.1) |
| 35-PEG1.5 | 60.3 (5.4) | 13.6 (8.4) | 78.0 (4.3) | 82.9 (15.4) |
| 35-PEG8.0 | 57.4 (7.1) | 15.9 (6.1) | 63.8 (5.7) | 18.3 (8.4) |
| 35-PEG20 | 56.7 (15.1) | 20.5 (3.0) | 68.6 (24.6) | 14.52 (4.8) |
Table 6.
Melting enthalpies and estimated PEG mass fraction in the thin opaque layers of 25-PEG1.5, 35-PEG1.5, 35-PEG8.0 and 35-PEG20 bars maintained at room temperature for 2 months after extrusion. ΔHmelting, PEG is 146, 158 and 159 J/g for PEG1.5, PEG8.0 and PEG20, respectively.
Table 6.
Melting enthalpies and estimated PEG mass fraction in the thin opaque layers of 25-PEG1.5, 35-PEG1.5, 35-PEG8.0 and 35-PEG20 bars maintained at room temperature for 2 months after extrusion. ΔHmelting, PEG is 146, 158 and 159 J/g for PEG1.5, PEG8.0 and PEG20, respectively.
| Formulation | ΔHmelting, thin layer (J/g) | χPEG (%) |
|---|
| 25-PEG1.5 | 5.0 | 3.4 |
| 35-PEG1.5 | 22.4 | 15.2 |
| 35-PEG8.0 | 0.3 | 0.3 |
| 35-PEG20 | 0.3 | 0.3 |