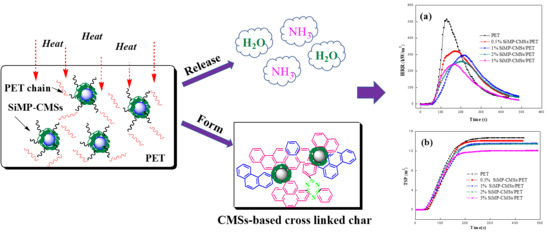
Construction of Carbon Microspheres-Based Silane Melamine Phosphate Hybrids for Flame Retardant Poly(ethylene Terephthalate)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. In Situ Construction of SiMP-CMSs Composites
2.3. Preparation of SiMP-CMSs/PET Composites
2.4. Materials Characterization
3. Results and Discussion
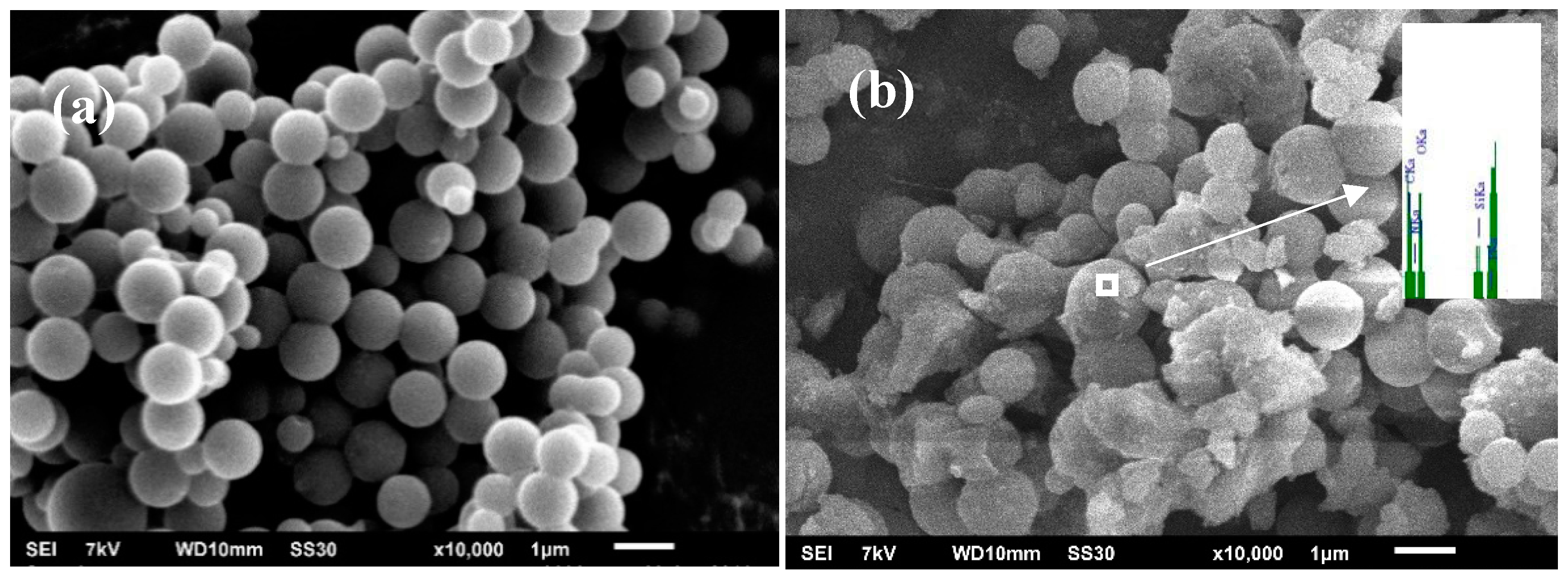
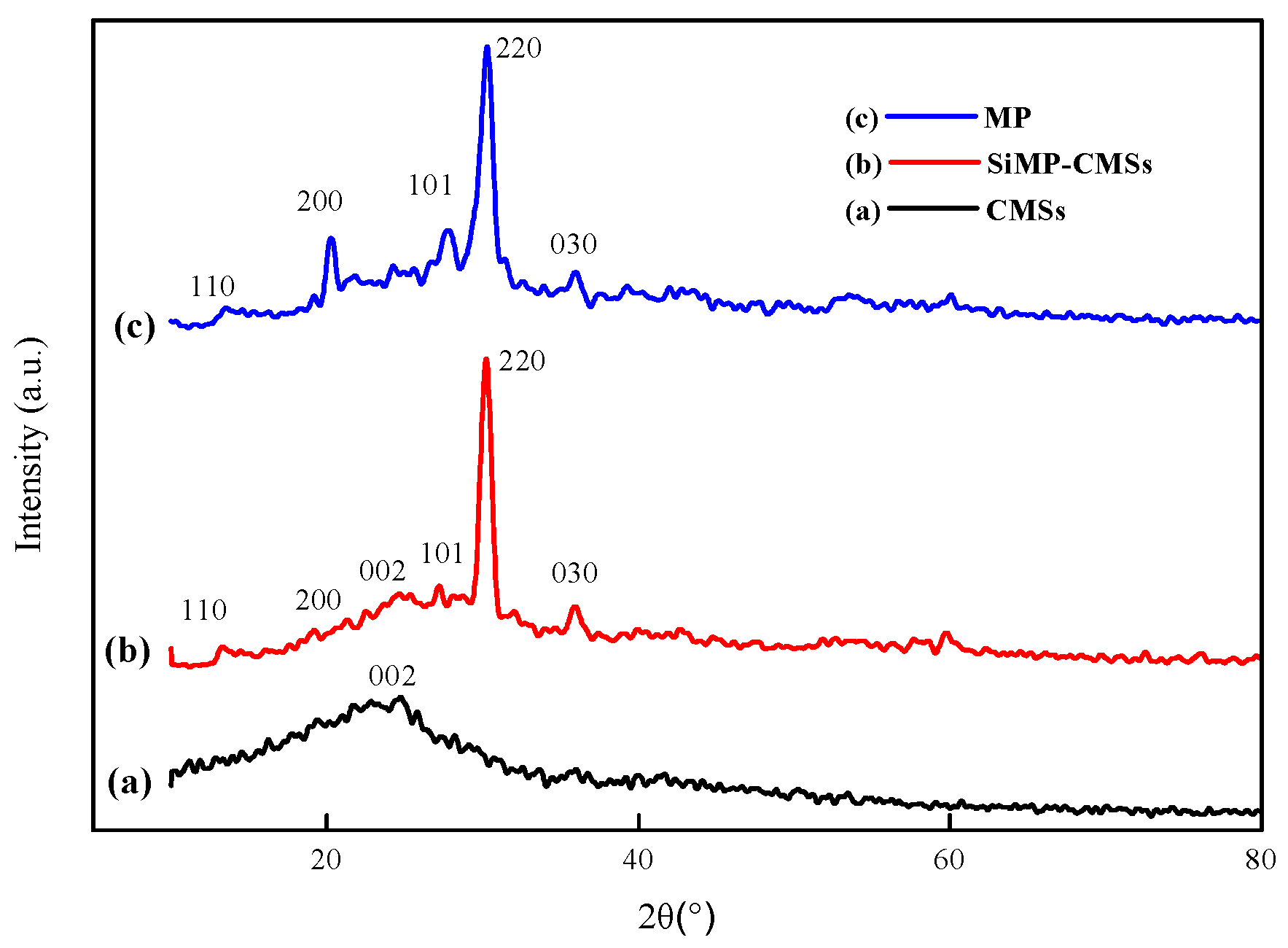
3.1. Morphology and Chemical Structure of SiMP-CMSs
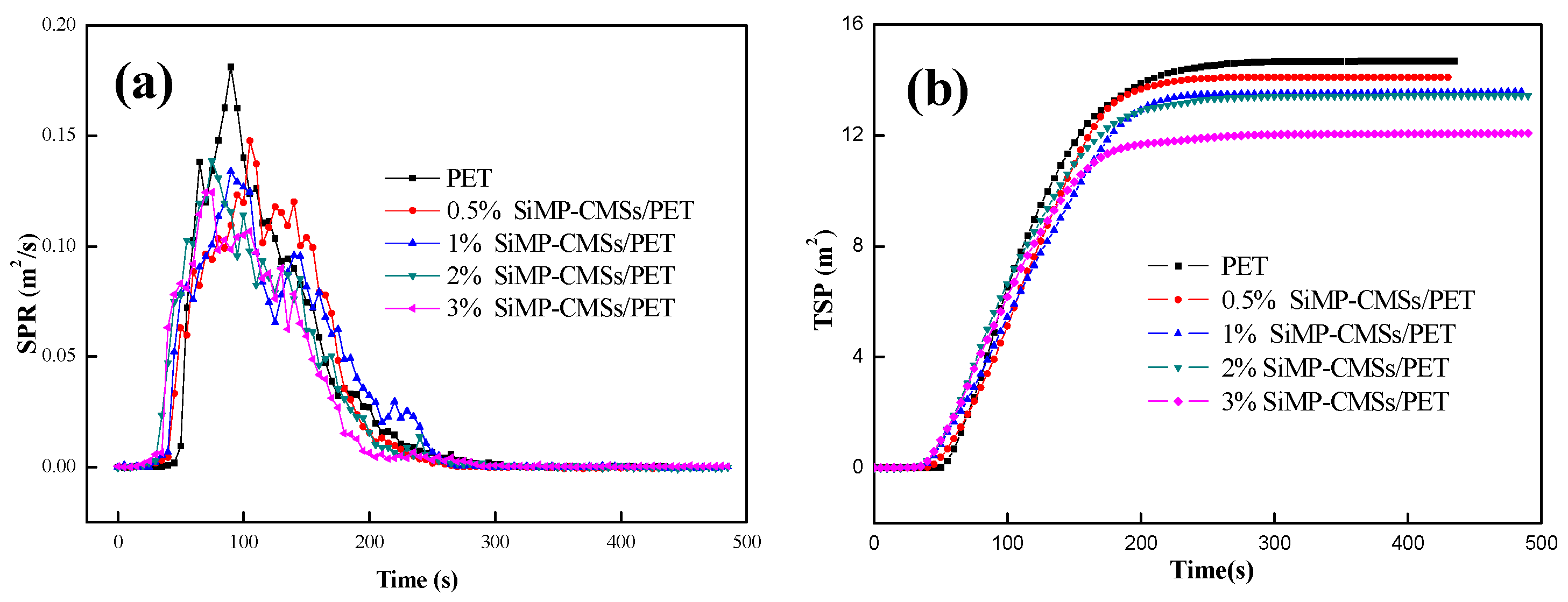
3.2. Flammability, Smoke, and Gas Production
3.3. Quantitative Analyses of Flame-Retardant Mode
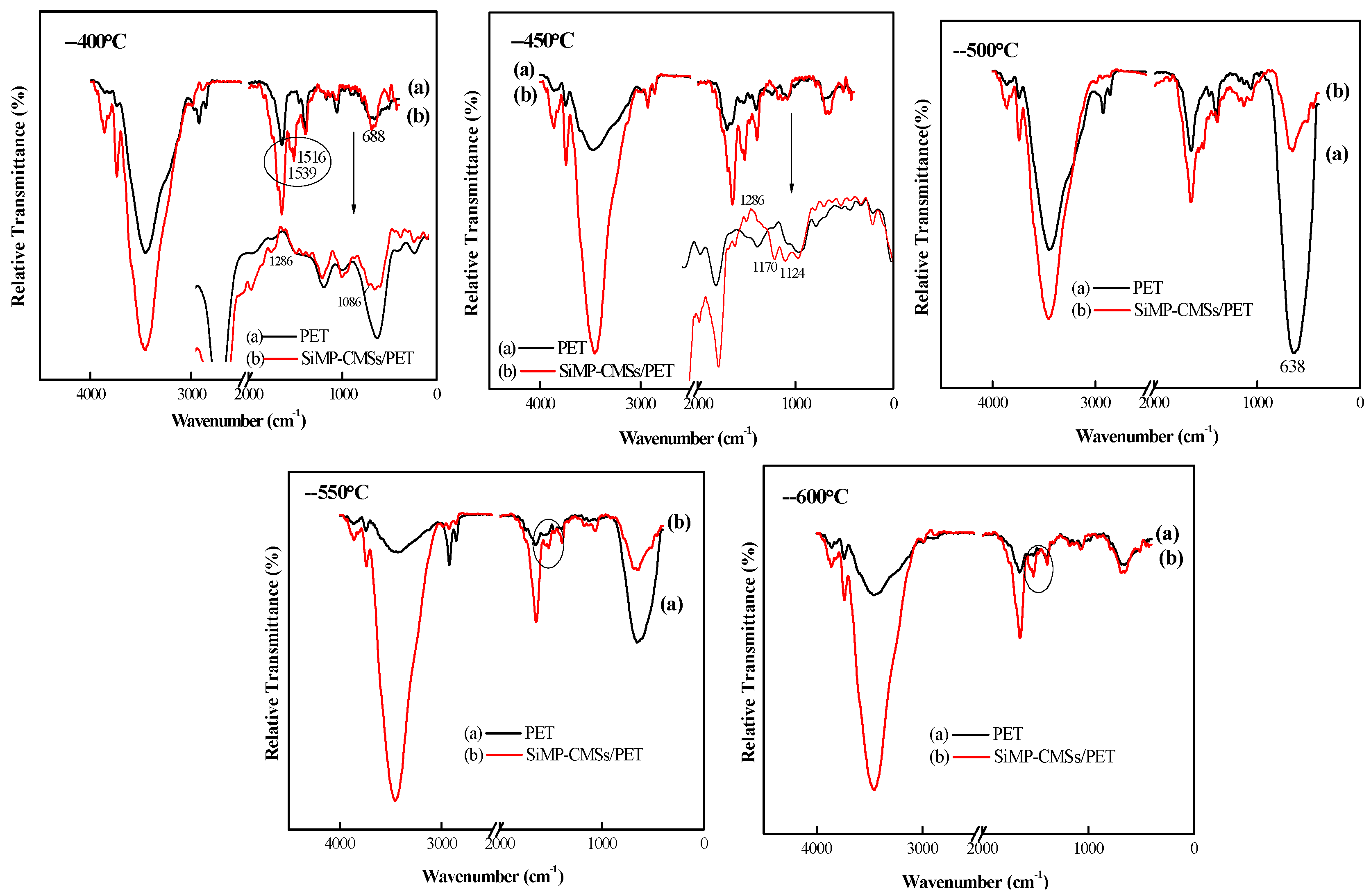
3.4. Thermal Degradation Behavior, Volatilized, and Solid Phase Product
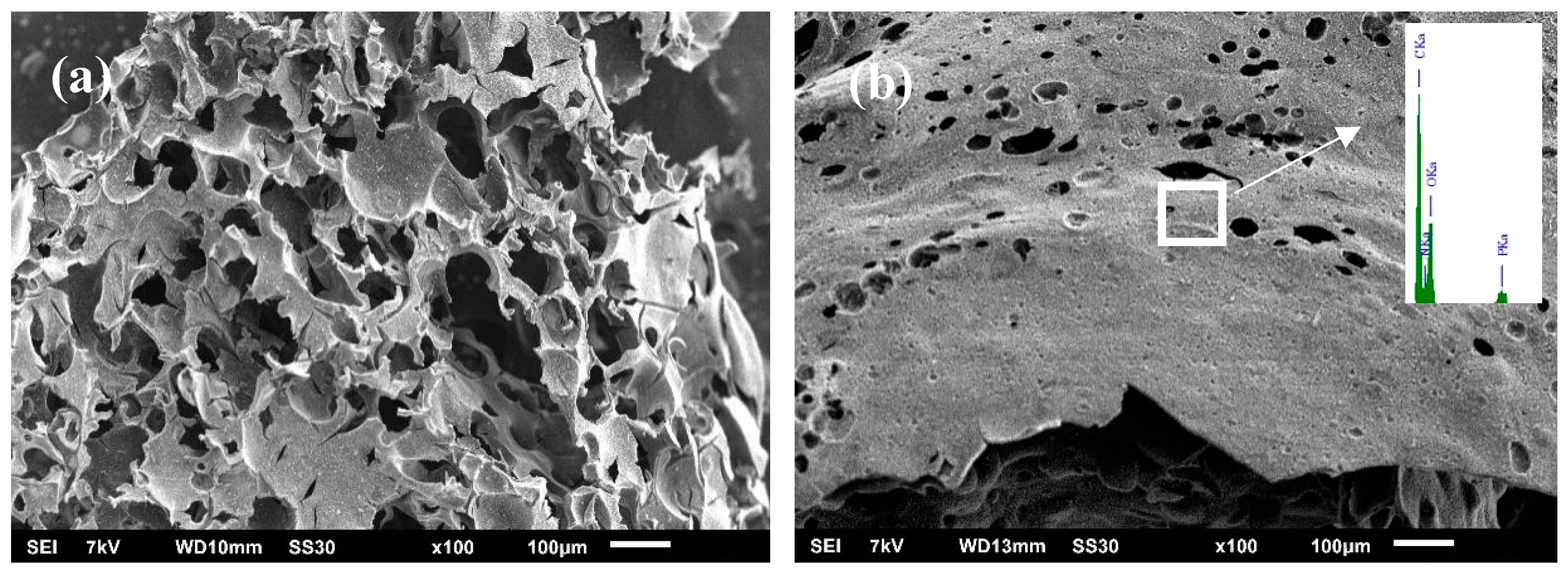
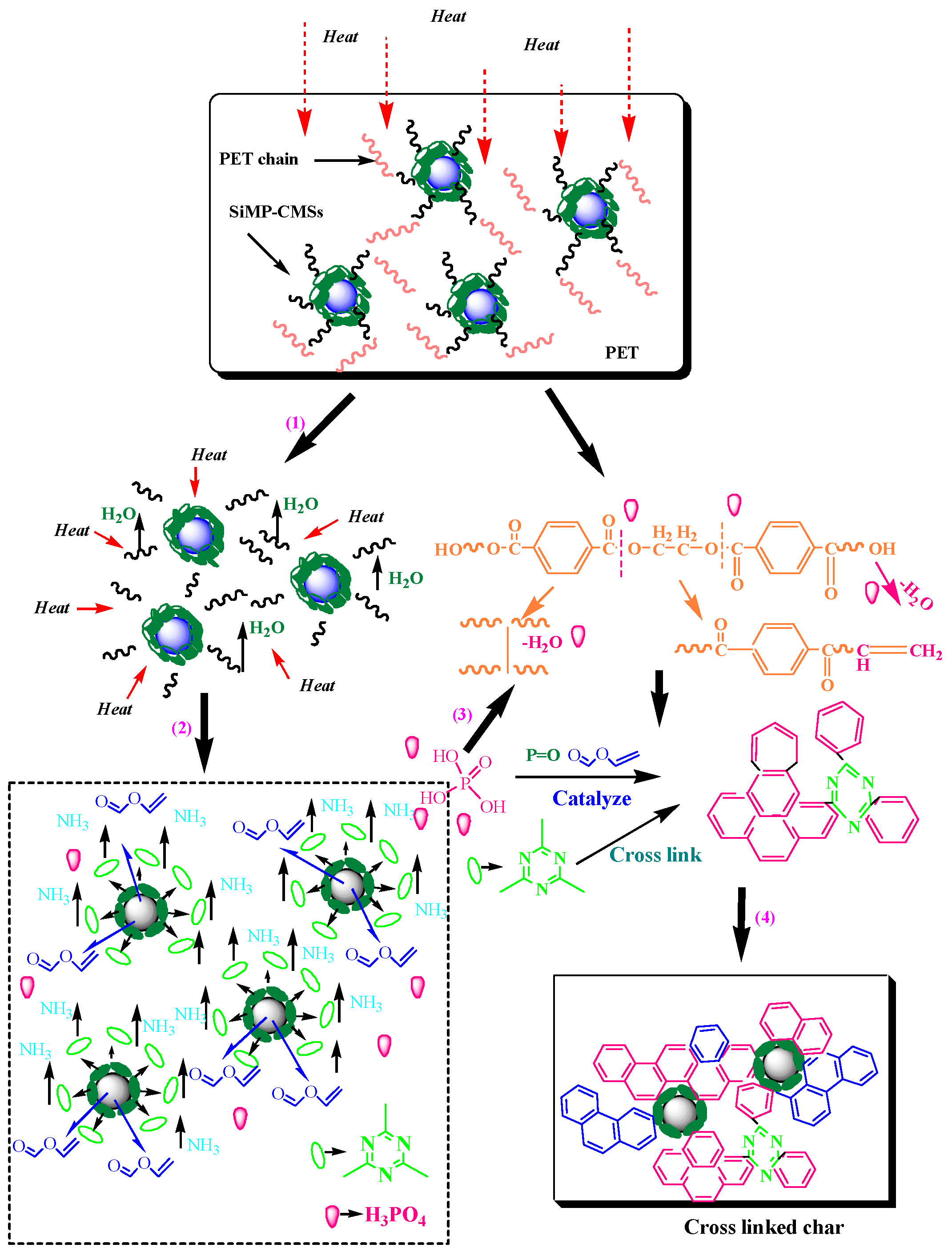
3.5. Char Structure, Flame-Retardant, and Smoke Suppression Mechanism
3.6. Tensile Strength
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aminoddin, H.-J.; Ruholla, S.-R.; Ahmad, M.-S. Improved microwave shielding behavior of carbon nanotube-coated PET fabric using plasma technology. Appl. Surf. Sci. 2014, 311, 593–601. [Google Scholar]
- Butstraen, C.; Salaun, F.; Devaux, E.; Giraud, S.; Vroman, P. Application of flame-retardant double-Layered shell microcapsules to nonwoven polyester. Polymers 2016, 8, 267. [Google Scholar] [CrossRef]
- Wang, W.; Wen, P.-Y.; Zhan, J.; Hong, N.-N.; Cai, W.; Gui, Z.; Hu, Y. Synthesis of a novel charring agent containing pentaerythritol and triazine structure and its intumescent flame retardant performance for polypropylene. Polym. Degrad. Stab. 2017, 144, 454–463. [Google Scholar] [CrossRef]
- Wang, W.; Peng, Y.; Zhang, W.; Li, J.-Z. Effect of Pentaerythritol on the properties of wood-flour/polypropylene/ammonium polyphosphate composite system. Bioresources 2015, 10, 6917–6927. [Google Scholar] [CrossRef]
- Wang, W.; Peng, Y.; Chen, H.; Gao, Q.; Li, J.-Z.; Zhang, W. Surface microencapsulated ammonium polyphosphate with beta-cyclodextrin and its application in wood-flour/polypropylene composites. Polym. Compos. 2017, 38, 2312–2320. [Google Scholar] [CrossRef]
- Hu, S.; Song, L.; Hu, Y. Preparation and characterization of chitosan-based flame retardant and its thermal and combustible behavior on polyvinyl alcohol. Polym. Plast. Technol. 2013, 52, 393–399. [Google Scholar] [CrossRef]
- Zhang, R.; Xiao, X.-F.; Tai, Q.-L.; Huang, H. Modification of lignin and its application as char agent in intumescent flame-retardant poly(lactic acid). Polym. Eng. Sci. 2012, 52, 2620–2626. [Google Scholar] [CrossRef]
- Zheng, Z.-H.; Liu, Y.; Zhang, L.; Zhang, H. Synergistic effect of expandable graphite and intumescent flame retardants on the flame retardancy and thermal stability of polypropylene. J. Mater. Sci. 2016, 51, 5857–5871. [Google Scholar] [CrossRef]
- Ma, H.-Y.; Tong, L.-F.; Xu, Z.-B.; Fang, Z.-P. Functionalizing carbon nanotubes by grafting on intumescent flame retardant: Nanocomposite synthesis, morphology, rheology, and flammability. Adv. Funct. Mater. 2008, 18, 414–421. [Google Scholar] [CrossRef]
- Dittrich, B.; Warting, K.-A.; Mülhaupt, R.; Schartel, B. Flame-retardancy properties of intumescent ammonium poly(phosphate) and mineral filler magnesium hydroxide in combination with graphene. Polymers 2014, 6, 2875–2895. [Google Scholar] [CrossRef]
- Niu, M.; Wang, X.; Yang, Y.-R.; Hou, W.-S.; Dai, J.-M.; Liu, X.-G.; Xu, B.-S. The structure of microencapsulated carbon microspheres and its flame retardancy in poly(ethylene terephthalate). Prog. Org. Coat. 2016, 95, 79–84. [Google Scholar] [CrossRef]
- Thirumal, M.; Khastgir, D.; Nando, G.-B.; Naik, Y.-P.; Singha, N.-K. Halogen-free flame retardant PUF: Effect of melamine compounds on mechanical, thermal and flame retardant properties. Polym. Degrad. Stab. 2010, 95, 1138–1145. [Google Scholar] [CrossRef]
- Huang, G.-B.; Liang, H.-D.; Wang, Y. Combination effect of melamine polyphosphate and graphene on flame retardant properties of poly(vinyl alcohol). Mater. Chem. Phys. 2012, 132, 520–528. [Google Scholar] [CrossRef]
- Wesolek, D.; Gieparda, W. Single- and multiwalled carbon nanotubes with phosphorus based flame retardants for textiles. J. Nanomater. 2014, 9, 1–6. [Google Scholar] [CrossRef]
- Xu, S.-H.; Wang, Z.-Z. Preparation of nano melamine phosphate and its application in phenolic foam. Chin. J. Mater. Res. 2015, 29, 377–382. [Google Scholar]
- Xue, B.-X.; Niu, M.; Yang, Y.-Z.; Bai, J.; Song, Y.-H.; Peng, Y.; Liu, X.-G. Coating magnesium hydroxide on surface of carbon microspheres and interface binding with poly (ethylene terephthalate) matrix. Appl. Surf. Sci. 2017, 412, 545–553. [Google Scholar] [CrossRef]
- Xue, B.-X.; Niu, M.; Yang, Y.-Z.; Bai, J.; Song, Y.-H.; Peng, Y.; Liu, X.-G. Multi-functional carbon microspheres with double shell layers for flame retardant poly (ethylene terephthalate). Appl. Surf. Sci. 2018, 435, 656–665. [Google Scholar] [CrossRef]
- Li, M.; Li, W.; Liu, S.-X. Hydrothermal synthesis characterization, and KOH activation of carbon spheres from glucose. Carbohydr. Res. 2011, 346, 999–1004. [Google Scholar] [CrossRef]
- Yang, Z.; Cai, J.; Zhou, C.-G.; Zhou, D.; Chen, B.-F.; Yang, H.; Cheng, R.-S. Effects of the content of silane coupling agent KH-560 on the properties of LLDPE/Magnesium hydroxide composites. J. Appl. Polym. Sci. 2010, 118, 2634–2641. [Google Scholar] [CrossRef]
- Wang, Z.-Z.; Lv, P.; Hu, Y.; Hu, K.-L. Thermal degradation study of intumescent flame retardants by TG and FTIR: Melamine phosphate and its mixture with pentaerythritol. J. Anal. Appl. Pyrol. 2009, 86, 207–214. [Google Scholar] [CrossRef]
- Wang, X.; Zhan, J.; Xing, W.; Wang, X.; Song, L.; Qian, X.-D.; Yu, B.; Hu, Y. Flame retardancy and thermal properties of novel UV-curable epoxy acrylate coating modified by a silicon-bearing hyperbranched polyphosphonate acrylate. Ind. Eng. Chem. Res. 2013, 52, 5548–5555. [Google Scholar] [CrossRef]
- Tai, Q.-L.; Yuen, R.K.K.; Song, L.; Hu, Y. A novel polymeric flame retardant and exfoliated clay nanocomposites: Preparation and properties. Chem. Eng. J. 2012, 183, 542–549. [Google Scholar] [CrossRef]
- Tang, S.; Wachtendorf, V.; Klack, P.; Qian, J.-L.; Dong, Y.-P.; Schartel, B. Enhanced flame-retardant effect of a montmorillonite/phosphaphenanthrene compound in an epoxy thermoset. RSC Adv. 2017, 7, 720–728. [Google Scholar] [CrossRef] [Green Version]
- Parvinzadeh, M.; Moradian, S.; Rashidi, A.; Yazdanshenas, M.-E. Surface characterization of polyethylene terephthalate/silica nanocomposites. Appl. Surf. Sci. 2010, 256, 2792–2802. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, Y.-Z.; Ban, D.-M. Burning behavior and pyrolysis products of flame-retardant PET containing sulfur-containing aryl polyphosphonate. J. Anal. Appl. Pyrol. 2006, 76, 198–202. [Google Scholar] [CrossRef]
- Badia, J.-D.; Martinez-Felipe, A.; Santonja-Blasco, L. Thermal and thermo-oxidative stability of reprocessed poly(ethylene terephthalate). J. Anal. Appl. Pyrol. 2013, 99, 191–202. [Google Scholar] [CrossRef]
- Li, J.-W.; Feng, P.; Zeng, X.-D. The flame-retardant properties and mechanisms of poly(ethylene terephthalate)/hexakis (para-allyloxyphenoxy) cyclotriphosphazene systems. J. Appl. Polym. Sci. 2015, 132, 42711–42725. [Google Scholar] [CrossRef]






| Sample | LOI (%) | T1/T2 (s) a | Tf (s) b | Igniting Cotton | UL-94 Rating |
|---|---|---|---|---|---|
| PET | 21.0 ± 0.1 | 11.9/7.2 | 85.5 | Yes | NR |
| 0.5%SiMP-CMSs/PET | 25.8 ± 0.2 | 6.9/4.4 | 56.5 | Yes | V-2 |
| 1%SiMP-CMSs/PET | 26.3 ± 0.1 | 5.7/3.6 | 46.5 | Yes | V-2 |
| 2%SiMP-CMSs/PET | 27.1 ± 0.2 | 3.8/2.4 | 31.0 | No | V-0 |
| 3%SiMP-CMSs/PET | 27.7 ± 0.3 | 3.0/1.7 | 23.5 | No | V-0 |
| Sample | pk-HRR (kW/m2) | THR (MJ/m2) | MEHC (MJ/kg) | FPI (m2s/kW) | Residue (%) |
|---|---|---|---|---|---|
| PET | 513.2 | 72.0 | 23.0 | 0.07 | 11.8 |
| 0.5%SiMP-CMSs/PET | 320.2 | 70.5 | 22.3 | 0.12 | 13.8 |
| 1%SiMP-CMSs/PET | 296.2 | 65.3 | 20.5 | 0.13 | 15.2 |
| 2%SiMP-CMSs/PET | 258.8 | 61.3 | 19.9 | 0.15 | 17.4 |
| 3%SiMP-CMSs/PET | 221.7 | 56.0 | 18.6 | 0.17 | 19.8 |
| Sample | TSP (m2) | av-SEA (m2/kg) | SP (kW/kg) | CO Yield (kg/kg) | CO2 Yield (kg/kg) |
|---|---|---|---|---|---|
| PET | 14.7 | 447.8 | 229,830.2 | 0.06 | 1.36 |
| 0.5%SiMP-CMSs/PET | 14.1 | 425.5 | 136,256.8 | 0.06 | 1.33 |
| 1%SiMP-CMSs/PET | 13.6 | 406.2 | 120,297.6 | 0.06 | 1.12 |
| 2%SiMP-CMSs/PET | 13.4 | 379.8 | 98,274.5 | 0.05 | 1.07 |
| 3%SiMP-CMSs/PET | 12.1 | 369.5 | 81,892.3 | 0.04 | 1.03 |
| Samples | Barrier Effect (%) | Flame Inhibition Effect (%) | Charring Effect (%) | |
|---|---|---|---|---|
| SiMP-CMSs/PET | 0.5% | 36.35 | 3.04 | 2.25 |
| 1% | 36.43 | 10.87 | 3.83 | |
| 2% | 40.80 | 13.47 | 6.81 | |
| 3% | 44.53 | 19.04 | 9.04 | |
| Sample | Tonesta (°C) | Tmax1b (°C) | Tmax2c (°C) |
|---|---|---|---|
| PET | 378.6 | 421.1 | 535.6 |
| SiMP-CMSs/PET | 387.7 | 433.5 | 572.3 |
| Peak No. | Main Pyrolysis Product | Content (%) | |
|---|---|---|---|
| PET | SiMP-CMSs/PET | ||
| 1 | Formic acid CH2O2 | 3.03 | 9.02 |
| 2 | Acetic acid C2H4O2 | 4.10 | --- |
| 3 | Propanediol C3H8O2 | 1.94 | 0.65 |
| 4 | Benzene C6H6 | 7.23 | 5.17 |
| 5 | BenzonitrileC6H5 | --- | 0.77 |
| 6 | Phenylglyoxal C8H6O2 | 7.25 | --- |
| 7 | Benzoyl methyl ketone C9H10O2 | --- | 7.11 |
| 8 | Benzene acid C7H6O2 | 34.12 | 38.57 |
| 9 | 2,2-diphenylethenone C12H14O | 1.00 | 1.13 |
| 10 | Biphenyl C12H10 | 4.39 | 3.15 |
| 11 | (4-cyanophenyl) oxygen-containing acetic acid C9H5NO3 | --- | 1.09 |
| 12 | 4-Carboxybenzaldehyde C8H5O3 | 8.05 | 2.84 |
| 13 | Diethyl phthalate C12H12O4 | --- | 1.10 |
| 14 | 4-acetylbenzoic acid C9H8O3 | 4.15 | 5.84 |
| 15 | Acridine C13H9N | --- | 0.16 |
| 16 | 9-Fluorenone C13H8O | 0.82 | 0.55 |
| 17 | 4-biphenyl formalin C13H10O | 5.52 | 3.51 |
| 18 | 4-biphenyl formic acid C13H10O2 | 2.64 | 3.29 |
| 19 | diethylene glycol dibenzoate C16H14O4 | 10.90 | 9.89 |
| 20 | Isopropyl phenyl ketone C10H12O | 1.32 | 0.72 |
| 21 | Vinyl benzoate C9H8O2 | --- | 1.02 |
| 22 | 4-phenyl-2-azetidinone C9H9NO | -- | 0.53 |
| 23 | N-[1-(ethoxyl)] ethyl] phthalic acid C13H15NO5 | --- | 1.15 |
| … | … | … | … |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, B.; Qin, R.; Wang, J.; Niu, M.; Yang, Y.; Liu, X. Construction of Carbon Microspheres-Based Silane Melamine Phosphate Hybrids for Flame Retardant Poly(ethylene Terephthalate). Polymers 2019, 11, 545. https://doi.org/10.3390/polym11030545
Xue B, Qin R, Wang J, Niu M, Yang Y, Liu X. Construction of Carbon Microspheres-Based Silane Melamine Phosphate Hybrids for Flame Retardant Poly(ethylene Terephthalate). Polymers. 2019; 11(3):545. https://doi.org/10.3390/polym11030545
Chicago/Turabian StyleXue, Baoxia, Ruihong Qin, Jie Wang, Mei Niu, Yongzhen Yang, and Xuguang Liu. 2019. "Construction of Carbon Microspheres-Based Silane Melamine Phosphate Hybrids for Flame Retardant Poly(ethylene Terephthalate)" Polymers 11, no. 3: 545. https://doi.org/10.3390/polym11030545